Abstract
Background:
Neutropenia, as a predisposing factor for invasive candidiasis, is defined as a reduction in neutrophil count to less than 1500/mm3. It is a common condition in patients with hematological malignancy and cytostatic chemotherapy. Extensive chemotherapy and prophylaxis with antifungals have increased the resistance of Candida isolates to antifungal drugs. Although, Candida albicans is the most common causative agent among neutropenic patients, there is an increasing rate of non-albicans species. Extracellular enzymes activity pattern and antifungal agent sensitivity profiles are two important factors for spreading resistant strains.Objectives:
The aim of the present study was to identify the Candida strains isolated from hospitalized neutropenic patients. The patterns of antifungal susceptibility of the causative agents to antifungals and the extracellular enzymes activity of the isolates were also evaluated.Patients and Methods:
In the present study, 243 urine and 243 oral swab samples were collected from neutropenic patients and inoculated on CHROMagar Candida. In addition, 100 blood samples were also inoculated in biphasic Brain Heart Infusion medium. Several yeast isolates were isolated from samples and identified by classical and molecular techniques. The profiles of extracellular enzymes and the susceptibility of recovered agents to amphotericin B, fluconazole and caspofungin were also evaluated.Results:
A total of 110 yeast strains isolated from urine and oral cavities were identified as C. albicans (51.8%), C. krusei (25.5%), C. glabrata (6.4%) and other yeasts (16.3%). No yeast species was isolated from blood samples. Our result showed that in 90% of the isolates, the range of secretion of extracellular enzymes was medium (2+) and high (3+), however only a few isolates were negative for this characteristic. All isolates were sensitive to caspofungin and fluconazole, whereas 54.7% of isolates were resistant to amphotericin B.Conclusions:
We found a marked increase in the incidence of non-albicans species (48.2%) among neutropenic patients. Only a few strains failed to produce extracellular enzymes. Finally, in addition to fluconazole, caspofungin can be considered as the first line treatment against Candida species among neutropenic patients.Keywords
Neutropenic Patients Antifungal Susceptibility Extracellular Enzymes Candida
1. Background
Neutropenia is defined as a reduction in blood neutrophil counts to less than 1500/mm3 (1). This disease is the most common condition in patients with hematological malignancy (2), cytostatic chemotherapy (3), hematopoietic stem cell transplantation (4) and acute leukemia (5). This condition is one of the most important risk factors for several fungal invasive infections (such as, invasive candidiasis and aspergillosis). As a result, such infections could be associated with high morbidity and mortality, and increase the duration of hospitalization in neutropenic patients (6). Generally, the incidence of systemic mycosis has increased dramatically over the past 30 to 40 years due to new invasive therapies.
Candida and Aspergillus species are the two most important fungal agents that are responsible for several fungal infections among neutropenic patients (3). Although, Candida albicans is the most common infectious agent among neutropenic patients, however, there has been an increasing rate of non-albicans species (C. krusei, C. glabrata and C. tropicalis) in the recent years. In addition, extensive antifungals prophylaxis (Amphotericin B and fluconazole) during/after chemotherapy increased the resistance to some isolates of Candida species especially non-albicans strains (7). Although, several host predisposing factors influence the pathogenicity of agents, extracellular enzymes have an important role in the pathogenesis. The role of phospholipase, esterase, proteinase and haemolysin, in the pathogenicity of Candida species, has been well documented (8). On the other hand, the extracellular enzymatic profile differs between different recovered isolates from different clinical samples.
Amphotericin B, fluconazole and itraconazole are routinely used for chemotherapy and chemoprophylaxis in neutropenic patients (7, 9, 10). Recently, caspofungin (blocker β-1,3-D glucan synthase enzyme) has been introduced as an effective antifungal against systemic candidiasis in neutropenic patients (2). On the other hand, nystatin mouthwash solutions are also used for the treatment of oral lesions. Therefore, in vitro assay of antifungal drugs against Candida species could be useful for best management and treatment by clinicians.
2. Objectives
The aim of the present study was to detect Candida species from hospitalized neutropenic patients at Shafa hospital in Ahvaz. In addition, antifungal susceptibility profiles in causative agents and the pattern of extracellular enzymes activity were also evaluated.
3. Patients and Methods
3.1. Patients and Sampling
This descriptive study was approved by the ethical committee of Ahvaz Jundishapur University of Medical Sciences (ajums.REC.1393.150). All patients or parents signed a consent form before sampling. In the present study, 243 hospitalised neutropenic patients (neutrophil count less than 1500 neutrophils per microliter) at Shafa hospital, affiliated to Ahvaz Jundishapur University of Medical Sciences, were sampled. All patients were under prophylactic treatment with fluconazole or amphotericin B. Different specimens including 100 blood samples, 243 urine samples and 243 swabs from oral cavity of patients were collected. Swabs from oral cavity and 10 µL of urine samples were cultured on CHROMagar Candida plates (CHROMagar Candida, France) and incubated at 37°C for 48 - 72 hours. In addition, 2 mL of blood samples were inoculated into biphasic brain heart infusion (BHI, Baharafshan, Iran) bottles and incubated at 37°C for seven days. Bottles were aerated using a sterile syringe needle every day.
3.2. Classical Identification
All isolated yeasts species were identified using routine mycological tests, such as germ tube formation on human serum, microscopy morphology on cornmeal agar (High Media, India) plus 1% Tween 80 (Merck, Germany), growth at 42 - 45°C and colony morphology on CHROMagar Candida.
3.3. DNA Extraction, Amplification and Sequencing
In the present study, 40 strains (all non-albicans species, nine strains of C. krusei and five strains of C. albicans) were subjected for polymerase chain reaction (PCR) analysis. Yeasts were cultured on sabouraud dextrose agar (SDA, Merck, Germany) and incubated at 37°C overnight. A small amount of each yeast colony was collected in special cryotube vials containing 300 µL of lysis buffer and 30 mg glass bed. Yeasts were homogenized with SpeedMill Plus system (Analytik Jena, Germany) for two minutes and then incubated at 100°C for two minutes. Overall, 150 µL of 3 M Sodium acetate was added to each tube and kept at -20°C for 10 minutes. Tubes were centrifuged at 12000 rpm for 10 minutes at 4°C and then supernatants were separated and extracted using phenol-chloroform-isoamyl alcohol (11).
All extracted DNA were kept at -20°C until use. The internal transcribed spacer (ITS) region of the rDNA was amplified by PCR with the primers V9G (5’-TTACGTCCCTGCCCTTTGTA-3’) and LS266 (5’-GCATTCCCAAACAACTCGACTC-3’) (12). The PCR products were sequenced by the Bioneer company (Bioneer, Daejeon, South Korea). The results of DNA sequencing were analyzed using the Chromas Lite program (Technelysium, Version 2.4) and similarity to the sequenced genes in GenBank library was evaluated.
3.4. Enzymatic Activities
The activity of extracellular enzymes (phospholipase, esterase, proteinase and haemolysin) of isolated strains was detected by several specific tests that were previously used by our team (13-15). Briefly, phospholipase activity was assessed by SDA supplemented with egg yolk. Bovine serum albumin agar medium (Merck, Germany) and Tween 80 (Merck, Germany) opacity test medium were used for the assessment of proteinase and esterase activities, respectively. sabouraud dextrose agar supplement with 3% glucose (Merck, Germany) and 7% defibrinated sheep blood was used for the detection of haemolytic activity. Finally, the extracellular enzymes indexes were calculated according to Price et al. (16)
3.5. Susceptibility Tests
In the present study, 95 yeast isolates including; C. albicans (50 isolates), C. krusei (20), C. glabrata (7), C. tropicalis (4), C. kefyr (3), C. dubliniensis (5), C. parapsilosis (2), C. orthopsilosis (1), C. lambica (1), C. hellenica (1) and Saccharomyces cerevisiae (1) were tested against amphotericin B, fluconazole and caspofungin in vitro using modified Clinical and laboratory standards institute (CLSI) method. A stock solution of 1.25 mg/mL for caspofungin (Sigma - Aldrich, Germany) and 32 mg/mL for amphotericin B (Sigma - Aldrich, Germany) and fluconazole (Serva, USA) were prepared in dimethyl sulfoxide (DMSO, Fluka, Germany). Antifungal stocks were kept at -20 until use. A serial dilution of each antifungal was prepared in RPMI (Bio Idea, Iran) containing 0.01% Alamar Blue (Resazurin) (Sigma - Aldrich, Germany) (17-19).
A serial dilution of caspofungin was prepared from 4 - 0.03125 µg/mL. In addition, serial dilution of each amphotericin B and fluconazole was separately prepared from 32 to 0.25 µg/mL. Then, 100 µL of antifungal and 100 µL of diluted standard yeast suspension were added to each microplate well. Microplates were incubated at 35°C for 24 to 48 hours, aerobically. Positive and negative controls contained, medium and suspension, and antifungal drug, respectively. The last unchanged blue color microplate well to red color was recorded as minimum inhibitory concentration (MIC) for each tested yeast against each antifungal drug (Figure 1).
Resazurin Dye Test for Determining Minumum Inhibitory Concentration of Fluconazole and Caspofungin to Candida Species
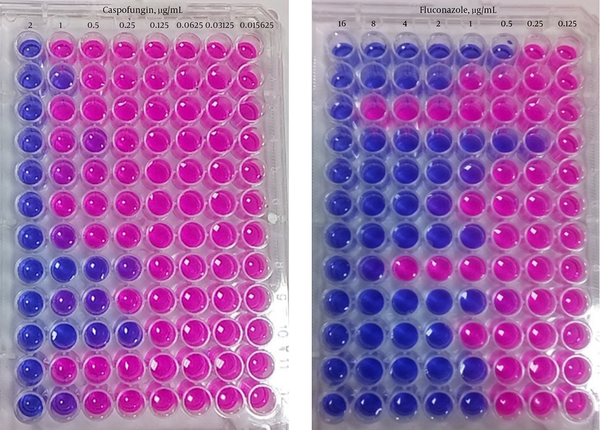
4. Results
4.1. Patients and Organisms
We examined 243 neutropenic patients (54.3% female and 45.7% male) with age range from >5 to 75 years. Fifteen (6.2%) cases of 243 sampled patients had candiduria, 12 cases were females and 3 cases were males. On the other hand, oral swabs from 95 cases yielded different yeast species. Our results showed that 110 different yeast species were isolated including; 109 (99.1%) Candida species and one (0.9%) non-Candida yeast. The most common species was C. albicans (57), followed by C. krusei (28), C. glabrata (7), C. tropicalis (4), C. kefyr (3), C. dubliniensis (5), C. parapsilosis (2), C. orthopsilosis (1), C. lambica (1), C. hellenica (1), and S. Cerevisiae (1) (Table 1). In the present study, we couldn’t recover any yeast from blood samples using biphasic BHI.
Recovered Yeast Species From Oral Cavity and Urine Samples of Neutropenic Patients
| Yeasts Species | Oral Cavity, No. (%) | Urine, No. (%) | Total, No. (%) |
|---|---|---|---|
| C. albicans | 50 (45.5) | 7 (6.4) | 57 (51.8) |
| C. krusei | 23 (20.9) | 5 (4.5) | 28 (25.5) |
| C. glabrata | 5 (4.5) | 2 (1.8) | 7 (6.4) |
| C. dubliniensis | 4 (3.6) | 1 (0.9) | 5 (4.5) |
| C. tropicalis | 4 (3.6) | 0 (0.0) | 4 (3.6) |
| C. kefyr | 3 (2.7) | 0 (0.0) | 3 (2.7) |
| C. parapsilosis | 2 (1.8) | 0 (0.0) | 2 (1.8) |
| C. lambica | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| C. orthopsilosis | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| C. hellenica | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| S. cerevisiae | 1 (0.9) | 0 (0.0) | 1 (0.9) |
| Total | 95 (86.4) | 15 (13.6) | 110 (100) |
4.2. Extracellular Enzymes
Table 2 summarizes the details of extracellular enzymes production in different species of Candida. The haemolytic and proteinase activities of oral isolates of Candida were observed to be much higher than urinary isolates. However, urinary strains showed higher esterase activity than oral species.
Extracellular Enzymes Activity of Isolated Yeasts
| Hi Value | Haemolytic Activity | Oral Isolates, No. (%) | Urine Isolates, No. (%) | Total, No. (%) |
|---|---|---|---|---|
| 1 | Negative | 8 (8.4) | 1 (6.7) | 9 (8.2) |
| 0.70 - 0.99 | Low (1+) | 11 (11.5) | 11 (73.3) | 22 (20) |
| 0.40 - 0.69 | Medium (2+) | 17 (17.9) | 1 (6.7) | 18 (16.4) |
| 0.10 - 0.39 | High (3+) | 59 (62.1) | 2 (13.3) | 61 (55.4) |
| Total | 95 (100) | 15 (100) | 110 (100) | |
| Ez Value | Esterase activity | |||
| 1 | Negative | 5 (5.3) | 1 (6.7) | 6 (5.4) |
| 0.70 - 0.99 | Low (1+) | 25 (26.3) | 4 (26.6) | 29 (26.4) |
| 0.40 - 0.69 | Medium (2+) | 50 (52.6) | 2 (13.3) | 52 (47.3) |
| 0.10 - 0.39 | High (3+) | 15 (42.8) | 8 (53.3) | 23 (20.9) |
| Total | 95 (100) | 15 (100) | 110 (100) | |
| Pz Value | Phospholipase activity | |||
| 1 | Negative | 4 (4.2) | 3 (20) | 7 (6.4) |
| 0.70 - 0.99 | Low (1+) | 17 (17.9) | 2 (13.3) | 19 (17.3) |
| 0.40 - 0.69 | Medium (2+) | 43 (45.3) | 6 (40) | 49 (44.5) |
| 0.10 - 0.39 | High (3+) | 31 (32.6) | 4 (26.6) | 35 (31.8) |
| Total | 95 (100) | 15 (100) | 110 (100) | |
| Pz Value | Proteinase activity | |||
| 1 | Negative | 2 (2.1) | 0 (0) | 2 (1.8) |
| 0.70 - 0.99 | Low (1+) | 32 (33.7) | 3 (20) | 35 (31.8) |
| 0.40 - 0.69 | Medium (2+) | 23 (24.2) | 7 (46.7) | 30 (27.3) |
| 0.10 - 0.39 | High (3+) | 38 (40) | 5 (33.3) | 43 (39.1) |
| Total | 95 (100) | 15 (100) | 110 (100) |
4.3. Antifungal Susceptibility
In the present study, 94 isolates of Candida species and one Saccharomyces cerevisiae were tested against three antifungal drugs including; amphotericin B, fluconazole and caspofungin. After 24 and 48 hours, 54.7% and 72.6% of the isolates were resistant to amphotericin B (< 8 µg/mL), respectively. Amphotericin B resistance was found after 24 to 48 hours of incubation in 57.1%, of C. glabrata isolates. However, drug resistance among the isolates of C. krusei increased from 50% to 65% when incubation period was extended to 48 hours. Fluconazole was shown to have good efficacy against all tested isolates, and dose dependent sensitivity to fluconazole (≤ 16 µg/mL) was only identified in one isolate of C. krusei after 48 hours of incubation. During this study, it was found that all of tested isolates were sensitive to caspofungin at a range of 2 - > 0.03125 µg/mL. Table 3 summarizes the results of MIC ranges, MIC50, MIC90 and geometric mean MIC. Overall, for all isolates, MICs 50%, 0.5 µg/mL was increased to 1 µg/mL after 48 hours, when MICs 90% was fixed 2 µg/mL after 24 and 48 hours incubation for caspofungin.
The Antifungal Susceptibility of Isolates to Amphotericin B, Fluconazole and Caspofungin
| Antifungal Agents | Minimum Inhibitory Concentration, µg/mL | |||
|---|---|---|---|---|
| MIC Ranges | MIC 50% | MIC 90% | Geometric Mean | |
| Candida albicans, n = 50 | ||||
| Amphotericin B | 16 - ≥ 0.125 | 16 | 16 | 13.52224 |
| Fluconazole | 2 - 0.125 | 0.5 | 1 | 0.59874 |
| Caspofungin | 2 - > 0.03125 | 0.5 | 1 | 0.46652 |
| Candida glabrata, n = 7 | ||||
| Amphotericin B | 16 - ≥ 0.125 | 16 | 16 | 9.68625 |
| Fluconazole | 4 - 0.125 | 0.125 | 2 | 0.41017 |
| Caspofungin | 2 - > 0.03125 | 1 | 2 | 0.3715 |
| Candida tropicalis, n = 4 | ||||
| Amphotericin B | 16 - 8 | 16 | 16 | 13.45434 |
| Fluconazole | 2 - 0.25 | 1 | 2 | 1 |
| Caspofungin | 2 - 0.25 | 1 | 2 | 0.8409 |
| Candida krusei, n = 20 | ||||
| Amphotericin B | 16 - ≥ 0.125 | 8 | 16 | 3.605 |
| Fluconazole | 16 - 0.125 | 0.25 | 4 | 0.46652 |
| Caspofungin | 2 - > 0.03125 | 0.25 | 1 | 0.19615 |
| Candida dubliniensis, n = 5 | ||||
| Amphotericin B | 16 - 4 | 8 | 16 | 8 |
| Fluconazole | 8 - 0.125 | 1 | 8 | 1 |
| Caspofungin | 2 - > 0.25 | 1 | 2 | 1 |
| Saccharomyces cerevisiae, n = 1 | ||||
| Amphotericin B | 16 | - | - | - |
| Fluconazole | 2 | - | - | - |
| Caspofungin | 2 | - | - | - |
| Candida kefyr, n = 3 | ||||
| Amphotericin B | 16 | - | - | - |
| Fluconazole | 16 - 0.125 | - | - | - |
| Caspofungin | 2 - >0.5 | - | - | - |
| Candida lambica, n = 1 | ||||
| Amphotericin B | 16 | - | - | - |
| Fluconazole | 1 | - | - | - |
| Caspofungin | 0.25 | - | - | - |
| Candida hellenica (n = 1 | ||||
| Amphotericin B | 8 | - | - | - |
| Fluconazole | 1 | - | - | - |
| Caspofungin | 0.25 | - | - | - |
| Candida orthopsilosis, n = 1 | ||||
| Amphotericin B | 4 | - | - | - |
| Fluconazole | 0.5 | - | - | - |
| Caspofungin | 1 | - | - | - |
| Candida parapsilosis, n = 2 | ||||
| Amphotericin B | 8 - 4 | - | - | - |
| Fluconazole | 8 - 4 | - | - | - |
| Caspofungin | 0.0625 - > 0.03125 | - | - | - |
| All isolated yeasts, n = 95 | ||||
| Amphotericin B | 16 - ≥ 0.125 | 16 | 16 | 6.28813 |
| Fluconazole | 16 - ≥ 0.125 | 0.5 | 2 | 0.64078 |
| Caspofungin | 2 - >0.03125 | 0.5 | 2 | 0.40465 |
5. Discussion
Since neutropenic patients are usually at high risk for invasive candidiasis, colonization of mucosal tissues with Candida species is more important for them. In this condition, transaction of Candida species via mucosal tissues into bloodstream causes candidemia. Indeed, some authors believe that candiduria among neutropenic patients could be evaluated as a marker for systemic candidiasis and suitable antifungals drugs must be prescribed (20, 21). In the present study, we investigated the epidemiological features of Candida colonization among hospitalized neu-tropenic patients. Our results showed that the urinary system in 6.2% of cases (12 females and 3 males) was colonized with different species of Candida (Candiduria), whereas oral cavity in 95 (39.1%) (females 27, 28.5% and males 68, 71.6 %) of the patients was colonized with different species of Candida and non-Candida species. Similar reports showed a difference in the colonization of Candida among neutropenic patients, for example colonization was indicated in 46.8% of pediatric patients with neutropenia (22), 66% of patients undergoing hematopoietic stem-cell transplantation (3) and 61.8% of neutropenic very low-birth-weight neonates (23). In addition, Zollner-Schwetz et al., believed that gastrointestinal tract colonization by Candida species in neutropenic patients is an important risk factor for invasive candidiasis. They also found that 48% of multi-colonized patients had same Candida species in their oral and intestinal samples (24).
Although, C. albicans is the first most common candidiasis agent and human colonizer, increasing non-albicans species such as C. glabrata, C. krusei, C. parapsilosis and C. tropicalis have been reported by several researchers during the last decades (25-27). In a study by Betts et al. (9) C. tropicalis (37%) was accounted as the major invasive candidiasis agent in neutropenic patients, followed by C. albicans (22%) and C. krusei (11%). The authors revealed that neutropenic patients were mainly colonized with C. albicans (51.8%) followed by non-albicans species of C. krusei (25.5%). On the other hand, very rare species of Candida, such as C. hellenica (28) and C. lambica (29), were reported as human pathogens. Candida lambica, C. hellenica, C. orthopsilosis and S. cerevisiae were rare non-albicans species that were recovered from our patients.
Nosocomial infections, candidemia and candiduria, by non-albicans species have considerably increased during the last decades. Moreover, virulence factors, such as phospholipase, esterase, proteinase and haemolysin have been well documented among vaginal and urine isolates of Candida (13-15, 30). Furthermore, host tissue colonization and tissue invasion have been associated with extracellular enzymes production by isolates. On the hand, little information is available concerning the role of extracellular enzymes in the colonization of Candida in neutropenic patients. A few isolates were phospholipase and protease positive in the study of Negri et al., (31), whereas in contrast, 100% and 72.9% of Candida species from pulmonary tuberculosis patients produced proteases and phospholipase as reported by Kumar et al. (32). More researchers believe that the virulence in C. albicans is correlated with the combination of several factors including, high phospholipase activity and germ tube production (33). Our study showed that only a few isolates were negative for the production of extracellular enzymes and the enzymatic activity of 90% of isolates were at medium (2+) and high (3+) ranges (Table 2).
Fluconazole, a triazole antifungal, is active against Candida species in neutropenic patients (34) as well as other infections. Furthermore, drugs are used as first line therapy for esophageal candidiasis among neutropenic patients (2). On the other hand, both fluconazole and amphotericin B are widely used for prophylaxis in neutropenic patients (7). Reports indicated that the sensitivity of C. glabrata, C. krusei and C. tropicalis was decreased to fluconazole and amphotericin B during several past decades (35-40). The frequency of fluconazole resistance was 20.3% among several species of Candida with different sources (41). Our results indicated that none of the isolates were resistant to fluconazole and only one isolate of C. krusei showed resistance in a dose dependent manner after 48 hours of incubation. Betts et al., reviewed several literature reports for the effectiveness of caspofungin in neutropenic patients with invasive candidiasis and aspergillosis. They concluded that caspofungin might represent an effective and well-tolerated antifungal drug for such patients (9). Our results confirmed the previous results that have shown that caspofungin is an effective antifungal drug for candidiasis therapy. Our study shows that 54.7% of isolates were resistant to amphotericin B (< 8 µg/mL). Furthermore, the most resistant isolates were C. glabrata and C. krusei. Haddadi et al. in a similar study showed that resistance to amphotericin B was present in C. albicans, C. glabrata and C. krusei (22).
In summary, during this study we found a marked increase in the incidence of non-species Candida (48.2%) among neutropenic patients. In addition, only a few strains were without extracellular enzymes. Finally, the results suggest caspofungin as the first line treatment against Candida species among neutropenic patients as well as fluconazole.
Acknowledgements
References
-
1.
Rezaei N, Farhoudi A, Pourpak Z, Aghamohammadi A, Moin M, Gharagozlou M, et al. Neutropenia in patients with primary antibody deficiency disorders. Iran J Allergy Asthma Immunol. 2004;3(2):77-81. [PubMed ID: 17301396].
-
2.
Walsh TJ, Gamaletsou MN. Treatment of fungal disease in the setting of neutropenia. Hematology Am Soc Hematol Educ Program. 2013;2013:423-7. [PubMed ID: 24319214]. https://doi.org/10.1182/asheducation-2013.1.423.
-
3.
Link H, Bohme A, Cornely OA, Hoffken K, Kellner O, Kern WV, et al. Antimicrobial therapy of unexplained fever in neutropenic patients--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society). Ann Hematol. 2003;82 Suppl 2:S105-17. [PubMed ID: 13680173]. https://doi.org/10.1007/s00277-003-0764-4.
-
4.
van Burik JAH, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, et al. Micafungin versus Fluconazole for Prophylaxis against Invasive Fungal Infections during Neutropenia in Patients Undergoing Hematopoietic Stem Cell Transplantation. Clinical Infectious Dis. 2004;39(10):1407-16. https://doi.org/10.1086/422312.
-
5.
Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, et al. 1,3-D-Glucan Antigenemia for Early Diagnosis of Invasive Fungal Infections in Neutropenic Patients with Acute Leukemia. Clinical Infectious Dis. 2008;46(6):878-85. https://doi.org/10.1086/527382.
-
6.
Spiess B, Seifarth W, Hummel M, Frank O, Fabarius A, Zheng C, et al. DNA microarray-based detection and identification of fungal pathogens in clinical samples from neutropenic patients. J Clin Microbiol. 2007;45(11):3743-53. [PubMed ID: 17715373]. https://doi.org/10.1128/JCM.00942-07.
-
7.
Vardakas KZ, Michalopoulos A, Falagas ME. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol. 2005;131(1):22-8. [PubMed ID: 16173959]. https://doi.org/10.1111/j.1365-2141.2005.05727.x.
-
8.
Meurman J, Siikala E, Richardson M, Rautemaa R. Non-Candida albicans Candida yeasts of the oral cavity. Communicating current research and educational topics and trends in applied microbiology Microbiology book series Badajoz. Spain: Formatex; 2007. p. 719-31.
-
9.
Betts R, Glasmacher A, Maertens J, Maschmeyer G, Vazquez JA, Teppler H, et al. Efficacy of caspofungin against invasive Candida or invasive Aspergillus infections in neutropenic patients. Cancer. 2006;106(2):466-73. [PubMed ID: 16353208]. https://doi.org/10.1002/cncr.21615.
-
10.
Vazin A, Davarpanah MA, Ghalesoltani S. Antifungal agent utilization evaluation in hospitalized neutropenic cancer patients at a large teaching hospital. Drug Healthc Patient Saf. 2015;7:97-102. [PubMed ID: 26064070]. https://doi.org/10.2147/DHPS.S80762.
-
11.
Waltimo T, Kuusinen M, Jarvensivu A, Nyberg P, Vaananen A, Richardson M, et al. Examination on Candida spp. in refractory periapical granulomas. Int Endod J. 2003;36(9):643-7. [PubMed ID: 12950580].
-
12.
Merseguel KB, Nishikaku AS, Rodrigues AM, Padovan AC, e Ferreira RC, de Azevedo Melo AS, et al. Genetic diversity of medically important and emerging Candida species causing invasive infection. BMC Infect Dis. 2015;15:57. [PubMed ID: 25887032]. https://doi.org/10.1186/s12879-015-0793-3.
-
13.
Seifi Z, Zarei Mahmoudabadi A. Extracellular Esterase Secretion by Vaginal Isolates of Candida Albicans. Jentashapir J Health Res. 2014;5(4). https://doi.org/10.17795/jjhr-21881.
-
14.
Seifi Z, Zarei Mahmoudabadi A, Zarrin M. Extracellular enzymes and susceptibility to fluconazole in Candida strains isolated from patients with vaginitis and healthy individuals. Jundishapur J Microbiol. 2015;8(3):20162. [PubMed ID: 25861438]. https://doi.org/10.5812/jjm.20162.
-
15.
Zarrin M, Rajabi M, Zarei Mahmoudabadi A. Study of haemolytic, esterase activities and germ tube formation in Candida albicans from patients with vaginitis and urinary infections. Brunei Int Med J.
-
16.
Price MF, Wilkinson ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982;20(1):7-14. [PubMed ID: 7038928].
-
17.
Refrence method for broth dilution abtifungal suceptibility testing of yeasts; approved standard-third edition. M27-A3.
-
18.
Scherr N, Roltgen K, Witschel M, Pluschke G. Screening of Antifungal Azole Drugs and Agrochemicals with an Adapted alamarBlue-Based Assay Demonstrates Antibacterial Activity of Croconazole against Mycobacterium ulcerans. Antimicrobial Agents Chemo. 2012;56(12):6410-3. https://doi.org/10.1128/aac.01383-12.
-
19.
Repp KK, Menor SA, Pettit RK. Microplate Alamar blue assay for susceptibility testing ofCandida albicansbiofilms. Med Mycol. 2007;45(7):603-7. https://doi.org/10.1080/13693780701581458.
-
20.
Kauffman CA. Candiduria. Clinical Infectious Dis. 2005;41(Supplement 6):S371-S6. https://doi.org/10.1086/430918.
-
21.
Georgiadou SP, Tarrand J, Sipsas NV, Kontoyiannis DP. Candiduria in haematologic malignancy patients without a urinary catheter: nothing more than a frailty marker? Mycoses. 2013;56(3):311-4. [PubMed ID: 23170870]. https://doi.org/10.1111/myc.12024.
-
22.
Haddadi P, Zareifar S, Badiee P, Alborzi A, Mokhtari M, Zomorodian K, et al. Yeast colonization and drug susceptibility pattern in the pediatric patients with neutropenia. Jundishapur J Microbiol. 2014;7(9):11858. [PubMed ID: 25485060]. https://doi.org/10.5812/jjm.11858.
-
23.
Manzoni P, Farina D, Monetti C, Priolo C, Leonessa M, Giovannozzi C, et al. Early-onset neutropenia is a risk factor for Candida colonization in very low-birth-weight neonates. Diagn Microbiol Infect Dis. 2007;57(1):77-83. [PubMed ID: 17178299]. https://doi.org/10.1016/j.diagmicrobio.2006.10.018.
-
24.
Zollner-Schwetz I, Auner HW, Paulitsch A, Buzina W, Staber PB, Ofner-Kopeinig P, et al. Oral and intestinal Candida colonization in patients undergoing hematopoietic stem-cell transplantation. J Infect Dis. 2008;198(1):150-3. [PubMed ID: 18491972]. https://doi.org/10.1086/588827.
-
25.
Ashour SM, Kheiralla ZM, Maklad SS, Ameen MR, Zaki SS. Relationship between virulence factors of Candida species with candiduria and myeloperoxidase concentrations. Int J Curr Microbiol App Sci. 2015;4(1):108-23.
-
26.
Ghiasian SA, Aghamirian MR, Eshghi GR. Nosocomial Candiduria in Critically Ill Patients Admitted to Intensive Care Units in Qazvin, Iran. Avicenna J Clin Micro Inf. 2014;1(2). https://doi.org/10.17795/ajcmi-21622.
-
27.
Zarei Mahmoudabadi A, Zarrin M, Ghanatir F, Vazirianzadeh B. Candiduria in hospitalized patients in educational hospitals of Ahvaz. Iran J Microbiol.
-
28.
Brandt ME, Kauffman CA, Pappas PG, Iqbal N, Arthington-Skaggs BA, Lee-Yang W, et al. Fungemia Caused by Zygoascus hellenicus in an Allogeneic Stem Cell Transplant Recipient. J Clin Microb. 2004;42(7):3363-5. https://doi.org/10.1128/jcm.42.7.3363-3365.2004.
-
29.
Vervaeke S, Vandamme K, Boone E, De Laere E, Swinne D, Surmont I. A case ofCandida lambicafungemia misidentified asCandida kruseiin an intravenous drug abuser. Med Mycol. 2008;46(8):853-6. https://doi.org/10.1080/13693780802342552.
-
30.
Pakshir K, Zomorodian K, Karamitalab M, Jafari M, Taraz H, Ebrahimi H. Phospholipase, esterase and hemolytic activities of Candida spp. isolated from onychomycosis and oral lichen planus lesions. J Mycol Med. 2013;23(2):113-8. [PubMed ID: 23706304]. https://doi.org/10.1016/j.mycmed.2013.04.007.
-
31.
Negri M, Martins M, Henriques M, Svidzinski TI, Azeredo J, Oliveira R. Examination of potential virulence factors of Candida tropicalis clinical isolates from hospitalized patients. Mycopathologia. 2010;169(3):175-82. [PubMed ID: 19851885]. https://doi.org/10.1007/s11046-009-9246-0.
-
32.
Kumar VG, Latha R, Vedhagiri K, Sathiamoorthi T, Jayarani G, Sasikala R, et al. Phospholipase C, proteinase and hemolytic activities of Candida spp. isolated from pulmonary tuberculosis patients. J de Mycologie Médicale/Journal of Medical Mycology. 2009;19(1):3-10.
-
33.
Vidotto V, Yumi Koga-Ito C, Milano R, Fianchino B, Ponton J. Correlation between germ tube production, phospholipase activity and serotype distribution in Candida albicans. Rev Iberoam Micol. 1999;16(4):208-10. [PubMed ID: 18473549].
-
34.
Yu DT, Seger DL, Peterson JF, Kumar RN, Bates DW. Fluconazole for empiric antifungal therapy in cancer patients with fever and neutropenia. BMC Infect Dis. 2006;6:173. [PubMed ID: 17147804]. https://doi.org/10.1186/1471-2334-6-173.
-
35.
Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325(18):1274-7. [PubMed ID: 1669837]. https://doi.org/10.1056/NEJM199110313251803.
-
36.
Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33(5):673-88. [PubMed ID: 24249283]. https://doi.org/10.1007/s10096-013-2009-3.
-
37.
Zhang L, Xiao M, Watts MR, Wang H, Fan X, Kong F, et al. Development of fluconazole resistance in a series of Candida parapsilosis isolates from a persistent candidemia patient with prolonged antifungal therapy. BMC Infect Dis. 2015;15:340. [PubMed ID: 26282840]. https://doi.org/10.1186/s12879-015-1086-6.
-
38.
Nucci M, Colombo AL. Emergence of resistant Candida in neutropenic patients. Braz J Infect Dis. 2002;6(3):124-8. [PubMed ID: 12144748].
-
39.
Yenisehirli G, Bulut N, Yenisehirli A, Bulut Y. In Vitro Susceptibilities of Candida albicans Isolates to Antifungal Agents in Tokat, Turkey. Jundishapur J Microbiol. 2015;8(9):28057. [PubMed ID: 26495115]. https://doi.org/10.5812/jjm.28057.
-
40.
Shokohi T, Bandalizadeh Z, Hedayati M, Mayahi S. In vitro antifungal susceptibility of Candida species isolated from oropharyngeal lesions of patients with cancer to some antifungal agents. Jundishapur J Microbiol. 2011;4(2):S19-26.
-
41.
Rezazadeh E, Sabokbar A, Moazeni M, Rezai M, Badali H. Microdilution in vitro Antifungal Susceptibility Patterns of Candida Species, From Mild Cutaneous to Bloodstream Infections. Jundishapur J Microbiol. 2016;9(7). https://doi.org/10.5812/jjm.34151.
