Abstract
Background:
Fatty acid synthase is a multifunctional protein that catalyzes de novo synthesis of long-chain fatty acids. FASN expression is higher in HER2-positive cells, such as SKBR3 and MCF-7/HER2 cells, than in MCF-7 cells, which express lower HER2 levels. Curcumin, a yellow-colored hydrophobic polyphenol derived from the rhizome turmeric, significantly suppressed growth of human breast cancer cells. In this study, we assessed the effect of curcumin on expression and activity of fatty acid synthase in SK-BR-3 breast cancer cells.Objectives:
In this study, we decided to determine the effects of Curcumin on Fatty Acid Synthase expression and enzyme activity in breast cancer cell line SKBR3.Methods:
We assessed the cytotoxicity effect of curcumin in SK-BR-3 cells by MTT. Apoptosis was performed by flow cytometry. FAS activity was measured by a spectrophotometer at 340 nm of NADPH absorption. Fatty acid synthase gene expression was analyzed by real-time PCR.Results:
Curcumin could decrease cell viability and induce apoptosis in SK-BR-3 cells. Curcumin also reduces the enzyme activity and expression of fatty acid synthase.Conclusions:
It is possible that inhibitory effects of curcumin on FAS may induce apoptosis in SK-BR-3 breast cancer cells.Keywords
1. Background
Curcumin, a yellow-colored hydrophobic polyphenol derived from the rhizome turmeric, has been used in medicine for centuries in Asia. Curcumin (Figure 1) (diferuloylmethane) significantly inhibited the growth of human breast cancer cells by inducing apoptosis in a dose- and time-dependent manner (1). This compound also suppresses Proliferation and Migration of breast cancer cells (2). As a result, curcumin can be used as a potential new treatment option for several cancers.
Chemical Structure of Curcumin
Fatty acid synthase is a multifunctional protein encoded by the FASN gene that catalyzes de novo synthesis of long-chain fatty acids. Lipid metabolism is mostly mutated in human carcinoma (3, 4). While most normal adult tissues use dietary lipids present by the blood stream, numerous cancers show enhanced amount of de novo fatty acid (FA) biosynthesis (5). The increased synthesis of fatty acids in tumor cells is essential for integration into membrane phospholipids and lipid signaling in constantly dividing cells.
FASN overexpression reasons resistance to multiple anticancer drugs via preventing treatment- induced ceramide production, caspase 8 activation, and apoptosis (6). Also, overexpression of FASN is intimately associated to progression of breast carcinoma and might be regarded as an independent prophesier of poor prognosis for breast carcinoma (7).
FASN expression is higher in HER2-positive cells, such as SKBR3 and MCF-7/HER2 cells, than in MCF-7 cells, which express lower HER2 levels (8, 9).
FASN inhibition that impedes the lypogenic pathway and obstructs fatty acid synthesis causes apoptosis in cancer cells that overexpress FAS, without affecting non‐malignant cells (10, 11).
Thus, targeting FASN has come to the consideration of many in the drug industries due to the discovery that the inhibition of de novo synthesis of fatty acids can be used to treat cancer patients. Since previous studies have shown apoptotic effects of curcumin via inhibition of FAS in only MDA-MB-231 human breast cancer cells and human hepatocellular carcinoma cell line (HepG2), we decided to study the effect of curcumin on FAS expression and activity in SK-BR-3 for the first time (12, 13).
2. Objectives
In this study, we decided to determine effect of Curcumin on fatty acid synthase expression and enzyme activity in breast cancer cell line SKBR3.
3. Methods
3.1. Chemicals and Materials
The analytical standard curcumin with 98.0% purity by HPLC was obtained from Sigma-Aldrich (St. Louis, MO, USA) and used in this study. Human breast cancer SKBR3 cells were obtained from pastor institute (Tehran, Iran).
Cell culture medium, Dulbecco’s modified eagle medium (DMEM), phosphate buffered saline (PBS) and fetal bovine serum (FBS) were obtained from Gibco laboratories (Grand Island, NY). MTT reagent was purchased from Sigma (St. Louis, MO, USA). RNA extraction kit was purchased from GeneALL Biotechnology Co. LTD (Seoul, Republic of Korea). Annexin V-FITC Apoptosis Detection Kit was purchased from eBioscience (San Diego, USA). RevertAid First Strand cDNA Synthesis kit was purchased from thermo scientific (Hudson, NH, USA).SYBR Green Master Mix was purchased from Takara (Japan). Ac-CoA, Mal-CoA, NADPH, and DMSO (Dimethyl Sulfoxide) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Cell Culture
Human breast cancer SKBR3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) added with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a moistened atmosphere containing 5% CO2.
3.3. Cell Viability Assay
SKBR3 cells were plated in 96-well plates in triplicate with density of 5,000 cells per well. The medium was removed after 24 hours, and the cells were treated with curcumin (0, 2.5, 10, 15, 20, 25, 30 μM) for 24, 48, and 72 hours. MTT assays were used to evaluate cell survival. After the desired time, 10 mL of MTT (5.0 mg/mL) was added to each well and then the plate was incubated for 3 hours. The supernatants were aspirated carefully, and 100 mL of Dimethyl sulfoxide was added to each well to dissolve the crystals formazan made by the metabolism of living cells. The absorbance was measured by ELISA reader at 570 nm.
3.4. Apoptptic Analysis Using Flow Cytometric Assay
SK-BR-3 cells (200,000 cells/well) were seeded in triplicate in 6-well plate. After 24 hours, cells were treated with appropriate concentrations of curcumin (0, 5, 10, 15, 20, 25 μM) for 48 hours. Then the cells were collected and washed twice with phosphate buffered solution (PBS). To each microtube, 5 µM annexinV was added and placed in the dark after 5 minutes. 5 µM propidium iodide was added and they were again placed in the dark after 20 minutes. Incubation cells were analyzed by flow cytometry (Annexin V-FITC Apoptosis Detection Kit).
3.5. RNA Extraction and Real Time PCR
To extract total RNA, SK-BR-3 cells (600,000 cells/well) were seeded in 6-well plate. After 24 hours, the cells were treated with curcumin (0, 5, 10, 15, 20, μM) for 48 hours. Afterwards, RNA extraction was performed by kit (GeneALL). Total RNA was used to manufacture cDNA By RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). The expression of fatty acid synthase and ABL as a reference gene was examined by real-time PCR using the following primers: FAS forward, 5’-TGG TAG TGA GTG GGA AGG TGT AC-3’ and reverse, 5’-CAG ACG CAG CTC CTT GTA AAC TT-3’ and ABL forward, 5’-CTT CTT GGT GCG TGA GAG TGA G-3’ and reverse, 5’-GAC GTA GAG CTT GCC ATC AGA AG-3’.
3.6. FAS Activity Assay
After 48 hours of exposure to curcumin, cells were collected by treatment with trypsin-EDTA solution, pelleted by centrifugation and were washed twice with cold phosphate buffered solution (PBS). Cells were centrifuged at 13,000 rpm for 30 minutes at 4°C providing particle-free supernatants. Enzyme activity was measured based on changes in absorbance of NADPH at 340 nm. 50 μL Particle-free supernatant, 25 mM potassium Phosphate buffer, 0.25 mM EDTA, 0.25 mM dithiothreitol (DTT), 30 μM acetyl-CoA, and 350 μM NADPH (pH 7.0) in a total volume of 500 μL were Observed at 340 nm for 100 s to measure NADPH oxidation. After the addition of 100 μM of malonyl-CoA, the reaction was tested for an additional 1 minutes to designate FAS-dependent oxidation of NADPH (14). The change in concentration of NADPH during oxidation was measured using the following formula: ∆C = ∆A/E
Where ∆C is change in the concentration of NADPH, ∆A is the change in absorbance, and E is extinction coefficient of NADPH (E 340 nm = 6.22 mM-1cm-1). FAS activity was reported as nmol NADPH oxidized/min/mg protein (6).
3.7. Statistical Analysis
Data analysis was implemented using SPSS v.18.One-way ANOVA (with Dunnett’s multiple range test for post hoc comparison) was implemented to compare groups at diverse concentrations of curcumin. P < 0.001 was considered statistically significant.
4. Results
4.1. Effect of Curcumin on Cell Viability of SK-BR-3 Cells
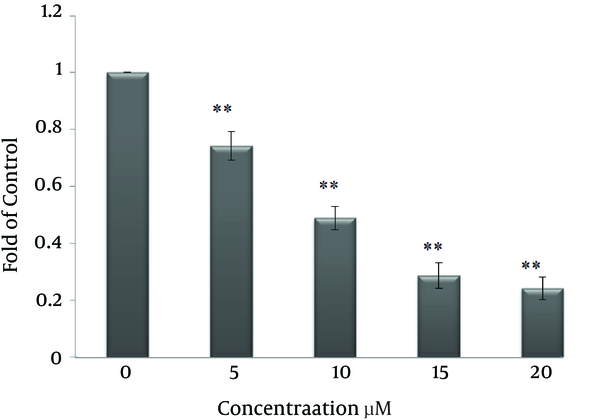
To assess the cytotoxicity of curcumin, breast cancer cells were treated with several concentrations of curcumin (0, 2.5, 5, 10, 15, 20, 25, 30 μM) for 24, 48 and 72 hours. Then cell viability was determined by a MTT assay. As shown in Figure 2 cytotoxic effect of curcumin on the growth of SK-BR-3 cells is dependent on time and concentration. The IC50 values of curcumin in SK-BR-3 cells were 15 μM in 48 hours and 10 μM in 72 hours.
Effect of curcumin on the viability of SK-BR-3 cells

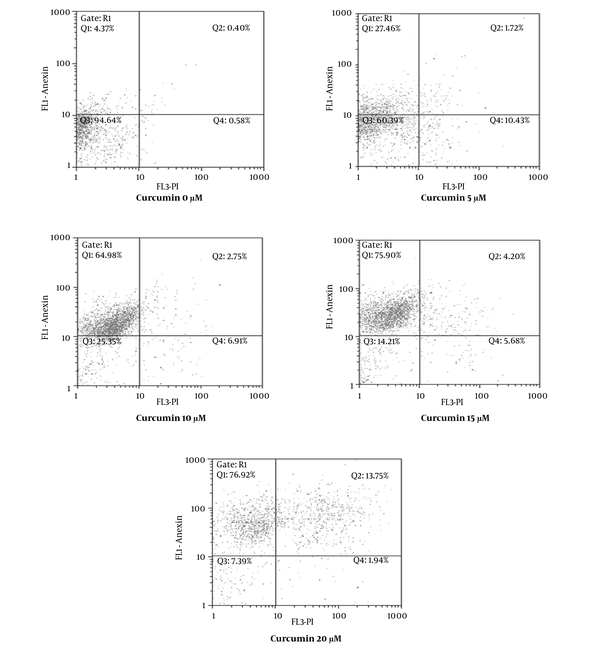
4.2. Curcumin Induced Apoptosis in SK-BR-3 Cells
In order to verify whether the growth inhibitory effect of curcumin was connected with apoptosis, SK-BR-3 cells were treated with various concentrations of curcumin (0, 5, 10, 15, 20 μM) for 48 hours and afterwards they were examined by flow cytometry. As shown in Figure 3 cells were designated intact cells (Annexin V-, PI-), early apoptotic (Annexin V+, PI-), damaged (Annexin V–, PI+) and late apoptotic (Annexin V+, PI+). The early apoptotic rate in SK-BR-3 cells treated with curcumin was 4.37% at 0 μM, 27.46% at 5 μM, 64.98% at 10 μM, 75.90% at 15 μM and 76.92% at 20 μM. According to the results, curcumin induced apoptosis in SK-BR-3 cells in a dose-dependent manner.
Curcumin Induced Apoptosis in SK-BR-3 Cells
4.3. Curcumin Suppressed FAS Expression and Inhibited Fatty Acid Synthase Activity in SK-BR-3 Cells
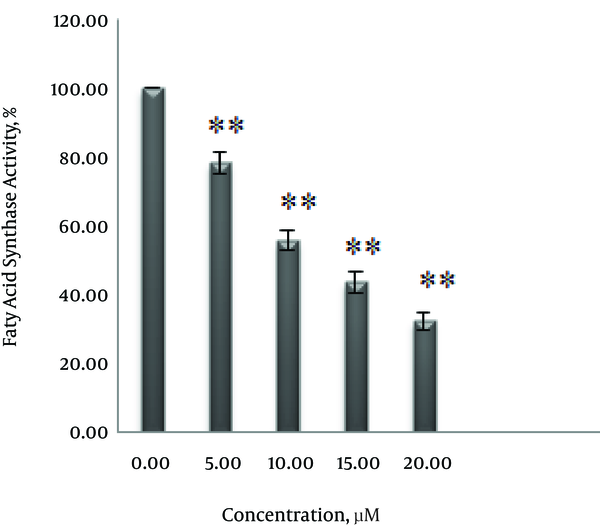
To explore the effect of curcumin on FAS expression and fatty acid activity, SK-BR-3 cells were treated with 0, 5, 10, 15 and 20 μM curcumin for 48 hours. As shown in Figure 4 curcumin decreased expression of FAS in a dose-dependent manner. Also, the inhibitory effect of curcumin on fatty acid synthase activity, according to the results in Figure 5 depends on concentration. Enzyme activity decreased by 21.7% at 5 μM, 41.4% at 10 μM, 56.4% at 15 μM and 67.7 % at 20 μM.
Effect of Curcumin on the Expression Levels of FAS in SK-BR-3 Cells
Effect of Curcumin on FAS Activity in SK-BR-3 Cells
5. Discussion
Despite various treatments, breast carcinoma is the most common cancer and a leading cause of mortality among females worldwide (15, 16). Fatty acid synthase is a multifunction protein that catalyzes de novo synthesis of long-chain fatty acids. Fatty acids play an important role in critical functions including reproduction, energetic metabolism through β‐oxidation, signal transduction, DNA synthesis, intracellular trafficking, protein acylation, cell polarization, cell cycle progression, and cell migration (17-19). Fatty acid synthase expression in tumor cells and normal cells were different; therefore, fatty acid synthase enzyme was used as a therapeutic target in the improvement of anticancer drugs (20-23). FAS inhibition reduced the production of lipids in the cancer cells and induced apoptosis in overexpressed FAS cancer cells, without affecting non-malignant cells (10). Overexpression of FAS is common in a wide range of cancers, especially breast cancers (24, 25). Increased fatty acids synthesis in tumor cells is essential for integration of phospholipids into the membrane and lipid signaling in constant cell divisions (17, 18, 21, 26). Curcumin, a yellow-colored hydrophobic polyphenol derived from the rhizome turmeric, has anti-cancer effects in vitro and in vivo on several types of cancers (27).
As shown in Figure 2 in our study, the IC50 values of curcumin in SK-BR-3 cells were 15 μM in 48 hours and 10 μM in 72 hours which indicated that curcumin reduced cell proliferation of cancer cells in a dose- and time-dependent manner.
Our results are in agreement with many studies (1, 28-31) for instance in the study conducted by Yan et al. with IC50 of 20 μM was obtained at 48 hours of treatment by curcumin in SK-BR-3 cells (29). Furthermore, consistent with the research by Plalange et al. curcumin with concentrations less than 30 μM had no significant effect on viability of cancer cells in 24 hours (31).
However, our results were in contrast with the results reported by Thomas who showed that the IC50 of curcumin was 3.0 ± 0.5 μM in 24 hours in SK-BR-3 cells.
The inhibitory effect of curcumin on the fatty acid synthase enzyme were previously observed in certain cell lines (12, 13). However, in this study for the first time, the inhibitory effect of curcumin on the expression and activity of fatty acid synthase in SK-BR-3 cells were examined. Following treatment with curcumin, expression and activity of fatty acid synthase were reduced in a dose-dependent manner (Figures 4 and 5). 5 μM, 10 μM, 15 μM and 20 μM concentrations of curcumin reduced enzyme activity by 21%, 41%, 56.4% and 67.7%, respectively.
In this study, curcumin with a concentration of 5 μM reduced enzyme activity by 21% in SK-BR-3 cells. While in human breast cancer MDA-MB-231 cells, enzyme activity was reduced by 6.8% at 5 µM concentration of curcumin (12). As a result, curcumin in breast cancer cell line SK-BR-3 compared with MDA-MB-231 cells is more capable of inhibiting the activity of fatty acid synthase.
Several studies have shown that inhibition of FAS may induce cell apoptosis via reducing fatty acids (12, 14, 32). The results obtained by the Annexin V/PI dual staining in our study suggests that curcumin induced apoptosis dose-dependently (Figure 3). 20 µM of curcumin concentration caused apoptosis in SK-BR-3 cells after 48 hours by 76.92%, while in contrast with our results in the study conducted by Guang Yan et al. curcumin, with concentration of 20 µM for 48 hours, had no significant effect on apoptosis of SK-BR-3 cells (29).
These results support the significant role of FAS in SK-BR-3 cells and propose that FAS is the aim that curcumin act on.
5.1. Conclusions
In summary, curcumin could induce apoptosis in SK-BR-3 cells. Curcumin treatment down-regulated the expression of FASN and inhibited fatty acid synthase activity in SK-BR-3 cells.
Therefore, it is possible that inhibitory effect of curcumin on FAS may induce apoptosis in human breast cancer SK-BR-3 cells.
Acknowledgements
References
-
1.
Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN, Wang HB, et al. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014;7(6):2818-24. [PubMed ID: 25031701].
-
2.
Guan F, Ding Y, Zhang Y, Zhou Y, Li M, Wang C. Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS One. 2016;11(1). e0146553. [PubMed ID: 26752181]. https://doi.org/10.1371/journal.pone.0146553.
-
3.
Currie E, Schulze A, Zechner R, Walther TC, Farese RJ. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18(2):153-61. [PubMed ID: 23791484]. https://doi.org/10.1016/j.cmet.2013.05.017.
-
4.
Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610-23. [PubMed ID: 22621751]. https://doi.org/10.1111/j.1742-4658.2012.08644.x.
-
5.
Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91(14):6379-83. [PubMed ID: 8022791].
-
6.
Liu H, Wu X, Dong Z, Luo Z, Zhao Z, Xu Y, et al. Fatty acid synthase causes drug resistance by inhibiting TNF-alpha and ceramide production. J Lipid Res. 2013;54(3):776-85. [PubMed ID: 23319743]. https://doi.org/10.1194/jlr.M033811.
-
7.
Hong Y, Qiao JL, Cui LF, Li TX, Zhang JN, Yang SM, et al. Increased expression of FAS is a prognostic marker for patients with breast cancer. Int J Clin Exp Med. 2016;9(1).
-
8.
Zhou L, Zhao YH, Wang XD, Jiang SF, Li H. [Expression of fatty acid synthase and adipocyte fatty acid-binding protein and the relationship with the clinicopathological characteristics in human infiltrating ductal breast cancer]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46(2):228-33. [PubMed ID: 25924435].
-
9.
Yan C, Wei H, Minjuan Z, Yan X, Jingyue Y, Wenchao L, et al. The mTOR inhibitor rapamycin synergizes with a fatty acid synthase inhibitor to induce cytotoxicity in ER/HER2-positive breast cancer cells. PLoS One. 2014;9(5). e97697. [PubMed ID: 24866893]. https://doi.org/10.1371/journal.pone.0097697.
-
10.
Relat J, Blancafort A, Oliveras G, Cufi S, Haro D, Marrero PF, et al. Different fatty acid metabolism effects of (-)-epigallocatechin-3-gallate and C75 in adenocarcinoma lung cancer. BMC Cancer. 2012;12:280. [PubMed ID: 22769244]. https://doi.org/10.1186/1471-2407-12-280.
-
11.
Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;38(6):765-72. [PubMed ID: 25448084]. https://doi.org/10.1016/j.canep.2014.09.006.
-
12.
Fan H, Liang Y, Jiang B, Li X, Xun H, Sun J, et al. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep. 2016;35(5):2651-6. [PubMed ID: 26985864]. https://doi.org/10.3892/or.2016.4682.
-
13.
Fan H, Tian W, Ma X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target Oncol. 2014;9(3):279-86. [PubMed ID: 23821378]. https://doi.org/10.1007/s11523-013-0286-5.
-
14.
Li P, Tian W, Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer. 2014;13:138. [PubMed ID: 24894151]. https://doi.org/10.1186/1476-4598-13-138.
-
15.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [PubMed ID: 21296855]. https://doi.org/10.3322/caac.20107.
-
16.
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-41. [PubMed ID: 22700443]. https://doi.org/10.3322/caac.21149.
-
17.
Porta R, Blancafort A, Casoliva G, Casas M, Dorca J, Buxo M, et al. Fatty acid synthase expression is strongly related to menopause in early-stage breast cancer patients. Menopause. 2014;21(2):188-91. [PubMed ID: 23982110]. https://doi.org/10.1097/GME.0b013e31829d17dc.
-
18.
Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130(2):387-98. [PubMed ID: 21188630]. https://doi.org/10.1007/s10549-010-1300-6.
-
19.
Zhou L, Jiang S, Fu Q, Smith K, Tu K, Li H, et al. FASN, ErbB2-mediated glycolysis is required for breast cancer cell migration. Oncol Rep. 2016;35(5):2715-22. [PubMed ID: 26936618]. https://doi.org/10.3892/or.2016.4627.
-
20.
Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202-8. [PubMed ID: 10705076].
-
21.
Menendez JA, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in her-2/neu-overexpressing breast cancer. Drug News Perspect. 2005;18(6):375-85. [PubMed ID: 16247515]. https://doi.org/10.1358/dnp.2005.18.6.927929.
-
22.
Puig T, Vazquez-Martin A, Relat J, Petriz J, Menendez JA, Porta R, et al. Fatty acid metabolism in breast cancer cells: differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res Treat. 2008;109(3):471-9. [PubMed ID: 17902053]. https://doi.org/10.1007/s10549-007-9678-5.
-
23.
Richardson RD, Smith JW. Novel antagonists of the thioesterase domain of human fatty acid synthase. Mol Cancer Ther. 2007;6(7):2120-6. [PubMed ID: 17620441]. https://doi.org/10.1158/1535-7163.MCT-07-0187.
-
24.
Puig T, Aguilar H, Cufi S, Oliveras G, Turrado C, Ortega-Gutierrez S, et al. A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res. 2011;13(6):R131. [PubMed ID: 22177475]. https://doi.org/10.1186/bcr3077.
-
25.
Cheng CS, Wang Z, Chen J. Targeting FASN in Breast Cancer and the Discovery of Promising Inhibitors from Natural Products Derived from Traditional Chinese Medicine. Evid Based Complement Alternat Med. 2014;2014:232946. [PubMed ID: 24778702]. https://doi.org/10.1155/2014/232946.
-
26.
Menendez JA, Mehmi I, Verma VA, Teng PK, Lupu R. Pharmacological inhibition of fatty acid synthase (FAS): a novel therapeutic approach for breast cancer chemoprevention through its ability to suppress Her-2/neu (erbB-2) oncogene-induced malignant transformation. Mol Carcinog. 2004;41(3):164-78. [PubMed ID: 15390078]. https://doi.org/10.1002/mc.20054.
-
27.
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267(1):133-64. [PubMed ID: 18462866]. https://doi.org/10.1016/j.canlet.2008.03.025.
-
28.
Lai HW, Chien SY, Kuo SJ, Tseng LM, Lin HY, Chi CW, et al. The Potential Utility of Curcumin in the Treatment of HER-2-Overexpressed Breast Cancer: An In Vitro and In Vivo Comparison Study with Herceptin. Evid Based Complement Alternat Med. 2012;2012:486568. [PubMed ID: 21876713]. https://doi.org/10.1155/2012/486568.
-
29.
Yan G, Graham K, Lanza-Jacoby S. Curcumin enhances the anticancer effects of trichostatin a in breast cancer cells. Mol Carcinog. 2013;52(5):404-11. [PubMed ID: 22290509]. https://doi.org/10.1002/mc.21875.
-
30.
Shajan AS. The Effects Of Curcumin On The Expression Of P53 And Bcl-2 Oncoproteins On The Human Breast Carcinoma Cell Line. Available from: http://csuepress.columbusstate.edu/cgi/viewcontent.cgi?article=1180&context=theses_dissertations.
-
31.
Palange AL, Di Mascolo D, Singh J, De Franceschi MS, Carallo C, Gnasso A, et al. Modulating the vascular behavior of metastatic breast cancer cells by curcumin treatment. Front Oncol. 2012;2:161. [PubMed ID: 23162792]. https://doi.org/10.3389/fonc.2012.00161.
-
32.
Wysham WZ, Roque DR, Han J, Zhang L, Guo H, Gehrig PA, et al. Effects of Fatty Acid Synthase Inhibition by Orlistat on Proliferation of Endometrial Cancer Cell Lines. Target Oncol. 2016;11(6):763-9. [PubMed ID: 27188391]. https://doi.org/10.1007/s11523-016-0442-9.