Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (6): 708-713.DOI: 10.6023/A22010054 Previous Articles Next Articles
Article
栾雪菲a,b, 王聪芝b, 夏良树a,*( ), 石伟群b,*(
), 石伟群b,*( )
)
投稿日期:2022-01-28
发布日期:2022-07-07
通讯作者:
夏良树, 石伟群
基金资助:
Xuefei Luana,b, Congzhi Wangb, Liangshu Xiaa( ), Weiqun Shib(
), Weiqun Shib( )
)
Received:2022-01-28
Published:2022-07-07
Contact:
Liangshu Xia, Weiqun Shi
Supported by:Share
Xuefei Luan, Congzhi Wang, Liangshu Xia, Weiqun Shi. Theoretical Studies on the Interaction of Uranyl with Carboxylic Acids and Oxime Ligands[J]. Acta Chimica Sinica, 2022, 80(6): 708-713.
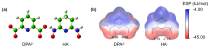
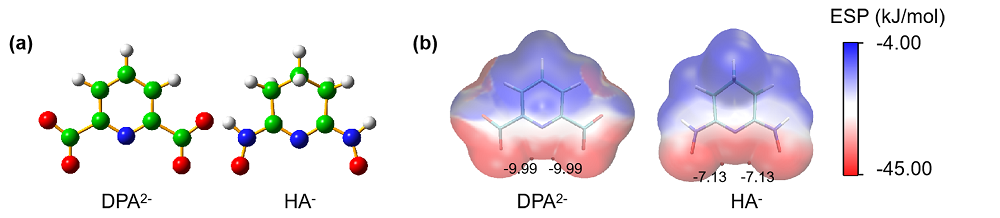
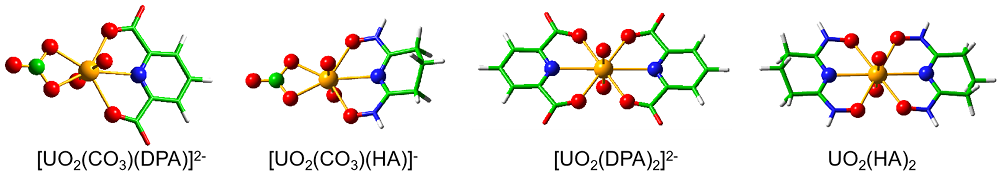
| 铀酰配合物 | U=Oax | U—N(L) | U—O(L) | U—O(CO32-) | vs | vas |
|---|---|---|---|---|---|---|
| [UO2(CO3)3]4- | 0.182 | 0.253 | 765.58 | 835.01 | ||
| [UO2(CO3)(DPA)]2- | 0.180 | 0.265 | 0.247 | 0.231 | 826.61 | 893.72 |
| [UO2(CO3)(HA)]- | 0.180 | 0.266 | 0.254 | 0.228 | 825.19 | 895.12 |
| [UO2(DPA)2]2- | 0.178 | 0.276 | 0.248 | 848.10 | 929.87 | |
| UO2(HA)2 | 0.178 | 0.266 | 0.250 | 851.62 | 932.91 |
| 铀酰配合物 | U=Oax | U—N(L) | U—O(L) | U—O(CO32-) | vs | vas |
|---|---|---|---|---|---|---|
| [UO2(CO3)3]4- | 0.182 | 0.253 | 765.58 | 835.01 | ||
| [UO2(CO3)(DPA)]2- | 0.180 | 0.265 | 0.247 | 0.231 | 826.61 | 893.72 |
| [UO2(CO3)(HA)]- | 0.180 | 0.266 | 0.254 | 0.228 | 825.19 | 895.12 |
| [UO2(DPA)2]2- | 0.178 | 0.276 | 0.248 | 848.10 | 929.87 | |
| UO2(HA)2 | 0.178 | 0.266 | 0.250 | 851.62 | 932.91 |
| 铀酰配合物 | U—N(L) | U—O(L) | U—O(CO32-) | Q(U) | ΔQ(L) | ΔQ(CO32-) |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 0.301 | 0.449 | 0.700 | 1.544 | 0.716 | 0.897 |
| [UO2(CO3)(HA)]- | 0.281 | 0.401 | 0.749 | 1.554 | 0.647 | 0.958 |
| [UO2(DPA)2]2- | 0.301 | 0.480 | 1.499 | 1.574 | ||
| UO2(HA)2 | 0.335 | 0.475 | 1.471 | 1.593 |
| 铀酰配合物 | U—N(L) | U—O(L) | U—O(CO32-) | Q(U) | ΔQ(L) | ΔQ(CO32-) |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 0.301 | 0.449 | 0.700 | 1.544 | 0.716 | 0.897 |
| [UO2(CO3)(HA)]- | 0.281 | 0.401 | 0.749 | 1.554 | 0.647 | 0.958 |
| [UO2(DPA)2]2- | 0.301 | 0.480 | 1.499 | 1.574 | ||
| UO2(HA)2 | 0.335 | 0.475 | 1.471 | 1.593 |
| 铀酰配合物 | ΔEPauli | ΔEelstat | ΔEorb | ΔEint | %ΔEelstat | %ΔEorb |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 1017.7 | –3837.3 | –1385.6 | –4205.1 | 73.47 | 26.53 |
| [UO2(CO3)(HA)]- | 1035.6 | –3311.6 | –1403.1 | –3679.0 | 70.24 | 29.76 |
| [UO2(DPA)2]2- | 664.2 | –3518.4 | –1179.6 | –4033.8 | 74.89 | 25.11 |
| UO2(HA)2 | 705.9 | –2563.6 | –1187.1 | –3044.8 | 68.35 | 31.65 |
| 铀酰配合物 | ΔEPauli | ΔEelstat | ΔEorb | ΔEint | %ΔEelstat | %ΔEorb |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 1017.7 | –3837.3 | –1385.6 | –4205.1 | 73.47 | 26.53 |
| [UO2(CO3)(HA)]- | 1035.6 | –3311.6 | –1403.1 | –3679.0 | 70.24 | 29.76 |
| [UO2(DPA)2]2- | 664.2 | –3518.4 | –1179.6 | –4033.8 | 74.89 | 25.11 |
| UO2(HA)2 | 705.9 | –2563.6 | –1187.1 | –3044.8 | 68.35 | 31.65 |
| 络合反应 | ΔGBE |
|---|---|
| [UO2(H2O)5]2++CO32-+DPA2-→[UO2(CO3)(DPA)]2-+5H2O | –452.1 |
| [UO2(H2O)5]2++2DPA2-→[UO2(DPA)2]2-+5H2O | –363.3 |
| [UO2(H2O)5]2++CO32-+HA-→[UO2(CO3)(HA)] -+5H2O | –472.6 |
| [UO2(H2O)5]2++2HA-→UO2(HA)2+5H2O | –446.6 |
| 络合反应 | ΔGBE |
|---|---|
| [UO2(H2O)5]2++CO32-+DPA2-→[UO2(CO3)(DPA)]2-+5H2O | –452.1 |
| [UO2(H2O)5]2++2DPA2-→[UO2(DPA)2]2-+5H2O | –363.3 |
| [UO2(H2O)5]2++CO32-+HA-→[UO2(CO3)(HA)] -+5H2O | –472.6 |
| [UO2(H2O)5]2++2HA-→UO2(HA)2+5H2O | –446.6 |
| 取代反应 | ΔG |
|---|---|
| [UO2(CO3)3]4-+H2DPA→[UO2(CO3)(DPA)]2-+2HCO3- | –103.0 |
| [UO2(CO3)(DPA)]2-+H2DPA→[UO2(DPA)2]2-+HCO3-+H+ | 41.4 |
| [UO2(CO3)3]4-+2H2DPA→[UO2(DPA)2]2-+3HCO3-+H+ | –61.1 |
| [UO2(CO3)3]4-+H2A→[UO2(CO3)(HA)] -+HCO3-+CO32- | 137.3 |
| [UO2(CO3)(HA)] -+H2A→UO2(HA)2+HCO3- | 8.4 |
| [UO2(CO3)3]4-+2H2A→UO2(HA)2+2HCO3-+CO32- | 145.7 |
| 取代反应 | ΔG |
|---|---|
| [UO2(CO3)3]4-+H2DPA→[UO2(CO3)(DPA)]2-+2HCO3- | –103.0 |
| [UO2(CO3)(DPA)]2-+H2DPA→[UO2(DPA)2]2-+HCO3-+H+ | 41.4 |
| [UO2(CO3)3]4-+2H2DPA→[UO2(DPA)2]2-+3HCO3-+H+ | –61.1 |
| [UO2(CO3)3]4-+H2A→[UO2(CO3)(HA)] -+HCO3-+CO32- | 137.3 |
| [UO2(CO3)(HA)] -+H2A→UO2(HA)2+HCO3- | 8.4 |
| [UO2(CO3)3]4-+2H2A→UO2(HA)2+2HCO3-+CO32- | 145.7 |
| [1] |
Li, H.; Wen, J.; Wang, X.-L. Chinese Sci. Bull. 2018, 63, 481. (in Chinese)
doi: 10.1360/N972017-01122 |
|
(李昊, 文君, 汪小琳, 科学通报, 2018, 63, 481.)
|
|
| [2] |
Hu, B.; Wang, H.; Liu, R.; Qiu, M. Chemosphere 2021, 274, 129743.
doi: 10.1016/j.chemosphere.2021.129743 |
| [3] |
Endrizzi, F.; Leggett, C. J.; Rao, L. Ind. Eng. Chem. Res. 2016, 55, 4249.
doi: 10.1021/acs.iecr.5b03679 |
| [4] |
Parker, B. F.; Hohloch, S.; Pankhurst, J. R.; Zhang, Z.; Love, J. B.; Arnold, J.; Rao, L. Dalton Trans. 2018, 47, 5695.
doi: 10.1039/c7dt04069e pmid: 29632905 |
| [5] |
Yuan, Y. H.; Niu, B. Y.; Yu, Q. H.; Guo, X.; Guo, Z. H.; Wen, J.; Liu, T.; Zhang, H. Q.; Wang, N. Angew. Chem.-Int. Ed. 2020, 59, 1220.
doi: 10.1002/anie.201913644 |
| [6] |
Tian, G.; Geng, J. X.; Jin, Y. D.; Wang, C. L.; Li, S. Q.; Chen, Z.; Wang, H.; Zhao, Y. S.; Li, S. J. J. Hazard. Mater. 2011, 190, 442.
doi: 10.1016/j.jhazmat.2011.03.066 pmid: 21497013 |
| [7] |
Zhu, J. H.; Liu, Q.; Li, Z. S.; Liu, J. Y.; Zhang, H. S.; Li, R. M.; Wang, J. J. Hazard. Mater. 2018, 353, 9.
doi: 10.1016/j.jhazmat.2018.03.042 |
| [8] |
Wang, C. Z.; Lan, J. H.; Wu, Q. Y.; Luo, Q.; Zhao, Y. L.; Wang, X. K.; Chai, Z. F.; Shi, W. Q. Inorg. Chem. 2014, 53, 9466.
doi: 10.1021/ic500202g |
| [9] |
Li, Z.-N.; Sha, H.-Y.; Yang, N.; Yuan, Y.; Zhu, G.-S. Acta Chim. Sinica 2019, 77, 469. (in Chinese)
doi: 10.6023/A19010028 |
|
(李樟楠, 沙浩岩, 杨南, 元野, 朱广山, 化学学报, 2019, 77, 469.)
doi: 10.6023/A19010028 |
|
| [10] |
Abney, C. W.; Mayes, R. T.; Saito, T.; Dai, S. Chem. Rev. 2017, 117, 13935.
doi: 10.1021/acs.chemrev.7b00355 |
| [11] |
Parker, B. F.; Zhang, Z.; Rao, L.; Arnold, J. Dalton Trans. 2018, 47, 639.
doi: 10.1039/c7dt04058j pmid: 29261203 |
| [12] |
Liu, Z.-Y.; Xie, Y.; Wang, Y.-F.; Hu, T.-Y.; Ye, G.; Chen, J. J. Tsinghua Univ. (Sci. & Technol.) 2021, 61, 279. (in Chinese)
|
|
(刘泽宇, 谢忆, 王一凡, 胡铜洋, 叶钢, 陈靖, 清华大学学报(自然科学版), 2021, 61, 279.)
|
|
| [13] |
Tang, N.; Liang, J.; Niu, C. G.; Wang, H.; Luo, Y.; Xing, W. L.; Ye, S. J.; Liang, C.; Guo, H.; Guo, J. Y.; Zhang, Y. F.; Zeng, G. M. J. Mater. Chem. A 2020, 8, 7588.
doi: 10.1039/C9TA14082D |
| [14] |
Sun, Q.; Aguila, B.; Earl, L. D.; Abney, C. W.; Wojtas, L.; Thallapally, P. K.; Ma, S. Adv. Mater. 2018, 30, e1705479.
|
| [15] |
Zhang, L.; Pu, N.; Yu, B.; Ye, G.; Chen, J.; Xu, S.; Ma, S. ACS Appl. Mater. Interfaces 2020, 12, 3688.
doi: 10.1021/acsami.9b20944 |
| [16] |
Xu, X.; Xu, L.; Ao, J.; Liang, Y.; Li, C.; Wang, Y.; Huang, C.; Ye, F.; Li, Q.; Guo, X.; Li, J.; Wang, H.; Ma, S.; Ma, H. J. Mater. Chem. A 2020, 8, 22032.
doi: 10.1039/D0TA07180C |
| [17] |
Tian, G. X.; Teat, S. J.; Zhang, Z. Y.; Rao, L. F. Dalton Trans. 2012, 41, 11579.
doi: 10.1039/c2dt30978e |
| [18] |
Xu, C.; Tian, G.; Teat, S. J.; Rao, L. Inorg. Chem. 2013, 52, 2750.
doi: 10.1021/ic4000389 |
| [19] |
Zhou, D.; Huang, C.; Wang, K.; Xu, G. Polyhedron 1994, 13, 987.
doi: 10.1016/S0277-5387(00)83020-X |
| [20] |
Guo, X.; Huang, L.; Li, C.; Hu, J.; Wu, G.; Huai, P. Phys. Chem. Chem. Phys. 2015, 17, 14662.
doi: 10.1039/C5CP00931F |
| [21] |
Murray, J. S.; Politzer, P. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2011, 1, 153.
doi: 10.1002/wcms.19 |
| [22] |
Pyykkö, P.; Li, J.; Runeberg, N. J. Phys. Chem. 1994, 98, 4809.
doi: 10.1021/j100069a007 |
| [23] |
Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
doi: 10.1103/PhysRevLett.77.3865 pmid: 10062328 |
| [24] |
Moellmann, J.; Grimme, S. J. Phys. Chem. C 2014, 118, 7615.
doi: 10.1021/jp501237c |
| [25] |
Wiberg, K. B. J. Am. Chem. Soc. 1968, 90, 59.
doi: 10.1021/ja01003a012 |
| [26] |
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
doi: 10.1063/1.449486 |
| [27] |
Ziegler, T.; Rauk, A. Theor. Chim. Acta 1977, 46, 1.
doi: 10.1007/BF02401406 |
| [28] |
Sun, X. Q.; Xu, C.; Tian, G. X.; Rao, L. F. Dalton Trans. 2013, 42, 14621.
doi: 10.1039/c3dt51767e |
| [29] |
Tian, G.; Teat, S. J.; Rao, L. Dalton Trans. 2013, 42, 5690.
doi: 10.1039/c3dt32940b |
| [30] |
Bernhard, G.; Geipel, G.; Reich, T.; Brendler, V.; Amayri, S.; Nitsche, H. Radiochim. Acta 2001, 89, 511.
doi: 10.1524/ract.2001.89.8.511 |
| [31] |
Kelly, S. D.; Kemner, K. M.; Brooks, S. C. Geochim. Cosmochim. Acta 2007, 71, 821.
doi: 10.1016/j.gca.2006.10.013 |
| [32] |
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 |
| [33] |
Lee, C. T.; Yang, W. T.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
pmid: 9944570 |
| [34] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 1, Revision B. 01,Gaussian Inc., Wallingford, CT, 2016.
|
| [35] |
Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Theor. Chim. Acta 1990, 77, 123.
doi: 10.1007/BF01114537 |
| [36] |
Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
doi: 10.1063/1.452288 |
| [37] |
Yang, C.; Pei, S.; Chen, B.; Ye, L.; Yu, H.; Hu, S. Dalton Trans. 2016, 45, 3120.
doi: 10.1039/C5DT04645A |
| [38] |
Guo, X.; Xiong, X.-G.; Li, C.; Gong, H.; Huai, P.; Hu, J.; Jin, C.; Huang, L.; Wu, G. Inorg. Chim. Acta 2016, 441, 117.
doi: 10.1016/j.ica.2015.11.013 |
| [39] |
Luan, X.-F.; Wang, C.-Z.; Wu, Q.-Y.; Lan, J.-H.; Chai, Z.-F.; Xia, L.-S.; Shi, W.-Q. J. Phys. Chem. A 2022, 126, 406.
doi: 10.1021/acs.jpca.1c08072 |
| [40] |
Baldridge, K.; Klamt, A. J. Chem. Phys. 1997, 106, 6622.
doi: 10.1063/1.473662 |
| [41] |
Andzelm, J.; Kolmel, C.; Klamt, A. J. Chem. Phys. 1995, 103, 9312.
doi: 10.1063/1.469990 |
| [42] |
Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995.
doi: 10.1021/jp9716997 |
| [43] |
Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. J. Comput. Chem. 2003, 24, 669.
doi: 10.1002/jcc.10189 |
| [44] |
Schreckenbach, G.; Shamov, G. A. Acc. Chem. Res. 2010, 43, 19.
doi: 10.1021/ar800271r |
| [45] |
Shamov, G. A.; Schreckenbach, G. J. Phys. Chem. A 2005, 109, 10961.
doi: 10.1021/jp053522f |
| [46] |
Camaioni, D. M.; Schwerdtfeger, C. A. J. Phys. Chem. A 2005, 109, 10795.
pmid: 16863129 |
| [47] |
te Velde, G.; Bickelhaupt, F. M.; Baerends, E. J.; Guerra, C. F.; Van Gisbergen, S. J. A.; Snijders, J. G.; Ziegler, T. J. Comput. Chem. 2001, 22, 931.
doi: 10.1002/jcc.1056 |
| [48] |
Guerra, C. F.; Snijders, J. G.; te Velde, G.; Baerends, E. J. Theor. Chem. Acc. 1998, 99, 391.
|
| [49] |
Vanlenthe, E.; Baerends, E. J.; Snijders, J. G. J. Chem. Phys. 1993, 99, 4597.
doi: 10.1063/1.466059 |
| [50] |
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
doi: 10.1021/cr00088a005 |
| [51] |
Lu, T.; Chen, F. W. J. Comput. Chem. 2012, 33, 580.
doi: 10.1002/jcc.22885 |
| [1] | Guanglong Huang, Xiao-Song Xue. Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source [J]. Acta Chimica Sinica, 2024, 82(2): 132-137. |
| [2] | Xuefeng Liang, Jian Jing, Xin Feng, Yongze Zhao, Xinyuan Tang, Yan He, Lisheng Zhang, Huifang Li. Electronic Structure of Covalent Organic Frameworks COF66 and COF366: from Monomers to Two-Dimensional Framework [J]. Acta Chimica Sinica, 2023, 81(7): 717-724. |
| [3] | Lei Yang, Jiaoyang Ge, Fangli Wang, Wangyang Wu, Zongxiang Zheng, Hongtao Cao, Zhou Wang, Xueqin Ran, Linhai Xie. A Theoretical Study on the Effective Reduction of Internal Reorganization Energy Based on the Macrocyclic Structure of Fluorene [J]. Acta Chimica Sinica, 2023, 81(6): 613-619. |
| [4] | Jie Yang, Lin Ling, Yuxue Li, Long Lu. Density Functional Theory Study on Thermal Decomposition Mechanisms of Ammonium Perchlorate [J]. Acta Chimica Sinica, 2023, 81(4): 328-337. |
| [5] | Shaoqin Zhang, Meiqing Li, Zhongjun Zhou, Zexing Qu. Theoretical Study on the Multiple Resonance Thermally Activated Delayed Fluorescence Process [J]. Acta Chimica Sinica, 2023, 81(2): 124-130. |
| [6] | Jinjing Liu, Na Yang, Li Li, Zidong Wei. Theoretical Study on the Regulation of Oxygen Reduction Mechanism by Modulating the Spatial Structure of Active Sites on Platinum★ [J]. Acta Chimica Sinica, 2023, 81(11): 1478-1485. |
| [7] | Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang, Man Yao. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials [J]. Acta Chimica Sinica, 2022, 80(6): 756-764. |
| [8] | Luocong Wang, Zhewei Li, Caiwei Yue, Peihuan Zhang, Ming Lei, Min Pu. Theoretical Study on the Isomerization Mechanism of Azobenzene Derivatives under Electric Field [J]. Acta Chimica Sinica, 2022, 80(6): 781-787. |
| [9] | Yinghui Wang, Simin Wei, Jinwei Duan, Kang Wang. Mechanism of Silyl Enol Ethers Hydrogenation Catalysed by Frustrated Lewis Pairs: A Theoretical Study [J]. Acta Chimica Sinica, 2021, 79(9): 1164-1172. |
| [10] | Qingmin Man, Zunyun Fu, Tiantian Liu, Mingyue Zheng, Hualiang Jiang. DFT Mechanism of Cu Catalyzed Coupling Reaction to Alkyl Aryl Ethers [J]. Acta Chimica Sinica, 2021, 79(7): 948-952. |
| [11] | Yan Wang, Yingqi Tian, Zhong Jin, Bingbing Suo. Hartree-Fock and Density Functional Calculations on Graphics Processing Unit [J]. Acta Chimica Sinica, 2021, 79(5): 653-657. |
| [12] | Yu Mohan, Cheng Yuanyuan, Liu Yajun. Mechanistic Study of Oxygenation Reaction in Firefly Bioluminescence [J]. Acta Chimica Sinica, 2020, 78(9): 989-993. |
| [13] | Lu Xiaoqing, Cao Shoufu, Wei Xiaofei, Li Shaoren, Wei Shuxian. Investigation on Oxygen Reduction Reaction Mechanism on S Doped Fe-NC lsolated Single Atoms Catalyst [J]. Acta Chimica Sinica, 2020, 78(9): 1001-1006. |
| [14] | Yang Zhice, Tian Jianan, Cai Hongxue, Li Li, Pan Qingjiang. Theoretical Probe for Tris(aryloxide)arene Complexed Low-valent Actinide Ions and Their Structural/Redox Properties [J]. Acta Chimica Sinica, 2020, 78(10): 1096-1101. |
| [15] | Bo Yifan, Liu Yuyu, Chang Yongzheng, Li Yinxiang, Zhang Xiaofei, Song Chunyuan, Xu Weifeng, Cao Hongtao, Huang Wei. Theoretical and Experimental Studies on Raman Spectroscopy of Cyclic Fluorene-Based Strained Semiconductors [J]. Acta Chim. Sinica, 2019, 77(5): 442-446. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||