Abstract
Keywords
Mycotoxin Contamination Aflatoxin T-2 Toxin Deoxynivalenol Fumonisin, Zearalenone Ochratoxin A Iran Rice
1. Context
As a very large and diverse category of secondary mold metabolites, mycotoxins can lead to a wide variety of toxicological symptoms such as carcinogenic, genotoxic, teratogenic, nephrotoxic, hepatotoxic, as well as immunotoxic effects across the world (1) (Table 1). Mycotoxins are considered not only a major threat to public health but also a factor that moderates the marketable quality and nutritional value of contaminated products, thereby causing substantial economic losses (2). Some studies have shed light on noticeable levels of mycotoxins such as AFTs (B1, B2, G1, and G2), citrinin, DON, FMs (B1, B2, and B3), fusarenon-X (Fus-X), nivalenol (NIV), OTA, sterigmatocystin (STE), and ZEN in rice worldwide (3). Climatic and storage-related conditions, as well as transportation, are regarded among factors leading to the production of mycotoxins in rice (4). Accordingly, post-harvest rice treatments, such as providing adequate drying and storage conditions, have been suggested as critical factors ascertaining storage stability (5). As mycotoxins are typically classified as stable compounds, different processes performed on grains before their consumption, such as cooking at a normal temperature (less than 150°C), cannot decrease toxicity in the majority of cases (6). In this regard, regular tests to exclude products with higher rates of toxicity than MTD are currently being conducted in countries and regions with adequate information about the occurrence of mycotoxins (7).
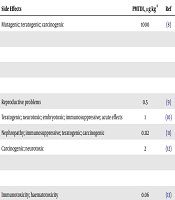
| Mycotoxin | Fungal Family | Carcinogenicity | Side Effects | PMTDI, µg kg-1 | Ref |
|---|---|---|---|---|---|
| AF | Aspergillus | Mutagenic; teratogenic; carcinogenic | 1000 | (8) | |
| AFB1 | Group 1 | ||||
| AFB2 | Group 1 | ||||
| AFG1 | Group 1 | ||||
| AFG2 | Group 1 | ||||
| ZON | Fusarium | Zearalenone caused increased incidences of hepatocellular and pituitary tumors in mice of both sexes when given in the diet, but no carcinogenic effect was seen in rats. | Reproductive problems | 0.5 | (9) |
| DON | Fusarium | More recent carcinogenicity studies of deoxynivalenol in mice have been negative (Iverson et al., 1995; Lambert et al., 1995) and reinforce the conclusion of the 1993 IARC Working Group that there is inadequate evidence in experimental animals for the carcinogenicity of deoxynivalenol. | Teratogenic; neurotoxic; embryotoxic; immunosuppressive; acute effects | 1 | (10) |
| OTA | Aspergillus penicillium | Group 2B | Nephropathy; immunosuppressive; teratogenic; carcinogenic | 0.112 | (11) |
| FB | Fusarium | Group 2B | Carcinogenic; neurotoxic | 2 | (12) |
| FB1 | |||||
| FB2 | |||||
| T-2 | Fusarium | T-2 toxin caused increased incidences of liver cell tumors and lung tumors in male mice when given in the diet. | Immunotoxicity; haematotoxicity | 0.06 | (13) |
Based on the nutritional and world rice trade, rice has a high consumption rate in Iran. Since the amount of Iranian rice is not sufficient for domestic consumption, it is often imported mostly from India, Pakistan, Bangladesh, and Thailand (14). These countries have frequent and heavy rainfalls that can provide the required conditions for the development of mycotoxin (15). Considering miscellaneous climatic conditions, as well as inappropriate storage requirements such as high temperature and humidity, rice can be contaminated by a number of fungi in Iran. In spite of this, there is scarce information on the fungal contamination of rice in this country, as well as the potentials of local fungal strains to produce mycotoxins. Various studies conducted on contamination of rice by mycotoxins in Iran are summarized in Table 2.
Different Studies Conducted on Mycotoxins Contamination in Rice in Iran
| Province | Origin | Concentration, ng.g-1 | Method | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DON | ZON | AFB1 | AFB2 | AFG1 | AFG2 | OTA | FB | BEA | ENN | T-2 | ||||
| Zabol | Imported | ND | ND | 0.59 | - | - | - | ND | - | - | - | - | HPLC | (16) |
| Tehran | Domestic | ND | 31.56 | 2.415 | - | - | - | 5.02 | 110.66 | - | - | - | LC-MS/MS | (17) |
| Tehran | Domestic | - | - | 3.90 | 0.93 | - | - | - | - | - | - | - | HPLC | (18) |
| Tehran | Imported | ND | ND | 0.59 | 0.07 | 0.35 | - | - | - | - | - | - | HPLC | (19) |
| Tehran | Domestic | ND | ND | 0.38 | ND | ND | - | - | - | - | - | - | HPLC | (19) |
| Amol | Imported | - | - | 1.89 | - | - | - | - | - | - | - | - | ELISA | (20) |
| Amol | Domestic | - | - | 1.08 | - | - | - | - | - | - | - | - | ELISA | (20) |
| Bushehr | Imported | - | - | 0.671 | - | - | - | - | - | - | - | - | HPLC | (21) |
| Tehran | Domestic | - | - | - | - | - | - | - | - | 0.11 | 0.06 | - | LC/ESIMS/MS | (18) |
| Qazvin | ? | - | - | 3.73 | 3.04 | 1.77 | 1.59 | - | - | - | - | - | HPLC-FLD | (22) |
| Tehran | ? | 345.0 | - | - | - | - | - | - | - | - | - | - | HPLC | (23) |
| Six provinces | ? | - | - | - | - | - | - | 0.34 | - | - | - | - | ELISA | (24) |
| Mazandaran | Domestic | - | - | - | - | - | - | Oct < 5; Nov 11.43 | - | - | - | - | ELISA | (25) |
| Mazandaran | Domestic | - | - | - | - | - | - | - | Old = 0.02; new = 0.02 | - | - | - | HPLC | (26) |
| Tehran | ? | - | ND | - | - | - | - | - | - | - | - | - | HPLC | (27) |
| Golestan | Domestic | - | - | - | - | - | - | - | 0.02 | - | - | - | HPLC | (12) |
| 30 provinces | Domestic | - | - | 1.4 | 0.1 | 0.4 | ND | - | - | - | - | - | HPLC | (28) |
| Mashhad | ? | - | - | - | - | - | - | 1.6 | - | - | - | - | HPLC | (29) |
| Mashhad | Domestic | - | - | 0.72 | 0.20 | 0.20 | ND | - | - | - | - | - | HPLC | (30) |
| 3 provinces | Imported | - | - | 1.89 | 0.14 | 0.05 | 0.01 | - | - | - | - | - | HPLC | (31) |
| Chaharmahal va Bakhtyari | Domestic | - | 89 | - | - | - | - | -- | - | - | - | - | ELISA | (32)) |
| Tehran | ? | - | - | - | - | - | - | - | - | - | - | 12.5 | ELISA | (33) |
| Mazandran | Domestic | - | - | New = 5.06; old = 6.8 | New = 3.55; old = 3.77 | New 5.49; old = 3.61 | New 2.97; old = 3.29 | - | - | - | - | - | HPLC | (34) |
| Zahedan | Imported | - | - | White = 0.58; yellow = 0.32 | White = 0.05; yellw =0.02 | - | - | - | - | - | - | - | HPLC | (35) |
| Ahvaz | Imported | - | - | 0.12 | 0.007 | 0.19 | 0.03 | - | - | - | - | - | HPLC | (36) |
| Tehran | Imported | - | - | - | - | - | - | - | - | - | - | 13 | ELISA | (37) |
| Tehran | Domestic | - | - | - | - | - | - | - | - | - | - | 11.2 | ELISA | (37) |
| Zahedan | Imported | - | - | White = 0.66; yellow = 0.34 | - | - | - | - | - | - | - | HPLC | (38) | |
| Qaemshahr | Domestic | 1.16 | - | - | - | - | - | - | - | - | ELISA | (39) | ||
| Yasuj | Domestic | - | - | 5.83 | - | - | - | - | - | - | - | ELISA | (40) | |
| Tehran | 2.99 | 1.38 | HPLC | (41) | ||||||||||
2. Aflatoxins
Aflatoxins (AFTs) are one of the most important categories of mycotoxins produced as secondary metabolites by three species of Aspergillus in a wide variety of foods and feeds (42). AFTs (B1, B2, G1, and G2) are considered potent teratogens, mutagens, hepatotoxins, and immunotoxins (43). AFTs (B1 and B2) are known as the most potent and typical toxins in contaminated grains, which have been the locus of research studies in most countries (44). Accordingly, the International Agency for Research on Cancer (IARC) incorporated AFB1 as primary groups of carcinogenic compounds (33, 45, 46). According to the national standards in Iran, the maximum residue limit (MRL) for AFTs and AFB1 in rice have been respectively reported by 30 and 5 ng/g ppb (Institute of Standard and Industrial Research of Iran (ISIRI) (47).
According to their frequent occurrence as well as severe effects on health status in animals and humans, AFTs have been the focus of attention among scholars. Faraji et al. (48) found that all rice samples (n = 60) had AFTs in which AFB1 and B2 levels were by 2.55 and 0.34 µg/kg, respectively. Besides, the amount of AFB1 in 8.3% of the samples was higher than the maximum annual concentration (MAC) in foodstuff in Iran. AFB1 was also reported as the highest amount of contamination in the study by Karajibani et al. (35), but AFG1 and G2 were not detected in the samples. The results similarly revealed that contaminations in white rice could be more than those yellow ones. Moreover, no imported rice had contamination caused by AFTs higher than standard levels, and the amount of AFB1 was below the internationally acceptable limits for human use, which were consistent with the findings by Mazaheri in which the mean of AFB1 was reported lower than Iranian maximum tolerated limit (MTL) (35). Furthermore, Feizy et al. (30) reported that 68.9% of the rice samples (n = 261) had AFB1 at levels greater than 0.2 ng.g-1. The mean AFB1 concentration in rice from the city of Mashhad by 0.72 mg.g-1 was also reported to be significantly different from the EU limit of 2 ng.g-1 (EEC, 1998) and the Iranian standard of -1. Currently, this mycotoxin is not assumed as a risk and even a serious public health issue, though routine monitoring such as food quality control measurement is required. It should be noted that rice is not considered a conductive product of Aspergillus growth and AFT contamination within normal conditions; however, it can be susceptible to AFT contamination once exposed to heavy rain or high moisture (49).
It had been reported that AFT contamination could be often associated with drought and temperature, and some synthetic or natural protectants could even help in inhibiting aflatoxigenic fungi and AFT formation during storage (2). The summer heat and high humidity could also lead to mold growth and AFT production; so, it seemed that the amount of AFB1 in white and yellow rice samples in the summer was more than that in the autumn and the winter (35). The rice grain might also contain some enzymes or chemicals inhibiting AFT production (2). Numerous methods have been proposed to detoxificate AFT in foodstuff, including citric, sodium hypochlorite, and ozone (50). The fumigation process is typically utilized for imported products, which can decrease AFT contamination rates. The results of the study by Karajibani et al. (35) in this domain suggested that AFTs had been reduced in imported rice using these methods. It was noted that the most prominent approach to control the amount of AFTs in rice was to control contamination in the field; however, this was very difficult as it could be primarily affected by climatic conditions such as humidity and temperature. Nevertheless, it had been reported that the highest concentrations of AFTs were associated with post-harvest growth of Aspergillus molds on poorly stored foodstuff (30).
3. Zearalenone
Zearalenone (ZEN) is the generic name for [6-(10-hydroxyl-6-oxo-trans-1-undecenyl)-B-resorcylic acid lactone], which is known as a nonsteroidal estrogenic mycotoxin produced by Fusarium spp. growing on cereal grains and animal feeds (51). Except for alkaline conditions or during extrusion, it is generally insoluble in water and stable during cooking (52). Also, ZEN may affect the uterus by decreasing progesterone secretion and changing the morphology of uterine tissues (53). It has also been classified by the IARC under group 3 carcinogens. Given the potential health hazards of ZEN, regulatory levels for ZEN have been recently documented in the range of 20 - 1000 ng/g in foods in most countries.
Iran has also made the regulatory limits of 200 ng/g for rice; however, there are rare data on ZEN contamination in rice in Iran. However, Yazdanpanahs validated HPLC for ZEN in rice using a monolithic column with sample cleanup on an immunoaffinity column and reported the contamination level lower than the MTL of ZEN in rice in Iran. The mean intake of ZEN from all the samples was also much lower than the tolerable daily intake (TDI) estimated by the Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) (0.002, μg/kg bw/day). In another study by Rashedi (32), a total number of 35 naturally contaminated samples in local markets of Chaharmahal and Bakhtyari Province, Iran, were collected during the spring and the summer, and their mycotoxins i.e. ZEN were detected via direct competitive (DC)-ELISA. The results of this study showed that ZEN had been observed in only two rice samples with a mean level of 89 μg,kg-1, and all negative samples had been collected during the summer. It had been ultimately suggested that warm weather conditions could diminish ZEN contamination (32). Tavakoli et al. (19) also showed that ZEN was not detectable in 80 rice samples collected from military centers of Tehran Province, Iran, using HPLC. Moreover, Sadeghi et al. (54) sampled 10 brands of Iranian and four brands of imported rice from markets located in northern, central, and southern parts of the city of Kermanshah to investigate contamination induced by mycotoxin and found that all the samples had been contaminated by ZEN, 4.43 and 3.88 µg/kg, respectively. The mean ZEN level in rice was observed only in two studies, which was reported lower than MTL (200 mg/kg) in Iran. The total amount of ZEN in rice was by 0.012 μg/kg bw/day, and it was much lower than the provisional maximum tolerable daily intake (PMTDI) estimated by the JECFA (0.5 g/kg bw/day), indicating no health risks for consumers at these levels.
Given the risks of ZEN to human health, it has been recommended to control ZEN production during all stages of rice cultivation. Therefore, determining the maximum amount of mycotoxins in food and attention to public health is of utmost importance. Studies have further demonstrated that rice exposed to ZEN would not be a health concern for public health in Iran
because Iranian rice is collected well and stored in good conditions.
4. T-2 Toxin
T-2 toxin is known as one of the most toxic Fusarium-derived trichothecenes found in grains and grain-based products (55). The lipophilic nature of T-2 toxin suggests that they can be easily absorbed through the cell bilayer membrane and consequently induce lipid peroxidation via generating free radicals, thus damaging cellular membrane (56, 57). T-2 toxin and other trichothecenes can also inhibit protein synthesis and consequently reduce DNA and RNA synthesis and apoptosis (56, 58). Typical clinical symptoms of T-2 toxicity are gastrointestinal and neurological, cardiopulmonary, and a weakened immune system (55, 59). The low rate of T-2 toxin contamination observed in the study by Riazipour et al. (37) did not support the idea that rice consumers were not at the risk of mycotoxin intoxications. It should be noted that screening domestic and imported rice in different stages was vital for identifying possible contaminations by mycotoxins and removing types of rice contaminated higher than acceptable limits (37). In a study conducted in the city of Tehran, Riazipour et al. (37) determined T-2 toxin in rice samples. In their study, the amount of T-2 toxin was measured using ELISA. The findings revealed that all the rice samples had been contaminated by T-2 toxin but not beyond the acceptable limit. They also suggested that the mean contamination of domestic and imported rice was 11.2 ± 2.3 and 13 ± 2.7 µg/kg, respectively (37). Furthermore, Riazipour et al. (33) investigated T-2 toxin contamination in grain and legume kitchen stores of 9 centers in the city of Tehran (23 samples), and finally, T-2 toxin positive samples were detected. Rice was also found to be affected by contamination levels of 12.5 (± 0.56) μg/kg.
5. Ochratoxin
Ochratoxin (OTAs) are among toxic secondary metabolites produced by seven species of Aspergillus and 6 species of Penicillium (60). Type A, is the most toxic form contaminating a variety of foodstuff (61). OTA is also known as a fat-soluble substance, which can be excreted and concentrated in fatty tissues. Nephrotoxic, immunosuppressive, teratogenic, and carcinogenic effects of OTA have also been reported in some studies (62). The IARC has classified AFTs into the group of primary risk factors for cancer and OTA as the secondary ones in humans (2B group) (63). According to the WHO, the TDI for OTA has been suggested about 5 ng OTA/kg body weight/day (64). Some studies conducted in Iran have further reported OTA in rice in which the mean concentration of OTA has been lower than MTL of 5 µg/kg (29). In 2013, OTA was tested in samples of rice in six different provinces in Iran, and the mean OTA in rice was reported by 0.84 - 11.37 ng/g. Therefore, the estimated TDI of OTA for an Iranian adult was 0.62 ng/kg bw/day, which was less than the PMTDI of 14 ng/kg bw/day established by the JECFA (2001) and less than the TDI of 17.1 ng/kg bw set by the EFSA (2006). The highest frequency of positive samples was found in Isfahan Province (11.37 ng/g) (24). In a study in Tehran, the amount of OTA was measured using HPLC. The levels of OTA in positive samples ranged from 0.2 to 4.8 ng.g-1, with the mean contamination of 1.6 ng.g-1 in all the samples (29). In the study by Gholampour Azizi et al. (25), a total number of 80 rice samples were analyzed for OTA in Mazandaran Province, Iran, using DC-ELISA. None of the samples were reported to be contaminated by > 5 μg/kg of OTA. In this regard, samples that had > 5 μg/kg of OTA contamination more than the acceptable limit were only reported in the cities of Sari, Nowshahr, and Ramsar (25). Another investigation showed that OTA contamination in 21.4% of Iranian rice samples was lower than standards set in Iran and the EU (European Union). The levels of OTA contamination in Iranian rice samples were also significantly 0.72% (ng/g) higher than that in the imported ones (65).
Based on incomplete information in the related literature, it was assumed that OTA was not of importance in terms of mycotoxin contamination of rice in Iran. However, conditions of rice contamination by OTA were reported significant in Isfahan Province because of their high average contents and ranges of positive samples, which needed continuous monitoring.
6. Fumonisin
Fumonisin (FMs) are toxic and carcinogenic metabolites produced by Fusarium genera (66). Different subtypes of FM have also been recognized, but only FM (B1, B2, and B3) can be naturally found in contaminated foods (12). FMB1 is the most abundantly occurring and the most significant toxicological derivative. Fumonisin can even be absorbed from the gastrointestinal tract and cause sphinganine and sphingosine accumulation and cell membrane dysfunction. Sphyngoid free bases can thus function as cancer promoters and mutations. FMB1 is toxic to the liver, kidney, as well as nervous and cardiovascular systems in humans and animals (67). In 2001, JECFA established a PMTDI for FMs of 2.0 µg/kg body weight/day (68).
In the study by Khosravi and colleagues, most rice samples (96.7%) were found to be positive for FMB1 with mean levels ranging from non-detected to 56.2 mg/kg for fresh and from 4.3 to 42.8 mg/kg for stored samples. This difference could be due to diversities in harvesting techniques, collection phases, storage conditions, testing methods, as well as sources of Fusarium section liseola isolates in soil and environmental conditions (temperature, humidity, and rainfall). In summary, this study demonstrated that rice grains could potentially have various fungi, Aspergillus and Fusarium species, and high levels of FMB1 toxin. Further studies on the decomposition of FMB1, as the most abundantly occurring FM, during processing, as well as cooking, were thus required to assess and minimize exposure to FMB1 in populations living on rice (26). Furthermore, Aghili et al. (69) reported the presence of F. moniliforme-type isolates in 65.1% of rice samples in three regions in Mazandaran Province, Iran. The mean levels of FM in regions 1, 2, and 3 were 108 - 6711, 950 - 7711 and 98 - 6595 µg/g, respectively.
Numerous studies have even focused on complicated and important roles of rainfall and temperature on FM in foods. The high rate of infection with Fusarium species in Golestan Province, Iran, was similarly in accordance with the climatic situation of this region. Since it had high average rainfall and humidity, which were suitable for cereals to be infected by FM. Moreover, FM contamination in most commonly used staple foods, especially rice, might be considered a potential risk factor for EU Commission in this high-risk region. In general, further studies were required to confirm this hypothesis (12).
7. Deoxynivalenol
Deoxynivalenol (DON), also called food-refusal factor or vomitoxin, has been recognized as one of the most important members of the trichothecene mycotoxins class produced by several FM species (70). Temperatures of 30°C and 0.995 water activity have also been reported as optimum extents for DON production. Deoxynivalenol can cause genotoxicity, teratogenicity, neurotoxicity, immunotoxicity, as well as induction of fetal skeletal deformities. At the cellular level, the main toxic effect of DON is due to the inhibition of protein and nucleic acid synthesis via binding to the ribosome and activating cellular kinases involved in signal transduction, which consequently results in decreased proliferation. According to FAO and EU Commission, the levels recommended for DON in cereal for human consumption are 100 - 2,000 ng/g and 500 μg/kg, respectively. In Iran, the MTD of DON in cereals is 1,000 ng/g. In this regard, DON in rice had been only reported in a study in the city of Tehran. Furthermore, Yazdanpanah et al. (23) analyzed rice samples using HPLC, and finally, DON was observed only in one rice sample. The mean dietary exposure of DON through rice consumption was less than the PMTDI of 1 μg/kg bw/day.
8. Conclusions
Rice can be a suitable substrate for mycotoxin-producing fungi in improper storage conditions. According to the data provided in this review, AFTs were observed from old samples rather than new ones. Moreover, the highest and the lowest levels of AFT contamination in rice samples were observed in the summer and the winter, respectively. It was also recommended to improve storage conditions to prevent spoilage and reduce AFT contamination. Owing to suitable climatic conditions in the northern regions of Iran with high temperature and humidity in the summer and extremely humid weather during the rainy season, this region was appropriate for mold growth. Consequently, high risks of contamination by these secondary metabolites are unavoidable, and it can be concluded that rice in Iran was generally safe, but more efforts are needed to assess the risks of rice contamination by mycotoxins. Considering the high daily consumption of rice, exposure to even these lower levels is a worrying health risk; therefore, our results strongly indicate that a more precise safety control system for imported and domestic rice should be considered during post-harvest handling, transfer, and long-term storage.
References
-
1.
Shirani K, Zanjani BR, Mahmoudi M, Jafarian AH, Hassani FV, Giesy JP, et al. Immunotoxicity of aflatoxin M1 : as a potent suppressor of innate and acquired immune systems in a subacute study. J Sci Food Agric. 2018;98(15):5884-92. [PubMed ID: 30014474]. https://doi.org/10.1002/jsfa.9240.
-
2.
Liu Z, Gao J, Yu J. Aflatoxins in stored maize and rice grains in Liaoning Province, China. J Stored Prod Res. 2006;42(4):468-79. https://doi.org/10.1016/j.jspr.2005.09.003.
-
3.
Tanaka K, Sago Y, Zheng Y, Nakagawa H, Kushiro M. Mycotoxins in rice. Int J Food Microbiol. 2007;119(1-2):59-66. [PubMed ID: 17913273]. https://doi.org/10.1016/j.ijfoodmicro.2007.08.002.
-
4.
Sun XD, Su P, Shan H. Mycotoxin Contamination of Rice in China. J Food Sci. 2017;82(3):573-84. [PubMed ID: 28135406]. https://doi.org/10.1111/1750-3841.13631.
-
5.
Samaddar A, Mohibbe Azam M, Singaravadivel K, Venkatachalapathy N, Swain BB, Mishra P. Postharvest Management and Value Addition of Rice and Its By-Products. The Future Rice Strategy for India. Academic Press; 2017. p. 301-34. https://doi.org/10.1016/b978-0-12-805374-4.00011-7.
-
6.
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front Microbiol. 2016;7:2170. [PubMed ID: 28144235]. [PubMed Central ID: PMC5240007]. https://doi.org/10.3389/fmicb.2016.02170.
-
7.
Ehsani M, Chaichi Nosrati A, Shirazi-Beheshtiha SH. Measuring toxin Zearalenone in wheat harvested from three regions of North, West and South of Iran. Bull Environ Pharmacol Life Sci. 2014;3:69-76.
-
8.
Yazdanpanah H, Zarghi A, Shafaati AR, Foroutan SM, Aboul-Fathi F, Khoddam A, et al. Analysis of aflatoxin b1 in Iranian foods using HPLC and a monolithic column and estimation of its dietary intake. Iran J Pharm Res. 2013;12(Suppl):83-9. [PubMed ID: 24250676]. [PubMed Central ID: PMC3813360].
-
9.
[No author listed]. Evaluation of certain food additives and contaminants. Thirty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ Tech Rep Ser. 1991;806:1-52. [PubMed ID: 2038840].
-
10.
Joint; World Health Organization; WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: seventy-third [73rd] report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; 2011.
-
11.
FOOD W. Safety evaluation of certain food additives and contaminants. WHO; 2007.
-
12.
Alizadeh AM, Rohandel G, Roudbarmohammadi S, Roudbary M, Sohanaki H, Ghiasian SA, et al. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac J Cancer Prev. 2012;13(6):2625-8. [PubMed ID: 22938431]. https://doi.org/10.7314/apjcp.2012.13.6.2625.
-
13.
Canady RA, Coker RD, Egan SK, Krska R, Olsen M, Resnik S, et al. T-2 and HT-2 toxins. Safety Evaluation of Certain Mycotoxins in Food. Joint Expert Committee on Food Additives (JECFA), WHO Food Additives Series,; 2001. 95 p.
-
14.
Shirani K, Shahbazi N, Khayat M, Khashayarmanesh Z, Hassani F, Moharerri N, et al. Concentration of heavy metals in Iranian market rice and associated population health risk. Qual Assur Safety Crops Foods. 2017;9(3):249-54. https://doi.org/10.3920/qas2015.0667.
-
15.
Reddy KRN, Reddy CS, Abbas HK, Abel CA, Muralidharan K. Mycotoxigenic Fungi, Mycotoxins, and Management of Rice Grains. Toxin Rev. 2008;27(3-4):287-317. https://doi.org/10.1080/15569540802432308.
-
16.
Amanloo S, Rezaei Kahhka MR, Ramezani AA, Mir L. The Mycotoxin contamination of the imported consumer rice and its producing fungi in Zabol. Pars Jahrom Univ Med Sci. 2014;12(1):17-25. https://doi.org/10.29252/jmj.12.1.17.
-
17.
Nazari F, Sulyok M, Yazdanpanah H, Kobarfard F, Krska R. A survey of mycotoxins in domestic rice in Iran by liquid chromatography tandem mass spectrometry. Toxicol Mech Methods. 2014;24(1):37-41. [PubMed ID: 24032669]. https://doi.org/10.3109/15376516.2013.844752.
-
18.
Nazari F, Sulyok M, Kobarfard F, Yazdanpanah H, Krska R. Evaluation of Emerging Fusarium mycotoxins beauvericin, Enniatins, Fusaproliferin and Moniliformin in Domestic Rice in Iran. Iran J Pharm Res. 2015;14(2):505-12. [PubMed ID: 25901158]. [PubMed Central ID: PMC4403067].
-
19.
Tavakoli H, Rostami H, Zabihi A, Bitarafan Z. A survey of aflatoxins, ochratoxin and Zearalenone Contamination in Imported and Iranian Rice in Iran. Agricult Engin Res J. 2014;4:61-4.
-
20.
Sani AM, Azizi EG, Salehi EA, Rahimi K. Reduction of aflatoxin in rice by different cooking methods. Toxicol Ind Health. 2014;30(6):546-50. [PubMed ID: 23024107]. https://doi.org/10.1177/0748233712462466.
-
21.
Mohammadi M, Mohebbi G, Hajeb P, Akbarzadeh S, Shojaee I. Aflatoxins in rice imported to Bushehr, a southern port of Iran. Am Eurasian J Toxicol Sci. 2012;4(1):31-5.
-
22.
Manoochehri M, Asgharinezhad AA, Safaei M. Determination of aflatoxins in rice samples by ultrasound-assisted matrix solid-phase dispersion. J Chromatogr Sci. 2015;53(1):189-95. [PubMed ID: 24771057]. https://doi.org/10.1093/chromsci/bmu018.
-
23.
Yazdanpanah H, Shafaati A, Foroutan SM, Zarghi A, Aboul-Fathi F, Khoddam A, et al. Occurrence of deoxynivalenol in foods for human consumption from tehran, iran. Iran J Pharm Res. 2014;13(Suppl):87-92. [PubMed ID: 24711833]. [PubMed Central ID: PMC3977057].
-
24.
Rahimi E. The occurrence of ochratoxin A in rice in six provinces of Iran. Toxicol Ind Health. 2016;32(7):1324-7. [PubMed ID: 25525057]. https://doi.org/10.1177/0748233714560078.
-
25.
Gholampour Azizi I, Ghadi H, Rouhi S. Ochratoxin A analysis in rice samples of different cities of Mazandaran (a province in Northern Iran). Nutr Food Sci. 2014;44(3):223-9. https://doi.org/10.1108/nfs-07-2013-0088.
-
26.
Khosravi AR, Shokri H, Zaboli F. Grain-Borne Mycoflora and Fumonisin B1 From Fresh-Harvested and Stored Rice in Northern Iran. Jundishapur J Microbiol. 1970;6(5). https://doi.org/10.5812/jjm.6414.
-
27.
Yazdanpanah H, Zarghi A, Shafaati AR, Foroutan SM, Aboul-Fathi F, Khoddam A, et al. Exposure assessment of the tehran population (iran) to zearalenone mycotoxin. Iran J Pharm Res. 2012;11(1):251-6. [PubMed ID: 24250447]. [PubMed Central ID: PMC3813106].
-
28.
Rahmani A, Soleimany F, Hosseini H, Nateghi L. Survey on the occurrence of aflatoxins in rice from different provinces of Iran. Food Addit Contam Part B Surveill. 2011;4(3):185-90. [PubMed ID: 24786005]. https://doi.org/10.1080/19393210.2011.599865.
-
29.
Feizy J, Beheshti HR, Fakoor Janati SS, Khoshbakht Fahim N. Survey of ochratoxin A in rice from Iran using affinity column cleanup and HPLC with fluorescence detection. Food Addit Contam Part B Surveill. 2011;4(1):67-70. [PubMed ID: 24779665]. https://doi.org/10.1080/19393210.2010.542252.
-
30.
Feizy J, Beheshti HR, Fahim NK, Janati SS, Davari G. Survey of aflatoxins in rice from Iran using immunoaffinity column clean-up and HPLC with fluorescence detection. Food Addit Contam Part B Surveill. 2010;3(4):263-7. [PubMed ID: 24779626]. https://doi.org/10.1080/19440049.2010.516456.
-
31.
Mazaheri M. Determination of aflatoxins in imported rice to Iran. Food Chem Toxicol. 2009;47(8):2064-6. [PubMed ID: 19481133]. https://doi.org/10.1016/j.fct.2009.05.027.
-
32.
Mojtaba R. Determination of zearalenone contamination in wheat and rice in Chaharmahal va Bakhtyari, Iran. J Cell Anim Biol. 2012;6(4). https://doi.org/10.5897/jcab11.097.
-
33.
Riazipour M, Fooladi AAI, Bagherpour G. A Survey on Occurrence of T-2 toxin in Cereals Destined for Human Consumption. Jundishapur J Microbiol. 2012;5(3):497-501. https://doi.org/10.5812/jjm.4251.
-
34.
Zaboli F, Khosravi AR, Gholampourazizi I, Norouzi M, Erfanmanesh A. Study of aflatoxins production in rice bran from Mazandran Province, Northern Iran. Glob Vet. 2010;5(1):39-44.
-
35.
Karajibani M, Merkazee A, Montazerifar F. Determination of Aflatoxin in the Imported Rice in Zahedan, South-East of Iran, 2011. Health Scope. 2013;2(3):125-9. https://doi.org/10.17795/jhealthscope-10736.
-
36.
Khorasgani ZN, Goudarzi M, Behfar A, Kalantari H, Ebrahim R, Mahdavi M. Occurrence of aflatoxins in imported rice at supermarkets of ahvaz city, khuzestan province, iran. Jundishapur J Nat Pharm Prod. 2015;10(2):1-5.
-
37.
Riazipour M, Fooladi AI, Razzaghi-Abyaneh M. Natural occurrence of T-2 toxin in domestic and imported rice. Iran J Public Health. 2009:111-6.
-
38.
Firouzi M, Harati H, Kalantari E. Measuring the Levels of Aflatoxin, Ochratoxin and Deoxynivaleno Mycotoxins in Pakistani Rice Samples Imported to Zahedan City. J Renew Nat Resources Bhutan.
-
39.
Eslami M, Mashak Z, Heshmati A, Shokrzadeh M, Mozaffari Nejad AS. Determination of aflatoxin B1levels in Iranian rice by ELISA method. Toxin Rev. 2015;34(3):125-8. https://doi.org/10.3109/15569543.2015.1074925.
-
40.
Mousavizadeh A, Peikar A, Hoseinian A, Ghaydie E, Pourmahmoudi A. Determination of total aflatoxin in rice consumption in Yasuj, Iran. Biosci Biotechnol Res Commun. 2017;10(1):195-8.
-
41.
Safara M, Zaini F, Hashemi S, Mahmoudi M, Khosravi A, Shojai-Aliabadi F. Aflatoxin Detoxification in Rice using Citric Acid. Iran J Public Health. 2010;39(2):24-9. [PubMed ID: 23113003]. [PubMed Central ID: PMC3481757].
-
42.
Zand MA, Kalantari HA, Abdollahi-Lorestani S. Determination of aflatoxins b1 and m1 in chiken livers produced in Ahwaz, Iran. Sci J Agricult. 1999;22(1).
-
43.
Jafari M, Rezaei M, Kalantari H, Tabarzad M, Daraei B. Optimization of Aflatoxin B1 Aptasensing. J Toxicol. 2017;2017:2461354. [PubMed ID: 28588614]. [PubMed Central ID: PMC5446859]. https://doi.org/10.1155/2017/2461354.
-
44.
Jayaraman P, Kalyanasundaram I. Natural occurrence of toxigenic fungi and mycotoxins in rice bran. Mycopathologia. 1990;110(2):81-5. [PubMed ID: 2114545]. https://doi.org/10.1007/BF00446995.
-
45.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. 56. World Health Organization; 1993.
-
46.
Shirani K, Riahi Zanjani B, Mehri S, Razavi-Azarkhiavi K, Badiee A, Hayes AW, et al. miR-155 influences cell-mediated immunity in Balb/c mice treated with aflatoxin M1. Drug Chem Toxicol. 2021;44(1):39-46. [PubMed ID: 30739504]. https://doi.org/10.1080/01480545.2018.1556682.
-
47.
Institute of Standard and Industrial Research of Iran (ISIRI). Maximum Tolerated Limits of Mycotoxins in Foods and Feeds. 2002. Report No.: National Standard No. 5925.
-
48.
Faraji H, Tabatabei YF, Kafilzadeh F, Nasiri MM. Investigation of total aflatoxins in consumed rice at Mashhad city in the summer and winter. J Food Sci Tech. 2010;2(5):11-6.
-
49.
Siruguri V, Kumar PU, Raghu P, Rao MV, Sesikeran B, Toteja GS, et al. Aflatoxin contamination in stored rice variety PAU 201 collected from Punjab, India. Indian J Med Res. 2012;136(1):89-97. [PubMed ID: 22885269]. [PubMed Central ID: PMC3461724].
-
50.
McKenzie KS, Sarr AB, Mayura K, Bailey RH, Miller DR, Rogers TD, et al. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem Toxicol. 1997;35(8):807-20. https://doi.org/10.1016/s0278-6915(97)00052-5.
-
51.
Yazar S, Omurtag GZ. Fumonisins, trichothecenes and zearalenone in cereals. Int J Mol Sci. 2008;9(11):2062-90. [PubMed ID: 19330061]. [PubMed Central ID: PMC2635619]. https://doi.org/10.3390/ijms9112062.
-
52.
Bucheli TD, Wettstein FE, Hartmann N, Erbs M, Vogelgsang S, Forrer HR, et al. Fusarium mycotoxins: overlooked aquatic micropollutants? J Agric Food Chem. 2008;56(3):1029-34. [PubMed ID: 18197623]. https://doi.org/10.1021/jf073082k.
-
53.
Hueza IM, Raspantini PC, Raspantini LE, Latorre AO, Gorniak SL. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins (Basel). 2014;6(3):1080-95. [PubMed ID: 24632555]. [PubMed Central ID: PMC3968378]. https://doi.org/10.3390/toxins6031080.
-
54.
Sadeghi E, Barkhordar S, Mohammadi G, Moradi M, Asadi F, Nesari S, et al. Determination of zearalenone levels in consumed rice samples in iran by high performance liquid chromatography (2015). Acta Med Mediterr. 2016;32(5):1945-9.
-
55.
Kalantari H, Mousavi M. Review on T-2 toxin. Jundishapur J Nat Pharm Prod. 2010;5(1):26-38.
-
56.
Moosavi M, Rezaei M, Kalantari H, Behfar A, Varnaseri G. l-carnitine protects rat hepatocytes from oxidative stress induced by T-2 toxin. Drug Chem Toxicol. 2016;39(4):445-50. [PubMed ID: 26888052]. https://doi.org/10.3109/01480545.2016.1141423.
-
57.
Kalantari H, Hemmati AA, Goudarzi M, Forouzandeh H, Kalantar M, Aghel N, et al. Healing Effect of Hawthorn (Crataegus pontica C. Koch) Leaf Extract in Dermal Toxicity Induced by T-2 Toxin in Rabbit. Jundishapur J Nat Pharm Prod. 2016;11(3). https://doi.org/10.17795/jjnpp-35688.
-
58.
Richard JL. Some major mycotoxins and their mycotoxicoses--an overview. Int J Food Microbiol. 2007;119(1-2):3-10. [PubMed ID: 17719115]. https://doi.org/10.1016/j.ijfoodmicro.2007.07.019.
-
59.
Boermans HJ, Leung MC. Mycotoxins and the pet food industry: toxicological evidence and risk assessment. Int J Food Microbiol. 2007;119(1-2):95-102. [PubMed ID: 17889389]. https://doi.org/10.1016/j.ijfoodmicro.2007.07.063.
-
60.
Taghizadeh SF, Rezaee R, Davarynejad G, Asili J, Nemati SH, Goumenou M, et al. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different Pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ Toxicol Pharmacol. 2018;61:61-6. [PubMed ID: 29852370]. https://doi.org/10.1016/j.etap.2018.05.010.
-
61.
Clark HA, Snedeker SM. Ochratoxin a: its cancer risk and potential for exposure. J Toxicol Environ Health B Crit Rev. 2006;9(3):265-96. [PubMed ID: 16621780]. https://doi.org/10.1080/15287390500195570.
-
62.
Li F, Ji R. [Ochratoxin A and human health]. J Hygiene Res. 2003;32(2):172-5. Chinese.
-
63.
Alshannaq A, Yu JH. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int J Environ Res Public Health. 2017;14(6). [PubMed ID: 28608841]. [PubMed Central ID: PMC5486318]. https://doi.org/10.3390/ijerph14060632.
-
64.
Chaleshtori R, Salehi E. Ochratoxin a in food products in Iran: A systematic review of the evidence. Int Arch Health Sci. 2018;5(2). https://doi.org/10.4103/iahs.iahs_4_18.
-
65.
Habib V, Mohammad G, Zeinal–Abedin B, Zahra M. Ochratoxin a detection in rice samples in mazandaran province. Pharmacophore. 2017;8(6):10-21.
-
66.
Marin P, Magan N, Vazquez C, Gonzalez-Jaen MT. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol Ecol. 2010;73(2):303-11. [PubMed ID: 20491926]. https://doi.org/10.1111/j.1574-6941.2010.00894.x.
-
67.
Ghiasian SA, Rezayat SM, Kord-Bacheh P, Maghsood AH, Yazdanpanah H, Shephard GS, et al. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia. 2005;159(1):31-40. [PubMed ID: 15750730]. https://doi.org/10.1007/s11046-004-3899-5.
-
68.
Hassanzadeh Eramssadati SFAN, Modiri L. Evaluations of fumonisins quantities in wheat crops harvested from North, West And South Regional provinces of Iran. Trends Life Sci. 2014;3(4):162-70.
-
69.
Aghili S, Khosravi AR, Shokohi T, Salmanian B, Shokri H, Nikaeen O. Corresponding Author: Isolated from Unpolished Rice in Mazandaran, Iran. Mazandaran Univ Med Sci. 2013.
-
70.
He J, Yang R, Zhou T, Tsao R, Young JC, Zhu H, et al. Purification of deoxynivalenol from Fusarium graminearum rice culture and mouldy corn by high-speed counter-current chromatography. J Chromatogr A. 2007;1151(1-2):187-92. [PubMed ID: 17306807]. https://doi.org/10.1016/j.chroma.2007.01.112.