Abstract
Background:
Tumor growth, invasion, and metastasis are angiogenesis dependent; so, using anti-angiogenic therapies can block these procedures. Vascular endothelial growth factor (VEGF) is a strong proangiogenic factor that is expressed by many cancer cells like bladder cancer.Objectives:
The aim of this study was to determine the prevalence of the VEGFR in transitional cell carcinoma (TCC) of bladder and its relationship with other prognostic factors.Methods:
This cross sectional study was carried out on 61 patients with TCC of bladder after radical cystectomy (RC). These patients were referred to the Urology Ward in Labbafineazhad Hospital in Tehran, Iran. The relationship of VEGF and the prognostic factors was determined, using the Fisher's Exact and Chi-square for data analyzing by SPSS software version 17.Results:
Among 61 patients with TCC of bladder, only 8 patients (13.1%) were VEGFR positive, and there was no significant relation between frequency of VEGFR expression and the other factors like age, gender, stage, lymph node involvement, lymphovascular, or perineural space invasion.Conclusions:
According to this study, the most patients with TCC of bladder did not express VEGFR; also, none of the prognostic factors showed any relation with VEGFR expression.Keywords
Vascular Endothelial Growth Factor Receptor Prognostic Factors Transitional Cell Carcinoma
1. Background
The most prevalent malignancy involving the urinary system is bladder cancer and it is one of the 10 most common tumors all over the world (1). The incidence of this cancer has been increasing during the last decades slowly and unfortunately this rising is parallel with the increasing of the age. In this sense, 6th and 7th decades of the life are the peaks for occurring (2). The most common histologic type of this malignancy is urothelial carcinoma or transitional cell carcinoma (TCC), especially in the United States and Western Europe; however, in the countries of the Middle East, non urothelial histologies such as squamous cell carcinomas (SCC) are more common. Some ethnic and racial diversities are present in this malignancy. Also, race and gender can affect the stage of the disease or prognosis. Invasive urothelial cancer (UC) is a high grade cancer, according to the classification of TCC into low grade and high grade forms. This is important because when these malignancies are diagnosed in the early onset, most of them are noninvasive, but high grade muscle invasive disease is a result of a delayed diagnosis. The high grade muscle invasive TCC can simply progress and eventually become lethal. According to James et al. (3), bladder cancer is a main cause of cancer mortality. The prognosis of advanced TCC is poor; so, the need for competent therapies are important. There are many molecular alterations in bladder cancer that engage in cellular processing like proliferation, differentiation, motility, angiogenesis, invasion, metastasis, and apoptosis according to Parvin et al. (4). To improve in the management of bladder cancer, we need to precisely detect some prognostic and/or predictive biomarkers. The inhibition of epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), or its receptors are two major targets that had received more attention. In many types of other malignancies like cervix, lung, colorectal, and gastric cancers, the prognostic value of VEGF expression had been mentioned (5, 6). Ramakrishnan et al. argued that up-regulation of VEGF is an important event after hypoxic conditions (7). The result of this action is producing new vessels. Capillary permeability is another effect of VEGF expression; thus, malignant cells migrate and infiltrating foci will be constructed (8, 9). As it was said, VEGF has an important role for progression, metastasis (10), and angiogenesis; hence, expression of VEGF is associated with dismal prognosis in many cancers. Increasing in oxygen delivery, chemotherapeutic agents’ transportation and normalization of tumor vessels are the results of VEGF blockage (10). Also, it is said that VEGF is a survival factor, as tumor cells and endothelial cells are protected by this factor against acidosis or hypoxia (11, 12). By the way, VEGF protects tumor cells against apoptosis induced by chemotherapy (13).
The aim of this article was to determine the prevalence of the VEGFR in transitional cell carcinoma of bladder and its relationship with other prognostic factors.
2. Methods
This cross sectional study has been conducted on 61 patients, who had transitional cell carcinoma of bladder by their pathology reports. Inclusion criteria were all adult patients (females and males) with documented TCC according to their biopsy or surgical reports, who had been referred to the Labbafinezhad Hospital in Tehran, Iran between 2007 and 2010. Exclusion criteria were tumors of the bladder other than TCC such as squamous cell carcinoma, adenocarcinoma, adenosquamous cell carcinoma, and melanoma, or any benign conditions as well as any unclear data in their records.
The sample size calculation was 61 cases according to this formula:
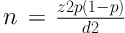
Where n = sample size, z = the appropriate value from the normal distribution for 95% confidence (1.96), p = he anticipated prevalence, and d = confidence interval.
The sampling method was done based on a nonprobability type, as convenience sampling.
The records of the patients, who had been referred to the urology ward from 2007 to 2010, were carefully screened by two of our co-workers according to the inclusion criteria and after data collection, TCC blocks’ from radical cystectomies (RC) were selected for further investigation and determination by two separate and specified pathologists. The mouse monoclonal anti-VEGF (Dako, Denmark) were used for staining and after using immunohistochemistry (IHC) techniques; the positive results were at least 10% staining of the blocks.
As mentioned for data collection, 61 patients after admittance had TCC of bladder according to their surgeries (RC). The samples have been determined by IHC for VEGF expression, and the other prognostic factors such as gender, age, grading, lymphovascular or perineural invasion, and their staging according to tumor -node- metastasis (TNM) staging were studied for the detection of any relationship with this expression. Based on grading, low grade tumors were G1 and G2, and high grade tumors were G3.
Written informed consent was obtained from all patients after the approval of the study by the Ethical Committee of Shahid Beheshti University of Medical Sciences under code ir.sbmu.unrc.87411.11.
The relationship of VEGF and the prognostic factors was determined by the Fisher's Exact and Chi-square for data analyzing. The SPSS software version was 17, and the P value for statistical significance was recognized as P < 0.05.
3. Results
Records of 61 patients were studied (57 men and 4 women). The mean age of both genders were 64 ± 19 years old. The mean age of men and women was 64.26 and 63.25, respectively. Totally, 8 patients (13.1%) were VEGFR positive. The mean age of VEGFR positive patients and VEGFR negative patients was 62.9 ± 10.6 and 64.4 ± 10.5, respectively. There was no significant relation between age and expression of VEGFR (P = 0.9). In VEGFR positive group, 7 patients (87.5%) were male and 1 patient (12.5%) was female. There was no significant relationship between the expression of VEGF and gender (P = 0.4). Based on the depth of invasion in positive receptors, 3 patients (37.5%) were T3 (invasion to perivesical tissue) and 2 patients (25%) were T4 (invasion to any of the following organs: Prostatic stroma, uterus, vagina, pelvis, or abdominal wall), but 11 (20.8%) and 4 patients (7.6%) in VEGFR negative group were T3 and T4, respectively; so, there was no significant association between expression of VEGFR and depth of invasion (P = 0.1). Among positive results for VEGFR, in 5 patients (62.5%), regional lymph nodes could not be assessed (Nx). Also, in the rest of them, 3 patients (37.5%) did not have any regional lymph nodes metastasis (N0). For negative receptors, 40 patients (75.5%) were Nx, 10 cases (18.7%) were N0, 2 cases (3.8%) and 1 patient (1.9%) were N1 and N2, respectively. Again, there was not any intercommunication between VEGFR expression and regional lymph nodes metastasis (P = 0.6). None of the positive receptor patients had G1 grading, but 4 patients (50%) were G2 and the rest of them were G3, and for the negative receptor patients, the results were as follow: 6 patients (11.3%), 10 patients (18.7%), and 37 cases (69.8%) were G1, G2 and G3, respectively. In this sense, no significant relation was detected between grading of the tumor and positive VEGFR expression (P = 0.1). Totally, 23 patients had lymphovascular invasion. In positive VEGFR group, 2 patients (25%) had lymphovascular invasion and in another group, 21 patients (39.6%) had this type of invasion. As we saw, no significant relationship was detected between lymphovascular invasion and expression of this receptor (P = 0.4). Entirely, perineural invasion was detected in 13 patients; 3 patients (37.5%) in VEGFR expression and 10 patients (18.7%) in non VEGFR expression had this type of invasion, but again no significant association was seen (P = 0.2) (Table 1).
VEGFR Expression and Other Prognostic Factors in 61 Patients with TCC
| Prognostic Factor | VEGFR Positivea | VEGFR Negativea | P Value |
|---|---|---|---|
| Age, y | 8 (100) | 53 (100) | 0.88 |
| ≤ 60 | 4 (50) | 24 (45.3) | |
| > 60 | 4 (50) | 29 (54.7) | |
| Gender | 8 (100) | 53 (100) | 0.46 |
| Female | 1 (12.5) | 3 (5.7) | |
| Male | 7 (87.5) | 50 (94.3) | |
| Invasion | 8 (100) | 53 (100) | 0.16 |
| Lamina propria | 3 (37.5) | 16 (30.2) | |
| Superficial muscle | 0 (0) | 12 (22.7) | |
| Deep muscle | 0 (0) | 10 (18.7) | |
| Perivesical tissue | 3 (34.5) | 11 (20.8) | |
| Other organs | 2 (25) | 4 (7.6) | |
| Primary Tumor | 8 (100) | 53 (100) | 0.26 |
| T1 | 2 (25) | 18 (34) | |
| T2 | 1 (12.5) | 20 (37.7) | |
| T3 | 3 (37.5) | 9 (17) | |
| T4 | 2 (25) | 6 (11.3) | |
| Regional lymph nodes | 8 (100) | 53 (100) | 0.62 |
| N0 | 3 (37.5) | 10 (18.7) | |
| N1 | 0 (0) | 2 (3.8) | |
| N2 | 0 (0) | 1 (1.9) | |
| Nx | 5 (62.5) | 40 (75.5) | |
| Distant metastasis | 8 (100) | 53 (100) | 0.85 |
| M0 | 0 (0) | 1 (1.9) | |
| M1 | 0 (0) | 1 (1.9) | |
| Mx | 8 (100) | 51 (96.2) | |
| Grade | 8 (100) | 53 (100) | 0.12 |
| G1 | 0 (0) | 6 (11.3) | |
| G2 | 4 (50) | 10 (18.7) | |
| G3 | 4 (50) | 37 (69.8) | |
| LVI | 8 (100) | 53 (100) | 0.42 |
| Positive | 2 (25) | 21 (39.6) | |
| Negative | 6 (75) | 32 (60.4) | |
| PNI | 8 (100) | 53 (100) | 0.23 |
| Positive | 3 (37.5) | 10 (18.7) | |
| Negative | 5 (62.5) | 43 (81.2) |
4. Discussion
The findings of this study revealed that expression of the VEGFR was seen only in 8 patients (13.1%) with TCC, and there was no significant relationship between this expression and the other prognostic factors such as age, gender, invasion of the tumor into the layers, involving the lymph nodes, grading and lymphovascular or perineural invasion. Vascular permeability factor (VPF) is another term of VEGF expressed by most cancer cell types. It is said that VPF increases microvascular permeability about 50000 times versus histamine (14). According to Bates, this factor has an important role for neovascularization, invasion, and angiogenesis (15) and it is a critical part of tumor metastasis. Over expression of VEGF in tissue, urine, and serum of patients with TCC has been confirmed and disease recurrence or progression related with this issue (16). Findings of a study conducted by Kopparapu et al. revealed that the expression of VEGF and its receptors are associated with recurrence or stage (17). As they said, VEGF and VEGFR1 mRNA levels were higher in TCC of bladder than normal mucosa and surprising in non-muscle invasive bladder cancer, the levels of VEGF, and its receptor, VEGFR1, were higher than muscle invasive TCC, but expression of VEGFR2 was significantly higher in muscle invasive TCC related to non-muscle invasive bladder cancer and expression of all of them was related with poorer recurrence free survival without any statistical significance. Xia et al. showed that increasing in VEGFR2 expression correlates with the factors that induced progression of TCC of bladder such as invasion to the muscle layer or disease stage (18). Finding of the present study did not show any significant association between this expression and the depth of invasion; as we saw in positive group expression, the rate of muscle invasion was zero. As illustrated by Ahmed Nabeel, there was a significant effect of tumor size (T) on the level of this expression. By the way, the serum level of VEGF increased with the stage progression, so this overexpression was associated with the advance stage (19). Not only serum level, but also urinary level of the VEGF was higher in patients with bladder cancer than the healthy group; also, the recurrence rates of TCC are higher parallel with the increased level of urinary VEGF secretion (20). Again, in another study conducted by Rahmani et al. (21), expression of VEGF in the normal epithelial cells of bladder was much lower than the tumor cells; hence, a significant difference were seen between them and this was not seen in our investigation. Many studies had confirmed the VEGF expression associated with grade and stage of the bladder malignancies (16, 22). This expression significantly increased when tumor grade progressed (21). This finding is in contrast with our results, in which no correlation was detected between grade of the tumor and VEGF expression. An average expression of VEGF gene in non-muscle invasive TCC of bladder was 4 times higher than in muscle invasive tumors and 10 times higher than normal urothelial epithelium, as illustrated in an article (16). However, as concluded by Sato et al. (23), invasive form of TCC expressed much more VEGF gene compared with the superficial TCC. According to the results pf the current research in positive expression group, invasion to the muscular layer was zero, but for the superficial TCC, that was much higher. Thus, there is controversy between these studies. Also, in an article (23), researchers showed that the expression of the VEGF gene correlated with the stage of the tumor, but this association was not seen with the grade. In another manuscript (19) that was examined on serum levels of VEGF in patients' bladder cancer, the statistical analysis showed important differences between the level of VEGF in two groups, patients versus control group, but there was not seen any correlation between age or gender and VEGF expression. Despite our results, the expression of VEGF was associated on the basis of age or gender, as indicated by Rahmani et al. (21). They concluded that there was no significant difference of this expression among two age groups under 50 years and above it only in females, but in males, the expression of VEGF in the group with age of 50 ≤ was higher, compared with the other group (21). Relationship between the expression of VEGF or its receptor and lymphovascular or perneural space invasion in TCC of bladder has not been studied anymore.
This study has some limitations. Firstly, the number of patients was low and this can explain why we could not find any relationship between VEGF expression with the other prognostic factors. Secondly, some data like the exact number of lymph node involvement or distant metastases in the records were incomplete; thus, further researches in this field need to be conducted with more patient samples to determine this association. Also, record a complete and comprehensive file for each patient is necessary.
4.1. Conclusions
The purpose of this study was to determine the prevalence of the VEGFR in transitional cell carcinoma of bladder and its relationship with other prognostic factors. The main conclusion to be drawn from this study was that only 13.1% of all patients with TCC of bladder expressed VEGFR. The following conclusions were the absence of any relation between this expression with the other prognostic factors such as age, gender, staging, and invasion to vascular or perineural space.
Acknowledgements
References
-
1.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. https://doi.org/10.3322/caac.21262.
-
2.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30. [PubMed ID: 23335087]. https://doi.org/10.3322/caac.21166.
-
3.
James N, Bryan RT, Viney R, Patel P, Hussain SA. Bladder cancer. In: Perez CA, Brady LW, Halperin EC, Wazer DE, editors. Perez and Brady's principles and practice of radiation oncology. 6th ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2013. p. 1259-79.
-
4.
Parvin M, Sabet-Rasekh P, Hajian P, Mohammadi Torbati P, Sabet-Rasekh P, Mirzaei H. Evaluating the prevalence of the epidermal growth factor receptor in transitional cell carcinoma of bladder and its relationship with other prognostic factors. Iran J Cancer Prev. 2016;9(1). e4022. [PubMed ID: 27366313]. [PubMed Central ID: PMC4922206]. https://doi.org/10.17795/ijcp-4022.
-
5.
Choi CH, Song SY, Choi JJ, Park YA, Kang H, Kim TJ, et al. Prognostic significance of VEGF expression in patients with bulky cervical carcinoma undergoing neoadjuvant chemotherapy. BMC Cancer. 2008;8:295. [PubMed ID: 18847499]. [PubMed Central ID: PMC2572070]. https://doi.org/10.1186/1471-2407-8-295.
-
6.
Gkiozos I, Tsagouli S, Charpidou A, Grapsa D, Kainis E, Gratziou C, et al. Levels of vascular endothelial growth factor in serum and pleural fluid are independent predictors of survival in advanced non-small cell lung cancer: Results of a prospective study. Anticancer Res. 2015;35(2):1129-37. [PubMed ID: 25667503].
-
7.
Ramakrishnan S, Anand V, Roy S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol. 2014;9(2):142-60. [PubMed ID: 24610033]. [PubMed Central ID: PMC4048289]. https://doi.org/10.1007/s11481-014-9531-7.
-
8.
Psatha A, Makris D, Kerenidi T, Daniil Z, Kiropoulos T, Gourgoulianis K. A potential role for VEGF in the diagnostic approach of pleural effusions. J Thorac Dis. 2016;8(7):1681-7. [PubMed ID: 27499957]. [PubMed Central ID: PMC4958865]. https://doi.org/10.21037/jtd.2016.05.73.
-
9.
Doi K, Noiri E, Fujita T. Role of vascular endothelial growth factor in kidney disease. Curr Vasc Pharmacol. 2010;8(1):122-8. [PubMed ID: 19485913]. https://doi.org/10.2174/157016110790226606.
-
10.
Monk BJ, Minion LE, Coleman RL. Anti-angiogenic agents in ovarian cancer: Past, present, and future. Ann Oncol. 2016;27 Suppl 1:i33-9. [PubMed ID: 27141068]. https://doi.org/10.1093/annonc/mdw093.
-
11.
Pronto-Laborinho AC, Pinto S, de Carvalho M. Roles of vascular endothelial growth factor in amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:947513. [PubMed ID: 24987705]. [PubMed Central ID: PMC4022172]. https://doi.org/10.1155/2014/947513.
-
12.
Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, Del Bufalo D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS One. 2010;5(7). e11772. [PubMed ID: 20668552]. [PubMed Central ID: PMC2910721]. https://doi.org/10.1371/journal.pone.0011772.
-
13.
Barr MP, Gray SG, Gately K, Hams E, Fallon PG, Davies AM, et al. Vascular endothelial growth factor is an autocrine growth factor, signaling through neuropilin-1 in non-small cell lung cancer. Mol Cancer. 2015;14:45. [PubMed ID: 25889301]. [PubMed Central ID: PMC4392793]. https://doi.org/10.1186/s12943-015-0310-8.
-
14.
Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50(6):1774-8. [PubMed ID: 2155059].
-
15.
Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87(2):262-71. [PubMed ID: 20400620]. [PubMed Central ID: PMC2895541]. https://doi.org/10.1093/cvr/cvq105.
-
16.
Yang H, Wang Z, Guo Y, Wang Z. Correlation and significance of urinary soluble fas and vascular endothelial growth factor in bladder urothelial cancer. Dis Markers. 2015;2015:383509. [PubMed ID: 26798188]. [PubMed Central ID: PMC4698549]. https://doi.org/10.1155/2015/383509.
-
17.
Kopparapu PK, Boorjian SA, Robinson BD, Downes M, Gudas LJ, Mongan NP, et al. Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res. 2013;33(6):2381-90. [PubMed ID: 23749886].
-
18.
Xia G, Kumar SR, Hawes D, Cai J, Hassanieh L, Groshen S, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175(4):1245-52. [PubMed ID: 16515971]. https://doi.org/10.1016/S0022-5347(05)00736-6.
-
19.
Nabeel Ahmed A. Clinical utility of altered expressions of P53, vascular endothelial growth factor and survivin in patients with bladder cancer. Cancer Res J. 2015;3(2):28. https://doi.org/10.11648/j.crj.20150302.12.
-
20.
Narayanan S, Srinivas S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther Adv Med Oncol. 2017;9(1):33-45. [PubMed ID: 28203296]. [PubMed Central ID: PMC5298449]. https://doi.org/10.1177/1758834016667179.
-
21.
Rahmani A, Alzohairy M, Khadri H, Mandal AK, Rizvi MA. Expressional evaluation of vascular endothelial growth factor (VEGF) protein in urinary bladder carcinoma patients exposed to cigarette smoke. Int J Clin Exp Pathol. 2012;5(3):195-202. [PubMed ID: 22558473]. [PubMed Central ID: PMC3341674].
-
22.
Khadim MT, Ahmed SA, Khan FA, Ikram A, Shaikh SY. Evaluation of vascular endothelial growth factors A, C and D as indicators of lymphangiogenesis and angiogenesis in invasive and non-invasive urothelial carcinoma bladder. J Pak Med Assoc. 2015;65(8):851-6. [PubMed ID: 26228330].
-
23.
Sato K, Sasaki R, Ogura Y, Shimoda N, Togashi H, Terada K, et al. Expression of vascular endothelial growth factor gene and its receptor (flt-1) gene in urinary bladder cancer. Tohoku J Exp Med. 1998;185(3):173-84. [PubMed ID: 9823778].