Cinnamaldehyde regulates mitochondrial quality against hydrogen peroxide induced apoptosis in mouse lung mesenchymal stem cells via the PINK1/Parkin signaling pathway
- Published
- Accepted
- Received
- Academic Editor
- Ramcés Falfán-Valencia
- Subject Areas
- Biochemistry, Cell Biology, Molecular Biology
- Keywords
- Cinnamaldehyde, Mitochondrial quality control, Mesenchymal stem cells, Mitochondrial autophagy, PINK1/Parkin
- Copyright
- © 2022 Ke et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Cinnamaldehyde regulates mitochondrial quality against hydrogen peroxide induced apoptosis in mouse lung mesenchymal stem cells via the PINK1/Parkin signaling pathway. PeerJ 10:e14045 https://doi.org/10.7717/peerj.14045
Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is a fatal respiratory disease without effective treatments. Mitochondrial dysfunction weakens the ability of mesenchymal stem cells (MSCs) to repair the distal lung epithelium, which is a probable pathogenesis of IPF. In previous research, we found that cinnamaldehyde (CA) can maintain the mitochondrial morphology of MSCs.
Methods
This present study evaluated the effect and mechanism of CA on murine lung MSCs using the hydrogen peroxide model. Antioxidant effects and mitochondrial function were determined using flow cytometry. The mRNA levels of mitochondrial dynamics and the expressions of autophagy-related proteins were also detected.
Results
CA can increase the levels of SOD, MMP and ATP, decrease the rate of ROS and apoptosis, and restore the mitochondrial structure. CA can also improve the mRNA expression of MFN1, MFN2, FIS1, DRP1, OPA1, and PGC-1α, increase the expression of LC3 II and p62 and promote the PINK1/Parkin signaling pathway. Our results demonstrated that CA can control mitochondrial quality and avoid apoptosis, which may be associated with the regulation of the PINK1/Parkin signaling pathway.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive respiratory disease with a median survival time of 2.8 years (Xaubet, Ancochea & Molina-Molina, 2017). Nintedanib and pirfenidone are approved IPF treatments, but neither drug has prospectively shown a survival benefit (Richeldi, Collard & Jones, 2017). Mesenchymal stem cells (MSCs) have attracted attention as a possible treatment for IPF in recent years (Averyanov et al., 2020; Tzouvelekis et al., 2018). There is a connection between the mitochondrial dysfunction of MSCs and IPF (Cárdenes et al., 2018). Mitochondrial quality affects the differentiation, repairing function and survival of MSCs (Li et al., 2017). Therefore, avoiding mitochondrial dysfunction, regulating the vitality of MSCs, and preserving their repairing effects on lung injuries may be a safer and better treatment method for IPF.
Cinnamaldehyde (CA) is a kind of monomer derived from Cinnamomum cassia with many biological properties, including antioxidant and anti-inflammatory properties (Doyle & Stephens, 2019; Ismail et al., 2022). The antioxidant effects of CA are widely recognized and it has even recently been used as a cancer-specific oxidative stress amplification nanomedicine (Wang et al., 2022a). One study suggested that CA can affect the viability, growth, and differentiation of human adipose-derived MSCs (Absalan et al., 2016). Additionally, several studies have shown the close relationship between CA and the mitochondria (Han et al., 2020; Choi, 2021). In previous research, we found that CA can maintain the mitochondrial morphology of MSCs. Therefore, we propose the following scientific hypotheses: CA can regulate the mitochondrial quality and enhance the vitality of MSCs. In this study, the effects of CA on murine lung mesenchymal stem cells (LMSCs) were investigated and its possible mechanisms were explored.
Materials & Methods
Cell extraction and identification
LMSCs were extracted from C57BL/6 mice and the cells were identified using flow cytometry. Healthy male C57BL/6 mice were provided by the Jiangxi University of Traditional Chinese Medicine (Jiangxi, China). Mice were reared in a barrier environment for one week and then euthanized by cervical dislocation after anesthesia with phenobarbital. Surviving animals continued to be reared for subsequent cell extraction. Mice were also euthanized if they developed depression, loss of appetite, or an inability to stand. More details are provided in Files S1.1–S1.2. This study was approved by the Ethics Committee for Animal Experimentation of Jiangxi University of Traditional Chinese Medicine (SYXK2017-0004).
Antioxidant effects of CA
In this study, hydrogen peroxide (H2O2) was used for modelling. The antioxidant effects of CA on cells were evaluated using measurements of cell viability, total SOD level, intracellular ROS level and apoptosis rate. N-Acetyl-L-cysteine (NAC), the classical antioxidant, was used as a positive control (Files S1.3–S1.6).
Study of cellular mitochondrial quality
The mitochondrial quality of murine LMSCs was assessed using the levels of mitochondrial membrane potential and ATP, and mitochondrial morphology was observed using a transmission electron microscope. The details are displayed in Files S1.7–S1.9.
Investigations of the expression levels of related mRNA and protein
The mRNA levels of mitochondrial dynamics and the expression levels of autophagy-related proteins were then measured and the preliminary exploration of possible mechanisms was carried out. The detailed methods of PCR and Western blotting used for these measurements are provided in Files 1.10–S1.11.
Statistical analysis
All experimental data were obtained from at least three independent experiments and presented as mean ± standard deviation, and between-groups data were evaluated using a one-way ANOVA test on GraphPad software version 8.0 (GraphPad, San Diego, CA, USA). P < 0.05 was defined as statistically different.
Results
Molecular characterization of CA and cell identification
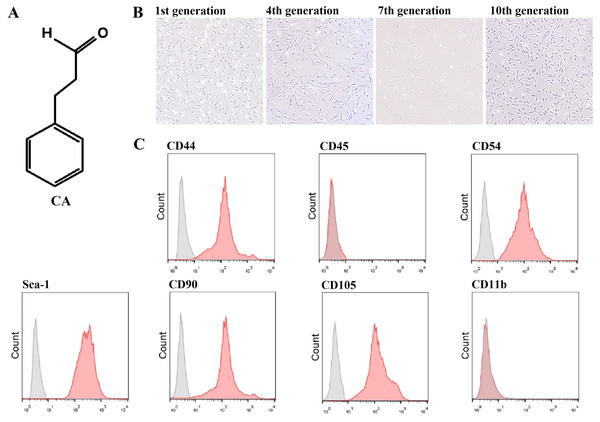
The molecular structure of CA is shown in Fig. 1A, and its molecular weight is 132.16. In addition, the microscopic morphology of murine LMSCs is shown in Fig. 1B (Files S2.1). Flow cytometry results showed that CD44, CD54, CD90, CD105, and Sca-1 were expressed, while CD11b and CD45 were not (Fig. 1C). These results are consistent with the phenotypic characteristics of MSCs.
Antioxidant effects of CA on H2O2-induced murine LMSCs
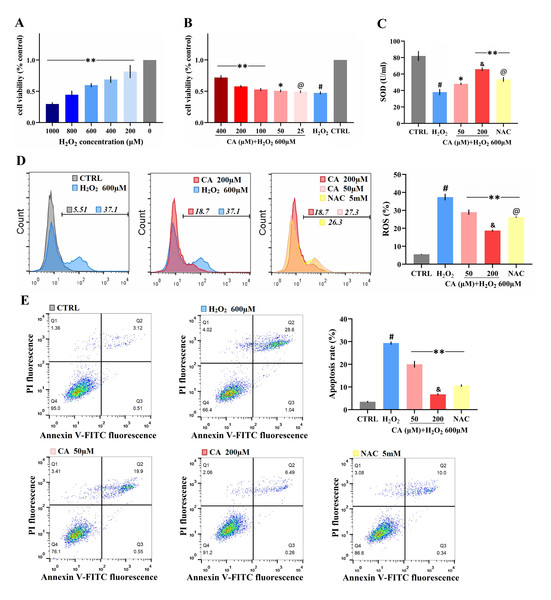
The survival rate of mouse LMSCs decreased gradually as the H2O2 dose increased (Fig. 2A), but the survival rates improved with a 50–400 µM CA intervention (Fig. 2B). The levels of SOD are shown in Fig. 2C; SOD levels in the CA and NAC groups were significantly higher than in the H2O2 group. ROS levels in the CA and NAC groups groups were significantly lower than in the H2O2 group (Fig. 2D). The apoptosis rates of the CA and NAC groups were also significantly lower than the H2O2 group (Fig. 2E). Among the above three indicators, the antioxidant capacity of 200 µM CA on H2O2-induced murine LMSCs was superior to that of NAC. More data are documented in Files S2.2–S2.5.
Figure 1: Molecular characterization of cinnamaldehyde (CA) and identification of murine LMSCs.
(A) Chemical structure of CA. (B) Microscopic morphology of murine LMSCs (magnification: 4×). (C) Phenotypes of mouse LMSCs.Figure 2: Antioxidant effects of CA on H2O2-induced murine LMSCs.
(A) The effects of various doses of H2O2 on cell viability. Data were obtained from seven independent experiments; **P < 0.01 versus 0 µM H2O2 group. (B) The protective effects of CA on H2O2-induced cells. Data were obtained from nine independent experiments; @P > 0.05, *P < 0.05, and **P < 0.01 versus H2O2 group. (C) Total SOD levels of groups. (D) Intracellular ROS production of each group. (E) Apoptosis rates of groups. Data were obtained from three independent experiments; #P < 0.01 versus CTRL groups; *P < 0.05, **P < 0.01 versus H2O2 groups; &P < 0.05 versus NAC groups; @P > 0.05 versus 50 µM CA groups.Protective effects of CA on mitochondrial function
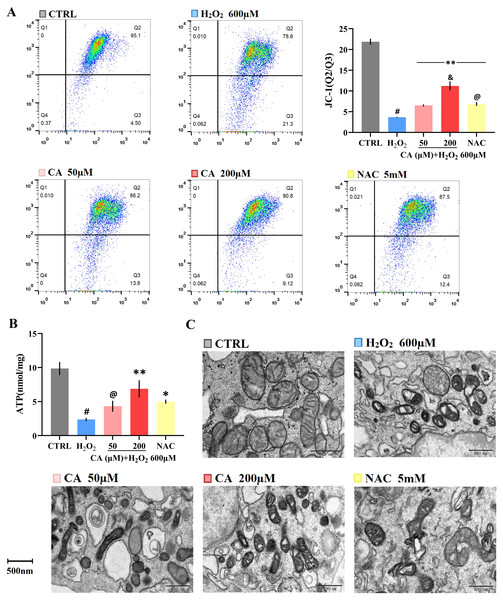
The MMP levels of the NAC and CA groups were significantly higher than the H2O2 group, and the level of the 200 µM CA group was higher than that of the NAC group (Fig. 3A). Additionally, the ATP levels of the 200 µM CA and NAC groups were obviously higher than in the H2O2 groups (Fig. 3B). The detailed data are presented in Files S2.6–S2.7.
Figure 3: Effects of CA on mitochondrial function and morphology of H2O2-treated murine LMSCs.
(A) The MMP levels of cells; #P < 0.01 versus CTRL group. Data were obtained from three independent experiments; **P < 0.01 versus H2O2 group; &P < 0.05 versus NAC group; @P > 0.05 versus 50 µM CA group. (B) Cellular ATP production of each group. Data were obtained from three independent experiments; #P < 0.01 versus CTRL group; *P < 0.05, **P < 0.01 versus H2O2 group; @P > 0.05 versus NAC group. (C) The mitochondria were observed by transmission electron microscopy (magnification: 14,000×).Effects of CA on mitochondrial morphology
Photos of the cells taken by transmission electron microscopy are provided in Fig. 3C. The mitochondria in the control group were healthy, mostly oval in shape, with clear cristae and intact membranes. The mitochondrial structure was seriously damaged in the H2O2 groups: the shape was deformed and twisted; the cristae of the mitochondria were loose, broken or missing; the density of the matrix was decreased or uneven; and numerous vacuoles were observed. In the 50 µM CA and NAC groups, the morphology of the mitochondria was still abnormal, but the cristae could be distinguished, and the matrix was relatively uniform. The morphology in the 200 µM CA group was relatively close to the control group, but with autophagosomes found in the 200 µM CA group.
Effects of CA on the mRNA levels of mitochondrial dynamics
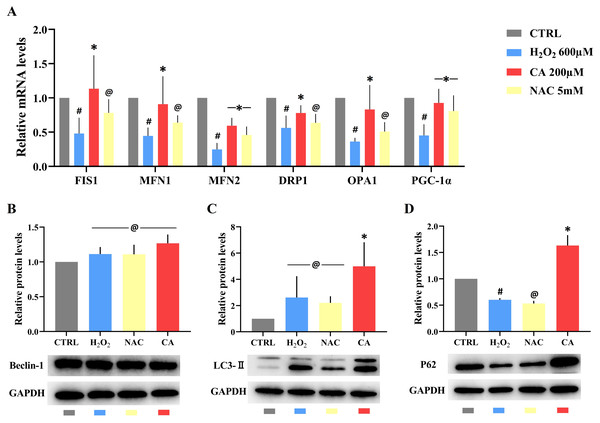
Compared with the control groups, the mRNA levels of FIS1, MFN1, MFN2, DRP1, OPA1, and PGC-1α were decreased in the H2O2 groups. All mRNA levels in the 200 µM CA groups were obviously higher than those in the H2O2 groups, but there was no significant difference in the mRNA levels of FIS1, MFN1, DRP1, and OPA1 between the NAC and H2O2 groups. These results are represented in Fig. 4A. (Files S2.8).
Figure 4: Effects of CA on mitochondrial dynamics genes and autophagy-related proteins in H2O2-treated murine LMSCs.
(A) The mRNA levels of FIS1, MFN1, MFN2, DRP1, OPA1 and PGC-1 α in groups. Data were obtained from six independent experiments; #P < 0.05 versus CTRL groups; *P < 0.05, @P > 0.05 versus H2O2 groups. (B) The protein expression levels of Beclin-1; @P > 0.05 versus CTRL group. (C) The protein expression levels of LC3-II; @P > 0.05 versus CTRL group, *P < 0.05 versus H2O2 group. (D) The protein expression levels of P62; #P < 0.05 versus CTRL group; *P < 0.05, @P > 0.05 versus H2O2 group. Protein expression levels were obtained from three independent experiments.Effects of CA on the expression levels of autophagy-related proteins
There was no statistical difference in the expression of Beclin-1 in all groups (Fig. 4B). There was also no obvious difference in the levels of LC3-II in the H2O2 and NAC groups compared with the control group (Fig. 4C), but the level of LC3-II in the 200 µM CA group was markedly higher than in the control group. P62 levels (Fig. 4D) were lower in the H2O2 group compared to the control group. There was no significant difference in P62 levels between the NAC and H2O2 groups, but the P62 level in the 200 µM CA group was significantly higher. These data are presented in Files S2.9.
Effects of CA on the PINK1/Parkin pathway of H2O2-treated murine LMSCs
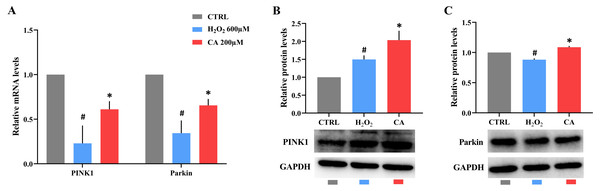
Compared with the control groups, the mRNA levels of PINK1 and Parkin were decreased in the H2O2 groups. However, all the mRNA levels in the 200 µM CA groups were obviously increased (Fig. 5A). The protein expression level of PINK1 in the H2O2 group was higher than the expression level in the control group, and the expression level of PINK1 in the 200 µM CA group was higher than in the H2O2 group (Fig. 5B). Moreover, the protein expression level of Parkin in the H2O2 group was lower than the level in the control group, and the expression level in the 200 µM CA group was higher than in the H2O2 group (Fig. 5C). More data are presented in Files S2.10.
Figure 5: Effects of CA on the PINK1/Parkin pathway of H2O2-treated murine LMSCs.
(A) The mRNA levels of PINK1 and Parkin in groups. (B) The protein expression levels of PINK1 in groups. (C) The protein expression levels of Parkin in groups. #P < 0.05 versus CTRL groups; *P < 0.05 versus H2O2 groups. Data were obtained from three independent experiments.Discussion
IPF is mainly manifested as progressive pulmonary fibrosis, in which healthy and soft lung tissue are replaced by diseased and stiff scars, ultimately endangering the life of the patient (Martinez et al., 2017). The connection between mitochondrial dysfunction and IPF has been a hot topic in recent years (Cárdenes et al., 2018). Mitochondrial dysfunction weakens the ability of MSCs to repair the distal lung epithelium, which is one of the key factors in the pathogenesis of IPF (Fergie et al., 2019). We first found that CA can control mitochondrial quality and avoid the apoptosis of LMSCs, which may be associated with the regulation of the PINK1/Parkin signaling pathway.
As the energy factories of cells, mitochondria produce most of the ATP required for cells. The potential difference between the two sides of the mitochondrial membrane is called the mitochondrial membrane potential (MMP), and is the basis for maintaining mitochondrial oxidation and ATP production. When the quality of mitochondria become worse, MMP and ATP levels decline (Van der Bliek, Sedensky & Morgan, 2017). We observed that CA can maintain MMP levels and increase ATP content in LMSCs. We also obtained more evidence from morphology that CA restored the mitochondrial structure of H2O2-induced LMSCs and the number of mitochondria was increased. Therefore, CA is beneficial to the function and morphology of mitochondria. Moreover, autophagosomes were found in the cells treated by CA. This means that CA is likely to maintain mitochondrial quality through the promotion of mitochondrial autophagy.
The occurrence of mitophagy depends on mitochondrial dynamics, including mitochondrial fission and fusion (Onishi et al., 2021). Mitochondria weed out impaired mitochondria that need to be cleared through continuous fusion and division, and then induce mitochondrial autophagy (Wai & Langer, 2016). The selective removal of damaged mitochondria by autophagosomes is the key to mitochondrial quality control (Kubli & Gustafsson, 2012). Mitochondrial dynamics are co-regulated by the MFN1, MFN2, FIS1, DRP1, and OPA1 proteins. We found that CA can improve the mRNA expressions of these proteins. MFN1 and MFN2 are located on the outer mitochondrial membrane and induce the fusion of the outer mitochondrial membrane (Song et al., 2017). DRP1 mainly mediates mitochondrial division and usually exists in the mitochondrial matrix. DRP1 proteins are recruited by FIS1 on the outer membrane to complete mitochondrial division together (Ikeda et al., 2015). OPA1 is located in the inner mitochondrial membrane and mediates the fusion of the inner membrane (Rovira-Llopis et al., 2017). However, the expression patterns of the genes related to mitochondrial fission and fusion were the same in this experiment, which differs from the opposite patterns commonly seen in studies related to mitochondrial dynamics (Wang et al., 2022b; Liu et al., 2022). We hypothesize that the reduction of those genes means the inhibition of mitophagy. DRP1 prevents apoptosis by promoting mitochondrial autophagy (Pernaute et al., 2022). Mfn2 is a pivotal mediator of mitophagy through the PINK1/Parkin signaling pathway; it facilitates Parkin recruitment from the cytosol to the depolarized mitochondria (Uchikado, Ikeda & Ohishi, 2022). These results indicate that the effect of CA in improving mitochondrial quality is most likely achieved by promoting mitophagy.
CA can also improve the mRNA expression of PGC-1α. The PGC-1α protein plays a key regulatory role in mitochondrial biosynthesis, which affects the energy supply for the initiation of mitochondrial autophagy (Li, Hou & Hao, 2017). In addition, PGC-1α is closely related to mitochondrial energy metabolism and glycolysis (Sreekumar et al., 2022). We are performing subsequent experiments relevant to this topic and will present the results when they are available. In our opinion, CA has positive effects on mitochondria biosynthesis, and it is likely to regulate the mitochondrial quality in multiple ways. Furthermore, there are some related genes that were not involved in this study, such as heat shock protein (HSP), voltage dependent anion channels (VDAC) and prohibitins (PHBs), which are expected to be explored in future studies. The role and impact of HSP is highly complex. Any change in the cellular dynamic equilibrium activates the synthesis of HSP, including mitochondrial stress (Minnaar & Szasz, 2022). The VDAC control the flux of most anionic respiratory substrates, ATP and small cations across the outer mitochondrial membrane, which is closely related to mitochondrial metabolism (Heslop et al., 2022). PHBs, which are evolutionarily strongly conserved proteins, are able to stabilize mitochondrial translation products (Aboouf et al., 2021).
In this study, we hypothesized that mitochondrial autophagy was the most critical link, because we found that CA can boost the expressions of autophagy-related proteins. Beclin-1 is an essential protein related to the formation of autophagosomes. A lack of Beclin-1 will inhibit the occurrence of autophagy (Sun et al., 2018). LC3-II represents the formation of autophagosomes, and it is located on the autophagosome membrane waiting for P62, then the two form a complex and finally degrade in autophagosomes (Rogov et al., 2017; Liu et al., 2017; Yamashita & Kanki, 2017).
Mitochondrial autophagy is interactively regulated by numerous mechanisms including the Parkin-dependent pathway, which is mainly mediated by PINK1-Parkin (Nah, Miyamoto & Sadoshima, 2017). PINK1 relies on the mitochondrial potential difference to transfer to the inner membrane, and is quickly degraded by related proteins in normal conditions (Shin & Chung, 2020). When the mitochondrial function is weakened and the level of MMP is decreased, PINK1 will accumulate on the outer membrane. Then, Parkin in the cytoplasm will be recruited and transferred to the outer membrane. Through a series of effects such as protein ubiquitination, it attracts the autophagy receptor P62, which initiates mitochondrial autophagy (Bayne & Trempe, 2019). In this experiment, we found that CA can up-regulate the PINK1/Parkin signaling pathway. PINK1 protein accumulated on the outer membrane can lead to autophagy through the PINK1/Parkin signaling pathway. PINK1 can also mediate MFN2 and DRP1 to regulate mitochondrial fusion and division, and assist in the occurrence of autophagy (Chen & Dorn, 2013).
We also confirmed the protective effects of CA on LMSCs under oxidative stress through the detection of SOD levels, ROS ratios, and apoptosis rates. SOD is a widespread metal enzyme. Its antioxidant ability stems from the catalysis of superoxide anion. SOD can catalyze the harmful superoxide anion to hydrogen peroxide, which eventually becomes water (Sirisena et al., 2019). In addition, ROS plays a key role in oxidative stress; the excessive production of ROS can trigger and strengthen the oxidative stress response and induce the pathological apoptosis of cells (He et al., 2018; Wu et al., 2018). In this experiment, CA strengthened the antioxidant capacity of cells by regulating SOD and ROS levels, thus reducing apoptosis under oxidative stress. However, it is not clear whether the antioxidant capacity of CA is due to the antioxidant effect of the drug itself or the enhancement of the mitochondrial quality of cells.
Conclusions
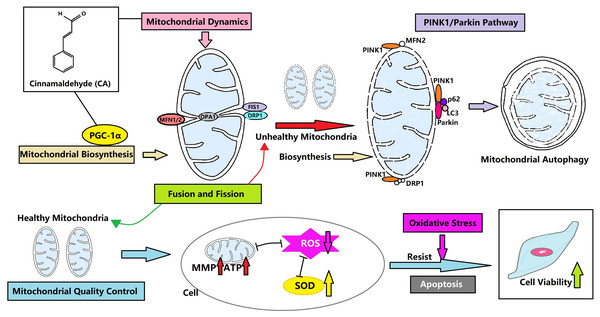
CA can control the mitochondrial quality of LMSCs to resist oxidative stress and avoid apoptosis by regulating the dynamics, biosynthesis and autophagy of mitochondria (Fig. 6). This may be a new direction for the treatment of IPF. We hypothesize that mitochondrial autophagy is the critical link, and CA may promote autophagy through the PINK1/Parkin signaling pathway. CA is likely to regulate the mitochondrial quality in multiple ways, and our team plans to conduct animal experiments and further research in this area in the future.
Figure 6: CA regulates the mitochondrial quality and enhances the vitality of LMSCs.
Supplemental Information
Original western blots of Beclin-1 for three repeats
Figure A represents western blot analysis shown in Fig. 4. B.
Original western blots of LC3-II for three repeats
Figure B represents western blot analysis shown in Fig. 4. C.
Original western blots of P62 for three repeats
Figure C represents western blot analysis shown in Fig. 4. D.
Original western blots of PINK1 for three repeats
Figure D represents western blot analysis shown in Fig. 5. B.
Original western blots of Parkin for three repeats
Figure E represents western blot analysis shown in Fig. 5. C.