Abstract
Background:
Integron is an important element in creating multi-drug resistant (MDR) bacteria. This study evaluated the relationship of class I integron and antibiotic resistance in extended-spectrum beta-lactamase producing (ESBL) isolates of Escherichia coli (E. coli).Methods:
A total of 66 ESBL-producing E. coli were isolated from urinary tract infection in Kermanshah and their antimicrobial susceptibility was assessed. The incidence of class I integron was determined in isolates using PCR. The class I integron-associated gene cassettes were also verified by DNA sequencing. Data were analyzed using statistical methods.Results:
Of 66 ESBL-producing isolates, 65 (98.5%) were MDR. The most prevalent antibiotic resistance of the isolates was observed for ampicillin (98.4%), ceftriaxone (98.4%), cefotaxime (95.4%), and co-trimoxazole (86.3%). The frequency of class I integron in isolates was 92.3%. The relationship between class I integron and resistance to streptomycin, co-trimoxazole, and ceftazidime was statistically significant. The genes encoding resistance to streptomycin and co-trimoxazole, as well as a gene encoding a protein with unknown function, were associated with class I integron. The most common gene cassette was dfrA17-aadA5.Conclusions:
The results indicate a high prevalence of antibiotic resistance among ESBL-producing isolates to the antibiotics commonly used for the empirical treatment of urinary tract infections. The frequency of class I integron and associated gene cassettes involving resistance to antibiotics is high. There is a high percentage of MDR among the ESBL-producing isolates, which mostly contain gene cassettes. These findings suggest a strong association of integron and ESBL genes in the isolates.Keywords
Integrons Gene Cassettes Escherichia coli Multidrug Resistance
1. Background
Escherichia coli (E. coli) is the most common bacterium isolated from urinary tract infections (UTIs) (1). Nowadays, the E. coli resistance to antibiotics used for the UTI treatment has been increased (2). Inappropriate using of antibiotics, as well as the horizontal gene transfer between bacteria, have led to the development of multi-drug resistant (MDR) strains (3). Mobile genetic elements such as plasmids, transposons, and integrons play an important role in the development of antibiotic-resistant strains (4). Integrons are not self-transmissible but can be transmitted by plasmids or transposons and their genetic structure provides a location for receiving and integration of resistant genes, which are referred to as gene cassettes (5).
Genetic structure of integrons is composed of a site for the entrance of gene cassettes (attI), an integron-integrase gene (int1), which encodes a recombinase enzyme, and a promoter (Pc) for the expression of gene cassettes (6). Gene cassettes have a specific sequence (attC) to combine with attI site in integron (6). Based on the integrase gene, integrons are categorized into four classes (I to IV) in clinical isolates. Class I and II integrons are the most frequent classes in pathogenic bacteria (4). Integrons have been frequently reported in Enterobacteriaceae strains. So far, more than 194 gene cassettes have been identified in integrons, including genes involved in resistance to aminoglycoside, beta-lactam, chloramphenicol, quinolone, and trimethoprim antibiotics (6, 7). Since several gene cassettes can be carried on integrons in a bacterium simultaneously, resistance to several antibiotics is possible (4). For example, genes encode dihydrofolate reductase (dfr), chloramphenicol acetyltransferase (cat, cml), beta-lactamase (bla), aminoglycoside-modifying enzymes (aac, aad, aphA), and ADP-ribose transferase (arr) have been found in integrons (8). Previous studies have shown that there is a remarkable correlation between the presence of integrons with the development of MDR strains of bacteria (9, 10).
In the Enterobacteriaceae, such as E. coli, the MDR strains are highly associated with the presence of integrons, especially in the case of resistance to aminoglycosides and sulfamethoxazole (11). On the other hand, extended-spectrum β-lactamase (ESBL)-producing isolates are usually resistant to various antibiotics. The ESBL genes can be carried by integron-containing isolates to make them multidrug resistance (12).
2. Objectives
Given the importance of integrons and their role in creating MDR isolates of E. coli, the aim of this study was to evaluate the gene cassettes of class I integrons and their association with drug resistance in ESBL-producing E. coli isolates in Iran.
3. Methods
3.1. Bacterial Strains
Two hundred forty E. coli isolates were isolated from urine samples of patients referred to two medical centers in Kermanshah province, West of Iran, from 2015 to 2016. Mid-stream clean catch of the urine samples was collected in sterile tubes and cultured on Eosin methylene blue (EMB) and Blood agar culture media and incubated for 24 hours. Then the colonies were counted to ensure the presence of 105 or more colonies in 1 milliliter of urine according to the previous report (13). E. coli isolates were identified based on standard protocols such as gram staining, morphology, culture characteristics, and complementary biochemical tests, including Oxidase, Simmons citrate, Urease, Phenylalanine deaminase, Lysine decarboxylase, Methyl Red Voges Proskauer (MR-VP), Triple Sugar Iron Agar (TSI), and Sulfide Indole Motility (SIM) assays (14). The demographic information of the patients, including age and gender, was also recorded.
3.2. Antimicrobial Susceptibility Test
Antibiotic susceptibility was done by disk diffusion method, according to the Clinical and Laboratory Standards Institute (CLSI) protocols (15) The ceftriaxone (30 µg), ceftazidime (30 µg), cefotaxime (30 µg), aztreonam (30 µg), ampicillin (10 µg), imipenem (10 µg), gentamicin (10 µg), tobramycin (10 µg), streptomycin (10 µg), ciprofloxacin (30 µg), and co-trimoxazole (25 µg) were tested (MAST, Merseyside, UK). The results were interpreted based on CLSI standard tables (15). The E. coli (ATCC 25922) strain was used as a control. Isolates resistant to at least three classes of antibiotics were considered to be MDR isolates.
3.3. ESBL Testing
ESBL-producing bacteria were identified using phenotypic confirmatory tests in which combined disks of ceftazidime (30 µg) + clavulanic acid (10 µg) or cefotaxime (30 µg) + clavulanic acid (10 µg) were used. If the inhibition zone diameter of combined disk was at least 5 mm or more than the diameter of a single disk, it was considered ESBL-producing isolate (15). The strain of E. coli (ATCC 35218) was used as the control for ESBL-producing isolates (15).
3.4. PCR Amplification of Integrase Genes
The bacterial genome was extracted using boiling method (16). The presence of class I integrons and related gene cassettes in isolates were identified by PCR using specific primers. The genome of a known citrobacter strain contained integrase gene (17) was used as a positive control. The amplification of intI, 5’cs, and 3’cs was done (Table 1). PCR products were sequenced (Applied Biosystem, ABI3130, USA) and analyzed using BLAST (http://www.ncbi.nlm.nih.gov/BLAST). The bands of PCR products of 5’cs and 3’cs were cut and extracted from agarose gel and purified using QIA quick PCR purification Kit (QIA-GEN, Germany). The sequencing of purified bands was done using DNAanalyzerABI 3730XI (Macrogen Inc, South Korea) and analyzed by BLAST bioinformatics tools (http://www.ncbi.nlm.nih.gov/BLAST).
3.5. Statistical Analysis
All data were statistically analyzed using SPSS version 20 software. Mann-Whitney U test was utilized to find differences between ESBL-producing and non-ESBL-producing isolates and chi-square test to find the difference between isolates with class I integron and without class I integron. The P ≤ 0.05 was considered statistically significant.
Primers
4. Results
Out of 240 isolates of E. coli isolated from UTIs, 66 (27.5%) were ESBL-producing isolates of E. coli. Moreover, 56 out of 66 (84.8%) isolates were isolated from women, while 10 out of 66 (15.2%) were isolated from men. The average age of the patients was 43.5 ± 22.1 years. The antibiotic susceptibility testing showed that the resistance of ESBL-producing isolates was much higher than non-ESBL-producing ones. Because the data for comparison of ESBL-producing and non-ESBL-producing isolates did not show a normal distribution, the non-parametric Mann-Whitney U test was used. This test showed a significant difference between ESBL-producing and non-ESBL-producing isolates in terms of antibiotic resistance (Table 2).
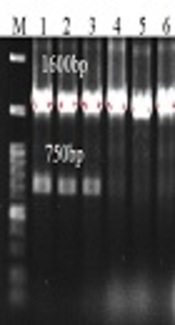
Furthermore, 65 out of 66 (98.5 %) ESBL-producing isolates were MDR, while only 17.2 % of non-ESBL-producing isolates were MDR (P = 0.01). Of 65 MDR ESBL-producing isolates, class I integron was identified in 60 isolates (92.3%) (Figure 1). Of these, 57 isolates contained gene cassettes with different sizes (Figure 2). These cassettes showed four electrophoretic patterns; a single band pattern (1600 bp), two-band pattern (750 and 1600 bp or 300 and 750 bp), and a three-band pattern (300, 750, and 1600 bp) (Table 2). The dfrA17-aadA5 and dfrA15-aadA1gene cassettes were found in 54 (94.7%) and 18 (31.6 %) isolates, respectively. These gene cassettes consisted of aadA (aadA1), dfr (dfrA15 and dfrA17) genes. The correlation between class I integron and resistance to 11 antibiotics was statistically analyzed (Table 3). Isolates contained class I integrons were more resistant to ceftazidime (P = 0.032), streptomycin (P = 0.018), and co-trimoxazole (P = 0.01). Moreover, there was a significant correlation between dfrA17-aadA5 and dfrA15-aadA1 genes with resistance to streptomycin (P = 0.015) as well as co-trimoxazole (P = 0.016) (Table 4).
Antibiotic Susceptibility of ESBL-Producing and Non-ESBL-Producing E. coli Isolates
| Isolates (No.), Susceptibility | Antibiotics, No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IMI | AP | ATM | CAZ | CTX | CRO | GM | TN | S | CIP | SXT | |
| ESBL-producing isolates (66) | |||||||||||
| Resistant | 0 | 65 (98.5) | 47 (71.2) | 33 (50) | 63 (95.5) | 65 (98.5) | 27 (40.9) | 35 (53) | 47 (71.2) | 43 (65.2) | 57 (86.4) |
| Intermediate | 0 | 0 | 11 (16.7) | 17 (25.7) | 1 (1.5) | 0 | 0 | 7 (10.6) | 0 | 4 (6) | 0 |
| Sensitive | 66 (100) | 1 (1.5) | 8 (12.1) | 16 (24.3) | 2 (3) | 1 (1.5) | 39 (59.1) | 24 (36.4) | 19 (28.8) | 19 (28.8) | 9 (13.6) |
| Non-ESBL-producing isolates (174) | |||||||||||
| Resistant | 0 | 134 (77) | 6 (3.5) | 5 (2.9) | 7 (4.1) | 6 (3.5) | 14 (8.1) | 18 (10.4) | 20 (11.5) | 36 (20.6) | 86 (49.5) |
| Intermediate | 0 | 6 (3.5) | 4 (2.3) | 5 (2.9) | 3 (1.7) | 4 (2.3) | 3 (1.7) | 26 (14.9) | 0 | 6 (3.5) | 10 (5.7) |
| Sensitive | 174 (100) | 34 (19.5) | 164 (94.2) | 164 (94.2) | 164 (94.2) | 164 (94.2) | 157 (90.2) | 130 (74.7) | 154 (88.5) | 132 (75.9) | 78 (44.8) |
| Statistical test | Z = 0; P = 1 | Z = -3.9; P < 0.001 | Z = -12.6; P < 0.001 | Z = -11.1; P < 0.001 | Z = -13.8; P < 0.001 | Z = -14.01; P < 0.001 | Z = -5.6; P < 0.001 | Z = -6.3; P < 0.001 | Z = -9.1; P < 0.001 | Z = -6.7; P < 0.001 | Z = -5.02; P < 0.001 |
Electrophoresis of intI gene PCR product. M, marker (100 bp); lane 1, negative control (non template control); lane 2, positive control (Citrobacter isolate containing integrase gene); lane 3, intI-containing samples.

Electrophoresis pattern of PCR products of class I integron gene cassettes. M, marker (100 bp); lanes 1 - 6, 8, 9, 12, 13, and 14 indicate clinical isolates containing gene cassettes with a length of 1600 bp (dfrA17-aadA5); lanes 1 - 3, 9, 11, 12, and 13 indicate clinical isolates containing gene cassettes with a length of 750bp (dfrA15-aadA1); lane 11 shows clinical isolate containing gene cassettes with a length of 300 bp (encodes a hypothetical protein); lanes 7 and 10 do not have class I integron gene cassettes.

The Correlation of Class I Integrons with Antibiotic Resistance in 65 ESBL-Producing E. coli Isolatesa
| Antibiotics | Number of Isolates Contained Class I Integronb | Number of Isolates Lack of Class I Integronb | P Value | ||
|---|---|---|---|---|---|
| Resistant | Sensitive | Resistant | Sensitive | ||
| Imipenem | 60 (92.3) | 0 | 0 | 5 (7.7) | 0.0001 |
| Ampicillin | 59 (90.8) | 1 (1.5) | 5 (7.7) | 0 | 0.773 |
| Aztreonam | 53 (81.5) | 7 (10.8) | 5 (7.7) | 0 | 0.158 |
| Ceftazidime | 45 (69.2) | 15 (23.1) | 5 (7.7) | 0 | 0.032 |
| Cefotaxime | 58 (89.2) | 2 (1.3) | 5 (7.7) | 0 | 0.612 |
| Ceftriaxone | 58 (89.2) | 2 (1.3) | 5 (7.7) | 0 | 0.796 |
| Streptomycin | 46 (70.8) | 14 (21.5) | 1 (1.5) | 4 (6.2) | 0.018 |
| Gentamicin | 25 (38.5) | 35 (53.8) | 2 (3.1) | 3 (6.4) | 0.943 |
| Tobramycin | 38 (58.5) | 22 (33.8) | 4 (6.2) | 1 (1.5) | 0.062 |
| Ciprofloxacin | 42 (64.6) | 18 (27.7) | 5 (7.7) | 0 | 0.103 |
| Co-trimoxazole | 56 (86.1) | 4 (6.2) | 1 (1.5) | 4 (6.2) | 0.001 |
The Characteristics of MDR E. coli Isolates Containing Class I Integrons
| Number of Isolates | 5’CS - 3’CS Size (bp) | Gene Cassette | Resistant to Antibiotics |
|---|---|---|---|
| 39 | 1600 | dfrA17-aadA5 | SXT, CIP, TN, CRO, CTX, CAZ, ATM, AP, S |
| 3 | 300, 750 | dfrA15-aadA1, hypothetical protein | SXT,CIP,CRO, CTX, ATM,CAZ, AP, S |
| 11 | 750, 1600 | dfrA17-aadA5, dfrA15-aadA1 | SXT, CIP, CRO, CTX, TN, CAZ, ATM, AP, S |
| 4 | 300, 750, 1600 | dfrA17-aadA5, dfrA15-aadA1, hypothetical protein | SXT, CRO, CTX, ATM, CAZ, AP, S |
| 3 | - | - | SXT, CRO, CTX, AP |
| 5 | - | - | CIP, TN, CRO, CTX, CAZ, ATM, AP |
5. Discussion
The frequency of MDR isolates of E. coli from UTIs is high in Iran (10, 19). In the present study, the highest resistance rate was seen for ceftriaxone, cefotaxime, and co-trimoxazole, which is consistent with previous reports in Iran (3, 20). Moreover, in accordance with previous reports in Iran, all isolates were sensitive to imipenem (2, 20, 21). Therefore, imipenem is recommended for the treatment of infections caused by MDR E. coli.
The studies on the frequency of class I integrin in E. coli in Iran have reported different results. As an example, in Yasuj, Shiraz, and Arak cities located in different parts of Iran, the frequency of class I integron in E. coli has been reported 52%, 22.5%, and 86%, respectively (2, 19, 22). Further, one study in Tehran (the capital city of Iran) showed the presence of class I integrons with a frequency of 20.5 % in E. coli and Klebsiella pneumoniae isolates (20). The higher frequency of class I integron in our study in comparison to other studies may refer to the fact that we only tested ESBL-producing isolates for integrons. Similarly, in two studies in Asian countries the higher frequency of class I integrons has been reported in ESBL-producing compared to non-ESBL-producing K. pneumoniae isolates (23, 24). In two studies in Iran and Egypt, it has been shown that there is a significant correlation between the presence of class I integrons and ESBL-producing bacteria (25, 26).
ESBL-producing isolates contain higher resistance genes than non-ESBL-producing ones. For all antibiotics tested in our study, antibiotic resistance was significantly higher in ESBL-producing in comparison to non-ESBL-producing isolates (P < 0.001). The only exception was imipenem that all isolates (both ESBL- and non-ESBL-producing isolates) were susceptible to it. This indicates the importance of ESBL as a good marker for MDR isolates of E. coli. Studies in Iran indicate that more than 90% of MDR isolates of K. pneumonia and E. coli isolates had class I integrons (9, 10, 26). The high frequency of MDR isolates in Kermanshah may be due to the dissemination of class I integrons in the bacteria. The correlation between MDR isolates and class I integrons has been previously reported (27). These findings emphasize the importance of integrons in the development of high resistant strains due to acquiring and expressing various resistance genes. So far, various gene cassettes encoding phenotypic resistance to antibiotics have been found in integrons (4). As an example, genes encoding resistance to streptomycin and trimethoprim are remarkably associated with class I integrons (28). Similarly, in our study, a significant correlation was observed between resistance to co-trimoxazole, streptomycin, and ceftazidime with class I integrons, which indicates the association of resistant genes with this class of integron. Data analysis revealed the presence of two variants of dfrA (dfrA17/dfrA15) and two variants of aadA (aadA1/aadA5). The high frequency of dfr gene cassette in isolates containing class I integron correlated with the higher resistance to trimethoprim (29). Another common gene cassette in class I integron is aadA, which encodes aminoglycoside 3’ adenyltransferase and associated with resistance to streptomycin and spectinomycin. Aminoglycosides (streptomycin and spectinomycin) are added to animal foods (30). The presence of aadA gene cassette in clinical isolates may show the transfer of resistance genes from animal sources to the clinical isolates. Furthermore, there is a strong association between dfr and aad genes that suggests the co-transmission of these cassettes in class I integron (31). Our results in concordance with the results of previous studies indicate that the frequency of dfrA and aadA is high in several parts of Iran (31-33). In our study, aadA gene cassettes (aadA1 and aadA5) were found in association with dfrA gene cassettes (dfrA17 and dfrA15). The dfrA17-aadA5 gene cassettes were the most frequent cassettes that is also consistent with a previous report (26). The presence of aadA5 and dfrA17 in E. coli isolates and other bacteria has been reported in several countries (30, 34, 35). Gene cassettes of dfrA15 and aadA1, which are exclusively found in E. coli (36), have been found in isolates contained class I integrons. Therefore, the presence of these gene cassettes in integrons may play an important role in horizontal transmission and development of resistant strains.
In conclusion, our findings indicate a high antibiotic resistance in ESBL-producing isolates to the drugs usually used for the treatment of UTIs. The prevalence of integrons and gene cassettes are remarkably associated with resistance to commonly used antibiotics. Most ESBL-producing isolates are MDR and the majority of them contain resistance gene cassettes. These results may indicate the transfer of integrons by ESBL-carrying plasmids. The diagnosis of ESBL-producing isolates in medical diagnostic laboratories may provide useful information to understand regional epidemiology and dissemination of MDR strains.
References
-
1.
Kumar S, Dave A, Wolf B, Lerma EV. Urinary tract infections. Dis Mon. 2015;61(2):45-59. [PubMed ID: 25732782]. https://doi.org/10.1016/j.disamonth.2014.12.002.
-
2.
Ranjbaran M, Zolfaghari M, Japoni-Nejad A, Amouzandeh-Nobaveh A, Abtahi H, Tabib Nejad M, et al. [Molecular Investigation of Integrons in Escherichia Coli and Klebsiella Pneumoniae Isolated from Urinary Tract Infections]. J Mazandaran Univ Med Sci. 2013;23(105):20-7. Persian.
-
3.
Haddadi A, Mikaili Ghezeljeh S, Shavandi M. Prevalence of class 1 and 2 integrons among the multidrug resistant uropathogenic strains of Escherichia coli. Asian Biomed. 2017;9(1):49-54. https://doi.org/10.5372/1905-7415.0901.367.
-
4.
Zeighami H, Haghi F, Hajiahmadi F. [Integrons and their role in antibiotic resistance]. Zanjan Univ Med Sci. 2014;5(22):61-71. Persian.
-
5.
Kargar M, Mohammadalipour Z, Doosti A, Lorzadeh S, Japoni-Nejad A. High prevalence of class 1 to 3 integrons among multidrug-resistant diarrheagenic Escherichia coli in Southwest of Iran. Osong Public Health Res Perspect. 2014;5(4):193-8. [PubMed ID: 25379369]. [PubMed Central ID: PMC4215003]. https://doi.org/10.1016/j.phrp.2014.06.003.
-
6.
Domingues S, da Silva GJ, Nielsen KM. Integrons: Vehicles and pathways for horizontal dissemination in bacteria. Mob Genet Elements. 2012;2(5):211-23. [PubMed ID: 23550063]. [PubMed Central ID: PMC3575428]. https://doi.org/10.4161/mge.22967.
-
7.
Partridge SR, Tsafnat G, Coiera E, Iredell JR. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev. 2009;33(4):757-84. [PubMed ID: 19416365]. https://doi.org/10.1111/j.1574-6976.2009.00175.x.
-
8.
Guo X, Xia R, Han N, Xu H. Genetic diversity analyses of class 1 integrons and their associated antimicrobial resistance genes in Enterobacteriaceae strains recovered from aquatic habitats in China. Lett Appl Microbiol. 2011;52(6):667-75. [PubMed ID: 21496063]. https://doi.org/10.1111/j.1472-765X.2011.03059.x.
-
9.
Mahluji Z, Firoozeh F, Khorshidi A, Zibaei M. [The frequency of class 1 integrons in multi-drug resistant Klebsiellapneumoniae isolated from clinical samples using polymerase chain reaction assay]. Sci J Kurdistan Univ Med Sci. 2016;21(3):68-78. Persian.
-
10.
Mehdipour Moghaddam MJ, Mirbagheri AA, Salehi Z, Habibzade SM. Prevalence of class 1 integrons and extended spectrum beta lactamases among multi-drug resistant Escherichia coli isolates from North of Iran. Iran Biomed J. 2015;19(4):233-9. [PubMed ID: 26220727]. [PubMed Central ID: PMC4649859].
-
11.
Ibrahim N, Wajidi MF, Yusof MY, Tay ST. The integron prevalence of extended-spectrum beta-lactamase producing enterobacterial isolates in a Malaysian teaching hospital. Trop Biomed. 2011;28(3):668-71. [PubMed ID: 22433898].
-
12.
Machado E, Canton R, Baquero F, Galan JC, Rollan A, Peixe L, et al. Integron content of extended-spectrum-beta-lactamase-producing Escherichia coli strains over 12 years in a single hospital in Madrid, Spain. Antimicrob Agents Chemother. 2005;49(5):1823-9. [PubMed ID: 15855502]. [PubMed Central ID: PMC1087637]. https://doi.org/10.1128/AAC.49.5.1823-1829.2005.
-
13.
Fauci S, Braunwald E, Kasper DL, Longo DL, Braunwald E, Hauser SL, et al. Harrison's principles of internal medicine. USA: McGraw-Hill; 2008.
-
14.
Washington C, Stephen A, Janda W, Koneman E, Procop G, Schreckenberger P. Koneman's color atlas and textbook of diagnostic microbiology. Lippincott: Williams & Wilkins; 2006.
-
15.
Wayne PA. CLSI informational supplement, performance standards for antimicrobial susceptibility testing. USA: Clinical and Laboratory Standards Institute; 2017.
-
16.
Ahangarzadeh Rezaee M, Langarizadeh N, Aghazadeh M. First report of class 1 and class 2 integrons in multidrug-resistant Klebsiella pneumoniae isolates from Northwest Iran. Japan J Infect Dis. 2012;65(3):256-9.
-
17.
Chegene Lorestani R, Akya A, Elahi A, Hamzavi Y. Gene cassettes of class I integron-associated with antimicrobial resistance in isolates of Citrobacter spp. with multidrug resistance. Iran J Microbiol. 2018;10(1):22.
-
18.
Mirnejad R, Mostofi S, Masjedian F. Antibiotic resistance and carriage class 1 and 2 integrons in clinical isolates of Acinetobacter baumannii from Tehran, Iran. Asian Pac J Trop Biomed. 2013;3(2):140-5. [PubMed ID: 23593593]. [PubMed Central ID: PMC3627174]. https://doi.org/10.1016/S2221-1691(13)60038-6.
-
19.
Khoramrooz SS, Sharifi A, Yazdanpanah M, Malek Hosseini SA, Emaneini M, Gharibpour F, et al. High frequency of class 1 integrons in Escherichia coli isolated from patients with urinary tract infections in Yasuj, Iran. Iran Red Crescent Med J. 2016;18(1). e26399. [PubMed ID: 26889395]. [PubMed Central ID: PMC4752967]. https://doi.org/10.5812/ircmj.26399.
-
20.
Eslami G, Seyedjavadi SS, Goudarzi H, Fallah F, Goudarzi M. [Distribution of integrons among multidrug resistant E. coli and Klebsiella strains]. Res Med. 2010;34(1):61-5. Persian.
-
21.
Adel El-Sokkary M, Abdelmegeed ES. Characterisation of class 1 integron among Escherichia coli isolated from Mansoura University Hospitals in Egypt. Adv Microbiol. 2015;5(4):269-77. https://doi.org/10.4236/aim.2015.54025.
-
22.
Japoni-Nejad A, Gudarzi M, Farshad S, Basiri E, Ziyaeyan M, Alborzi A, et al. Assay for integrons and pattern of antibiotic resistance in clinical Escherichia coli strains by PCR-RFLP in Southern Iran. Japan J Infect Dis. 2008;61(1):85.
-
23.
Bhattacharjee A, Sen MR, Prakash P, Gaur A, Anupurba S, Nath G. Observation on integron carriage among clinical isolates of Klebsiella pneumoniae producing extended-spectrum beta-lactamases. Indian J Med Microbiol. 2010;28(3):207-10. [PubMed ID: 20644307]. https://doi.org/10.4103/0255-0857.66472.
-
24.
Yao F, Qian Y, Chen S, Wang P, Huang Y. Incidence of extended-spectrum beta-lactamases and characterization of integrons in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolated in Shantou, China. Acta Biochim Biophys Sin (Shanghai). 2007;39(7):527-32. [PubMed ID: 17622472]. https://doi.org/10.1111/j.1745-7270.2007.00304.x.
-
25.
Abd El-Rahman S, Ismail Y, Fathy Alhusseini N, Abbas S. Role of integrons in multidrug resistant extended-spectrum β-lactamase producing enterobacteriaceae. J Microbiol Antimicrobials. 2014;6(7):104-10. https://doi.org/10.5897/jma2014.0325.
-
26.
Zeighami H, Haghi F, Hajiahmadi F. Molecular characterization of integrons in clinical isolates of betalactamase-producing Escherichia coli and Klebsiella pneumoniae in Iran. J Chemother. 2015;27(3):145-51. [PubMed ID: 24571248]. https://doi.org/10.1179/1973947814Y.0000000180.
-
27.
Oliveira-Pinto C, Diamantino C, Oliveira PL, Reis MP, Costa PS, Paiva MC, et al. Occurrence and characterization of class 1 integrons in Escherichia coli from healthy individuals and those with urinary infection. J Med Microbiol. 2017;66(5):577-83. [PubMed ID: 28485709]. https://doi.org/10.1099/jmm.0.000468.
-
28.
Lima AM, de Melo ME, Alves LC, Brayner FA, Lopes AC. Investigation of class 1 integrons in Klebsiella pneumoniae clinical and microbiota isolates belonging to different phylogenetic groups in Recife, State of Pernambuco. Rev Soc Bras Med Trop. 2014;47(2):165-9. [PubMed ID: 24861289].
-
29.
Sung JY, Oh JE. Distribution and characterization of integrons in enterobacteriaceae isolates from chickens in Korea. J Microbiol Biotechnol. 2014;24(7):1008-13. [PubMed ID: 24851814].
-
30.
Yan H, Li L, Zong M, Alam MJ, Shinoda S, Shi L. Occurrence and characteristics of class 1 and 2 integrons in clinical bacterial isolates from patients in South China. J Health Sci. 2010;56(4):442-50. https://doi.org/10.1248/jhs.56.442.
-
31.
Hajiahmadi F, Safari N, Alijani P, Rabiei M, Masomian N, Arabestani MR. Assessment of the prevalence of class i and ii integrons of Escherichia coli and klebsiella pneumoniae isolates from hospitals of Hamadan. Avicenna J Clin Med. 2016;23(3):193-201. https://doi.org/10.21859/hums-23036.
-
32.
Masoumian N, Haghi F. [Analysis of integrons and associated gene cassettes in clinical isolates of Escherichia coli]. J Zanjan Univ Med Sci. 2015;23(99):74-82. Persian.
-
33.
Salimizand H, Shahcheraghi F, Kalantar E, Badmasti F, Mousavi SF. Molecular characterization of class 1 integrons and gene cassettes in multidrug resistant (MDR) Klebsiella spp. isolated from hospitalized and outpatients in Iran, 2009. Iran J Microbiol. 2013;5(1):48-55. [PubMed ID: 23466743]. [PubMed Central ID: PMC3577558].
-
34.
Chen J, Su Z, Liu Y, Wang S, Dai X, Li Y, et al. Identification and characterization of class 1 integrons among Pseudomonas aeruginosa isolates from patients in Zhenjiang, China. Int J Infect Dis. 2009;13(6):717-21. [PubMed ID: 19208492]. https://doi.org/10.1016/j.ijid.2008.11.014.
-
35.
Chen X, Li GX, Zhang H, Yuan M, Hou XP, Yu HL, et al. Characterization of class 1 integron gene cassettes among clinical bacteria isolated from one large hospital in northern China. Biomed Environ Sci. 2013;26(12):1003-7. [PubMed ID: 24393512]. https://doi.org/10.3967/bes2013.038.
-
36.
Ammar AM, Attia AM, Abd El-Aziz NK, Abd El Hamid MI, El-Demerdash AS. Class 1 integron and associated gene cassettes mediating multiple-drug resistance in some food borne pathogens. Int Food Res J. 2016;23(1):332-9.