Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2018; 27(2): 129-138
Published online April 30, 2018
https://doi.org/10.5607/en.2018.27.2.129
© The Korean Society for Brain and Neural Sciences
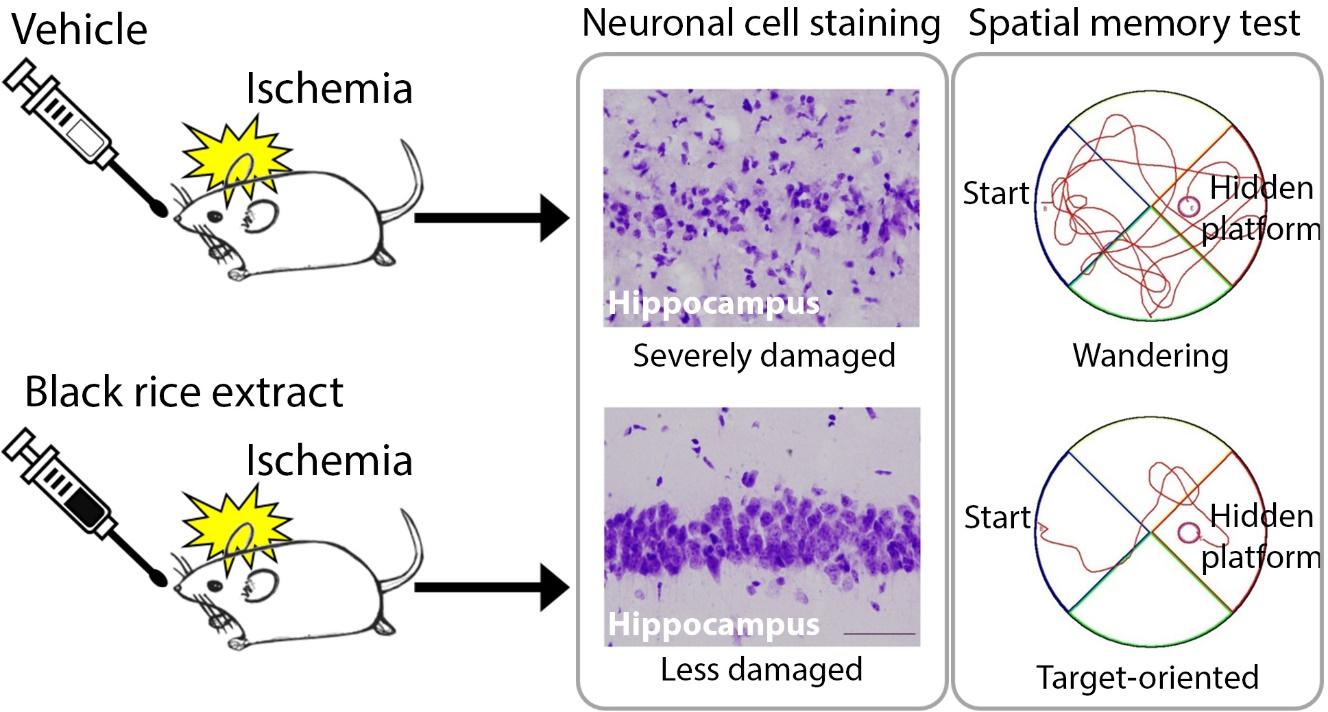
Black Rice (Oryza sativa L ., Poaceae) Extract Reduces Hippocampal Neuronal Cell Death Induced by Transient Global Cerebral Ischemia in Mice
Sun-Nyoung Hwang1†, Jae-Cheon Kim1†, Mohammad Iqbal Hossain Bhuiyan1,Joo Youn Kim1, Ji Seon Yang2, Shin Hee Yoon2, Kee Dong Yoon3 and Seong Yun Kim1*
Departments of 1Pharmacology and 2Physiology, Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, 3College of Pharmacy, The Catholic University of Korea, Bucheon 14662, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-2258-7324, FAX: 82-2-536-2485
e-mail: syk@catholic.ac.kr
†These authors contributed equally
Abstract
Rice is the most commonly consumed grain in the world. Black rice has been suggested to contain various bioactive compounds including anthocyanin antioxidants. There is currently little information about the nutritional benefits of black rice on brain pathology. Here, we investigated the effects of black rice (
Graphical Abstract
Keywords: Oryza sativa, brain ischemia, neuroprotection, hippocampus, memory and learning tests
INTRODUCTION
Although brain ischemia is frequently induced by sudden cardiac arrest, which is the main cause of death and disability in developed countries [1,2], no neuroprotective agents are currently clinically available [3]. The hippocampus, one of the brain areas responsible for cognitive function [4], is severely damaged following cerebral ischemia [5]. Various mechanisms, including excitotoxicity [6], oxidative stress [7,8], and inflammation [9] have been rigorously studied to explain the neuronal cell death induced by brain ischemia. Among these mechanisms, oxidative stress has been considered a main pathogenic mechanism of brain ischemia/reperfusion (I/R) injury [7]. Overproduction of reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide, and hydroxyl radicals and/or decreases in the scavenging activity of antioxidants take place during I/R injury [7]. Consequent oxidative stress leads to cellular dysfunction through oxidation of proteins, lipids, and nucleic acids, eventually resulting in cell death [7]. On the other hand, antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase play important roles in scavenging ROS and maintaining redox homeostasis [7]. In addition, there have been many reports on the neuroprotective effects of antioxidants against cerebral I/R injury [7,10,11]. Because the level of endogenous antioxidants is insufficient to relieve oxidative stress during I/R injury, interest in dietary natural antioxidants as alternative preventive and/or therapeutic agents has grown [12].
Rice is widely consumed by about half of the world's population [13]. Interest in rice increased after epidemiological studies showed a correlation between a low incidence of cancers and coronary heart disease and rice consumption [14,15]. In particular, black rice (
The aim of this study was to investigate the effects of black rice extract (BRE) on neuronal cell death in the mouse hippocampus using the bilateral common carotid artery (BCCAO) model of transient global cerebral ischemia. Additionally, we performed the Morris water maze test to examine whether BRE could alleviate BCCAO-induced spatial learning and memory impairment.
MATERIALS AND METHODS
Black rice (
Male C57BL/6 mice (Koatec, Kyunggi-do, Korea) weighing 20 to 22 g (9 weeks old) were housed in groups of five under standard conditions (temperature 22±1℃, relative humidity 50±3%, 12-h light-dark-cycle, lights on at 08:00 am) with free access to food and water. All animal procedures were approved by the Ethics Committee of the Catholic University of Korea and were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23).
Mice were randomly divided into three groups: (1) vehicle-treated sham-operated (n=15), (2) vehicle-treated BCCAO (n=13), and (3) BRE-treated BCCAO (n=6). BRE (300 mg/kg) and vehicle (distilled water) were administered orally by gavage once per day for 21 consecutive days. The experimental schedule is shown in Fig. 1.
We employed the BCCAO technique established by Cho et al. [23], with slight changes to induce transient global cerebral ischemia. Briefly, on the 8th day of BRE or vehicle administration, the animal was anesthetized by exposure to 2% isoflurane in a mixture of oxygen/nitrous oxide (30:70) for 3 min and 1% isoflurane was maintained with the same gas mixture with an anesthetic facemask. Body temperature was maintained at 37.0±0.5℃ with a heating pad during surgery. Laser Doppler flowmetry (PF5010, Perimed, Järfälla, Sweden) was used to measure regional cerebral blood flow (rCBF). To this end, two flexible probes (407) were attached to the skull bilaterally 3.5 mm from the bregma with cyanoacrylate adhesives. rCBF was recorded throughout the operation by a computer-based data acquisition system (Perisoft, Perimed). A midline neck incision was made. Then, both the common carotid arteries were isolated and clamped with micro-serrefines. Sham-operated animals underwent the same procedure except for occlusion of the common carotid arteries. The extent of rCBF reduction after occlusion was expressed as a percentage calculated by dividing the average perfusion unit (PU) obtained during the 1 min immediately after occlusion by the baseline value and multiplying by 100. Mice who had rCBF exceeding 10% on one or both sides were excluded from the study. Reperfusion was initiated by removing the clamps after 23 min of occlusion. The skin was sutured, and the animals were kept in an incubator at 31℃ until they recovered to normal activity levels.
To evaluate the effect of BRE on spatial learning and memory dysfunction caused by transient global cerebral ischemia, the Morris water maze (MWM) acquisition (learning) and retention (memory) tests were performed as described by Morris [24], with slight modifications. Briefly, a circular water tank 120 cm in diameter and 40 cm high was filled with opaque water at 22±2℃. A platform 10 cm in diameter was submerged 1 cm below the water surface. Visual cues were placed around the water tank to help mice find the hidden platform. The MWM acquisition test was performed on the 15th~19th day of BRE or vehicle administration. Mice underwent 4 trials per day for 5 consecutive days. The mouse was randomly placed in each quadrant of the pool facing the wall. The mouse was given 120 s to find the hidden platform and a 20-s rest between trials. If the mouse did not find the platform within the given time, it was manually placed on the platform for 20 s for reinforcement. The escape latency and swimming speed of each mouse were recorded via SMART video tracking software (Panlab, Barcelona, Spain) connected to a video camera hanging from the ceiling. On the 22nd day of BRE or vehicle administration, the MWM retention test was carried out to assess whether the animals remembered the platform location. The platform was removed from the pool and the mouse was allowed to swim freely for 120 s. The swimming path and speed of each mouse were recorded by the video camera. Memory scores for each group were calculated as described by Shin et al. [25]. The memory score of each mouse was calculated by multiplying the amount of time spent in a specific zone by a weighting factor, which was inversely proportional to the distance from the correct platform location, and summing the values.
After the MWM retention test, mice were deeply anesthetized with 15% chloral hydrate and underwent intracardiac perfusion with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brain tissue was sampled and post-fixed for 24 h at 4℃ in the same fixative. For cryoprotection, the fixed brain tissue was incubated in 30% sucrose in 0.1 M PB for 2 days at 4℃, embedded in Tissue-Tek OCT compound (Sakura Fine Technical, Tokyo, Japan), quickly frozen in liquid nitrogen, and stored at −80℃. Coronal brain sections 20 µm thick were collected with a cryostat and stored in phosphate-buffered saline (PBS, pH 7.4) with 0.04% sodium azide at 4℃ until use.
Cresyl violet staining was performed to examine neuronal morphology. Briefly, tissue sections were attached to a slide glass and dried overnight at 37℃. The sections were hydrated by immersing the slides sequentially in 95%, 90%, 80%, and 70% ethanol in distilled water for 3 min each. Then, they were incubated in 0.1% cresyl violet solution for 15 min followed by destaining for 3 min with 95% ethanol containing 0.1% glacial acetic acid. The sections were incubated for 3 min each in 100% ethanol, 50:50 (vol/vol) ethanol-xylene, and 100% xylene (two times) and then covered with Canada balsam and coverslipped. The sections were examined with light microscopy (BX51, Olympus, Tokyo, Japan).
Free-floating sections were washed with 0.01 M PBS 3 times for 5 min each. The sections were blocked with 10% normal sheep serum (Jackson ImmunoResearch) in 0.01 M PBS for 1 h at room temperature and incubated overnight at 4℃ with one of the following antibodies: mouse anti-neuronal nuclei (NeuN, 1:1000, Millipore), rabbit anti-glial fibrillary acidic protein (GFAP, 1:1000, Millipore), or goat anti-GPx (1:40, R&D Systems). The primary antibodies were diluted in 0.01 M PBS containing 1% normal sheep serum. The next day, the sections were rinsed with 0.01 M PBS three times for 5 min each and labeled with corresponding secondary antibodies for 2 h at room temperature: Cy3 conjugated anti-mouse IgG (1:500, Jackson ImmunoResearch), Alexa fluor 488-conjugated anti-rabbit IgG (1:500, Invitrogen), or Alexa fluor 488-conjugated anti-goat IgG (1:500, Invitrogen). After a final rinse with 0.01 M PBS 3 times for 5 min each, the sections were mounted on glass slides with Kaiser's glycerol gelatin (Merck, Germany) and coverslipped. The sections were then observed under a confocal microscope (LSM 510 Meta, Carl Zeiss Co., Ltd., Jena, Germany).
The number of cells was counted as described by Jeong et al. [26], with minor modifications. Three coronal sections, −1.46 and −1.94 mm from bregma [27], were collected at 100-µm intervals for histological analysis of the hippocampal CA1 area. Fluorescence photomicrographs of each section in the medial CA1 region, including the stratum oriens, pyramidal layer, and stratum radiatum, were taken at ×400 magnification (225×225 µm2) with a fluorescence microscope (AxioImager, Carl Zeiss, Jena, Germany). The number of NeuN- and GFAP-positive cells in each field was counted with ImageJ software (NIH). GPx immunoreactivity in the pyramidal cell layers of the medial CA1 was measured by the mean gray value in ImageJ (W. Rasband, National Institutes of Health, http://imagej.nih.gov/ij/).
All data are expressed as mean±SEM. All statistical analyses were conducted with SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was assessed with a one-way analysis of variance (ANOVA) for multiple group comparisons followed by Tukey's post hoc test. Differences were considered significant at p<0.05.
RESULTS
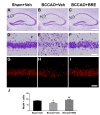
To assess the effect of BRE on hippocampal CA1 pyramidal cell death following transient global cerebral ischemia, we examined the morphology of hippocampal CA1 pyramidal neurons by cresyl violet staining and NeuN (a neuron-specific marker) immunofluorescence staining. Cresyl violet staining revealed that most medial CA1 pyramidal cells in vehicle-treated, sham-operated mice retained an intact morphology (Fig. 2A and D), whereas vehicle-treated BCCAO mice had numerous cells with a shrunken apoptotic morphology (Fig. 2B and E). In BRE-treated BCCAO mice, most medial CA1 pyramidal cells appeared to be intact (Fig. 2C and F). Fluorescence photomicrographs of NeuN-immunostained sections in the vehicle-treated sham-operated group demonstrated that the medial CA1 pyramidal cell layer was dense with NeuN-positive cells (Fig. 2G). NeuN-positive cells were sparse in the medial CA1 pyramidal cell layer of the vehicle-treated BCCAO group (Fig. 2H). In line with the cresyl violet staining, the medial CA1 pyramidal cell layer of the BRE-treated BCCAO group was filled with NeuN-positive cells (Fig. 2I). To quantify the neuroprotective effects of BRE against transient global cerebral ischemia, we counted the NeuN-positive cells in the medial CA1 pyramidal cell layer. The vehicle-treated BCCAO group had 66.88±29.04 NeuN-positive cells, which was significantly lower than the vehicle-treated sham-operated group (85.31±18.54) and the BRE-treated BCCAO group (93.33±18.41) (Fig. 2J).
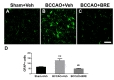
To investigate the effect of BRE on astrocyte reactions to I/R injury, hippocampal tissues were immunostained for GFAP, an astrocyte marker [5]. In the vehicle-treated sham-operated group, most GFAP-positive cells had small cell bodies with fine ramified processes (Fig. 3A), whereas the vehicle-treated BCCAO group had GFAP-positive astrocytes with enlarged cell bodies and thickened processes (Fig. 3B). GFAP-expressing cells in the BRE-treated BCCAO group had similar morphology to astrocytes in the vehicle-treated sham-operated group (Fig. 3C). To clarify the histological changes, we counted GFAP-positive cells in the medial CA1 subregion. The vehicle-treated BCCAO group had 129.21±75.36 GFAP-positive cells, which was considerably more than the vehicle-treated sham-operated group (65.64±23.46) and the BRE-treated BCCAO group (48.78±15.19) (Fig. 3D).
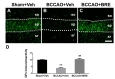
To explore the neuroprotective mechanisms of BRE, we investigated whether BRE administration regulates expression of GPx, an endogenous antioxidant enzyme, in mice. We examined GPx expression in the medial CA1 subregion via immunofluorescence staining. As shown in Fig. 4A, strong and dense GPx immunoreactivity was seen almost exclusively in the CA1 pyramidal layer of the vehicle-treated sham-operated group, whereas the vehicletreated BCCAO group had weak and scattered GPx signals throughout CA1, including the stratum oriens, pyramidal layer, and stratum radiatum (Fig. 4B). In the BRE-treated BCCAO group, the GPx immunostaining pattern was similar to that of the vehicle-treated sham-operated group (Fig. 4C). Quantitative analysis confirmed that GPx immunoreactivity in the vehicle-treated sham-operated group (9.90±0.44) and the BRE-treated BCCAO groups (10.38±0.73) was significantly stronger than that of the vehicle-treated BCCAO group (3.90±0.47), whereas there was no significant difference between the vehicle-treated sham-operated and BRE-treated BCCAO groups (Fig. 4D).
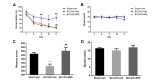
To investigate the effect of BRE on the spatial learning and memory deficit induced by transient global cerebral ischemia, mice were subjected to the MWM acquisition test on the 15th~19th day of BRE or vehicle administration and the retention test on the 22nd day of BRE or vehicle administration. In the acquisition test, vehicle-treated BCCAO mice had significantly longer escape latency to find the hidden platform than did vehicle-treated sham-operated mice on the 17th~19th day (Fig. 5A). BRE-treated BCCAO mice had significantly shorter escape latency than did the vehicle-treated BCCAO group on the 18th~19th day (Fig. 5A). In the retention test, the memory score of the vehicle-treated BCCAO group was 305.00±31.42, which was significantly lower than that of the vehicle-treated sham-operated group (464.53±22.36) (Fig. 5C). Similar to acquisition scores, the memory score of the BRE-treated BCCAO group (502.50±45.56) was significantly higher than the vehicle-treated BCCAO group (Fig. 5C). Swimming speed during the acquisition and retention tests did not differ among groups (Fig. 5B and D).
DISCUSSION
Our results demonstrated that administering BRE before and after 23-min BCCAO reduced neuronal cell death in the hippocampal CA1 pyramidal layer, indicating that BRE could be a promising potential neuroprotective agent against cerebral ischemic/reperfusion damage. We also found that BRE suppressed glial activation and prevented a loss of GPx expression in the CA1 area following ischemic damage. Furthermore, we observed that chronic administration of BRE could ameliorate BCCAO-induced memory impairment, supporting the hypothesis that some ingredients of BRE might protect against ischemic brain damage.
The neuroprotective effects of antioxidants against cerebral I/R injury have been reported by many researchers [7,10,11]. Murakami et al. [10] showed that SOD1 overexpression significantly protected hippocampal CA1 neurons from cell death following global cerebral ischemia. Dawson et al. [11] suggested the neuroprotective role of GPx in transient focal cerebral ischemia using a glutathione peroxidase mimic. Several studies have presented the antioxidant effects of black rice. Chiang et al. [28] showed that BRE not only reduced superoxide anions and ROS but also enhanced antioxidant enzyme activity in both HepG2 cells and C57BL/6 mice. They also suggested that cyanidin-3-O-glucoside (C3G) chloride and peonidin-3-O-glucuside chloride, which are typical anthocyanins in black rice, are major contributors to these antioxidant effects. A paper published by Kangwan et al. [21] reported that administering BRE could reduce the oxidative stress induced by global cerebral ischemia due to its antioxidant components. Yoon et al. [20] showed the cytoprotective effect of BRE against oxidative stress-induced cell death
Astrocytes are supporting cells that play several important roles, including maintaining homeostasis [31]. Astrocyte hypertrophy is a typical result of cerebral ischemia-induced neuronal cell death [5]. We speculated that if BRE protected hippocampal neurons against transient global cerebral ischemia, it might also inhibit glial activation in the hippocampus. In the present study, we found that GFAP-positive astrocytes had an activated morphology with enlarged cell bodies and thickened processes in vehicle-treated BCCAO mouse hippocampi. However, in the BRE-treated BCCAO group, GFAP-positive cells in hippocampal tissues appeared to have a normal stellate-shape with long, fine processes. These findings are consistent with our results showing that BRE has neuroprotective effects against transient global cerebral ischemia. Thus, BRE-treated mice are likely to exhibit reduced reactive astrocytosis due to BRE preventing neuronal cell death after transient forebrain ischemia.
GPx, an endogenous antioxidant enzyme that scavenges harmful ROS, was identified as an important protective factor against focal cerebral I/R damage [32]. Eating black rice has been shown to improve GPx activity in normal rat tissue [33]. In addition, eating a black and brown rice mixture showed higher GPx activity clinically than eating white rice [34]. Therefore, we investigated whether GPx might be involved in BRE protection against transient cerebral ischemia. In the present study, GPx immunoreactivity was reduced in the hippocampal CA1 area after transient forebrain ischemia. This finding was consistent with results provided by two other research groups [35,36], and also indicated that BRE reversed a decrease in GPx immunoreactivity resulting from BCCAO. Collectively, these results suggest that BRE neuroprotection against transient global cerebral ischemia might involve GPx signaling.
Cognitive impairment, which can significantly lower quality of life, is one of the main sequelae of transient global cerebral ischemia [37]. Several studies have reported that these cognitive deficits are related to neuronal damage in the hippocampal CA1 sector [4]. Since we showed a BRE-mediated reduction in the hippocampal neuronal cell death induced by BCCAO, BRE might ameliorate the cognitive impairment caused by transient forebrain ischemia. In our study, cognitive function was significantly disrupted in the mice subjected to BCCAO as determined through the MWM. In addition, mice treated with BRE performed significantly better than did vehicle-treated BCCAO mice. This result was supported by Kangwan et al. [21], who showed that BRE ameliorated BCCAO-induced cognitive dysfunction. In addition to neuronal damage, another hypothesis to explain the cause of memory impairment in the BCCAO model is an aberrant increase in gamma-aminobutyric acid (GABA) released from reactive astrocytes. There is evidence to suggest that GABA from reactive astrocytes contributes to memory impairment in Alzheimer's disease by inhibiting synaptic transmission mediated through neuronal GABAA and GABAB receptors [38]. Also, it has been recently reported that excessive GABA was released from reactive astrocyte in the ischemic brain areas [39]. Therefore, we speculate that the ameliorative effect of BRE on cognitive dysfunction might be due to the suppression of reactive astrocytes and the consequent reduction of GABA level in the ischemic brain. However, further studies are needed to confirm this hypothesis.
Our study provided evidence that BRE could protect hippocampal neuronal cells and mitigate cognitive dysfunction induced by BCCAO. In addition, administering BRE also prevented loss of GPx expression in the CA1 subregion following I/R. Although further investigation is necessary to uncover the specific mechanisms, our findings potentially indicate a neuroprotective role of black rice in cerebral ischemia through GPx signaling.
Figures

References
- Array. Health, United States, 2016: with chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics, 2017; 2017. p. 18.
- Tong JT, Eyngorn I, Mlynash M, Albers GW, Hirsch KG. Functional neurologic outcomes change over the first 6 months after cardiac arrest. Crit Care Med 2016;44:e1202-e1207.
- Stocchetti N, Taccone FS, Citerio G, Pepe PE, Le Roux PD, Oddo M, Polderman KH, Stevens RD, Barsan W, Maas AI, Meyfroidt G, Bell MJ, Silbergleit R, Vespa PM, Faden AI, Helbok R, Tisherman S, Zanier ER, Valenzuela T, Wendon J, Menon DK, Vincent JL. Neuroprotection in acute brain injury: an up-to-date review. Crit Care 2015;19:186.
- Hartman RE, Lee JM, Zipfel GJ, Wozniak DF. Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res 2005;1043:48-56.
- Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma 2002;19:85-98.
- Kim JY, Ho H, Kim N, Liu J, Tu CL, Yenari MA, Chang W. Calcium-sensing receptor (CaSR) as a novel target for ischemic neuroprotection. Ann Clin Transl Neurol 2014;1:851-866.
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001;21:2-14.
- Yoo DY, Yoo KY, Park JH, Kwon HJ, Jung HY, Kim JW, Choi GM, Moon SM, Kim DW, Yoon YS, Won MH, Hwang IK. Time- and cell-type specific changes in iron, ferritin, and transferrin in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neural Regen Res 2016;11:924-930.
- Rolova T, Dhungana H, Korhonen P, Valonen P, Kolosowska N, Konttinen H, Kanninen K, Tanila H, Malm T, Koistinaho J. Deletion of nuclear factor kappa B p50 subunit decreases inflammatory response and mildly protects neurons from transient forebrain ischemia-induced damage. Aging Dis 2016;7:450-465.
- Murakami K, Kondo T, Epstein CJ, Chan PH. Overexpression of CuZn-superoxide dismutase reduces hippocampal injury after global ischemia in transgenic mice. Stroke 1997;28:1797-1804.
- Dawson DA, Masayasu H, Graham DI, Macrae IM. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett 1995;185:65-69.
- Cherubini A, Ruggiero C, Morand C, Lattanzio F, Dell'aquila G, Zuliani G, Di Iorio A, Andres-Lacueva C. Dietary antioxidants as potential pharmacological agents for ischemic stroke. Curr Med Chem 2008;15:1236-1248.
- Mohanty S. Trends in global rice consumption. Rice Today 2013;12:44-45.
- Array. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, D.C: American Institute for Cancer Research, 2007; 2007. p. 66-197.
- Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci Nutr 2014;2:75-104.
- Ling WH, Wang LL, Ma J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J Nutr 2002;132:20-26.
- Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol 2010;10:959-966.
- Thummayot S, Tocharus C, Pinkaew D, Viwatpinyo K, Sringarm K, Tocharus J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology 2014;45:149-158.
- Zuo Y, Peng C, Liang Y, Ma KY, Yu H, Edwin Chan HY, Chen ZY. Black rice extract extends the lifespan of fruit flies. Food Funct 2012;3:1271-1279.
- Yoon J, Ham H, Sung J, Kim Y, Choi Y, Lee JS, Jeong HS, Lee J, Kim D. Black rice extract protected HepG2 cells from oxidative stress-induced cell death via ERK1/2 and Akt activation. Nutr Res Pract 2014;8:125-131.
- Kangwan N, Pintha K, Preedapirom W, Tantipaiboonwong P, Chumphukam O, Suttajit M. Learning and memory enhancing effects of anthocyanin in black rice extract on cerebral ischaemia in mice. Sci Asia 2015;41:315-321.
- Jeon H, Choi J, Choi SJ, Lee CU, Yoon SH, Kim J, Yoon KD. Rapid isolation of cyanidin 3-glucoside and peonidin 3-glucoside from black rice (
oryza sativa ) using high-performance countercurrent chromatography and reversed-phase column chromatography. Nat Prod Sci 2015;21:30-33. - Cho KO, Kim SK, Cho YJ, Sung KW, Kim SY. Regional differences in the neuroprotective effect of minocycline in a mouse model of global forebrain ischemia. Life Sci 2007;80:2030-2035.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47-60.
- Shin JW, Cheong YJ, Koo YM, Kim S, Noh CK, Son YH, Kang C, Sohn NW. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of proinflammatory cytokines and microglial activation. Biomol Ther 2014;22:17-26.
- Jeong KH, Lee KE, Kim SY, Cho KO. Upregulation of Krüppel-like factor 6 in the mouse hippocampus after pilocarpine-induced status epilepticus. Neuroscience 2011;186:170-178.
- Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed. San Diego, CA: Academic Press, 2008.
- Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006;41:797-803.
- Bhuiyan MI, Kim JY, Ha TJ, Kim SY, Cho KO. Anthocyanins extracted from black soybean seed coat protect primary cortical neurons against in vitro ischemia. Biol Pharm Bull 2012;35:999-1008.
- Bhuiyan MI, Kim HB, Kim SY, Cho KO. The neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J Physiol Pharmacol 2011;15:353-361.
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 1997;20:570-577.
- Crack PJ, Taylor JM, de Haan JB, Kola I, Hertzog P, Iannello RC. Glutathione peroxidase-1 contributes to the neuroprotection seen in the superoxide dismutase-1 transgenic mouse in response to ischemia/reperfusion injury. J Cereb Blood Flow Metab 2003;23:19-22.
- Kim JY, Do MH, Lee SS. The effects of a mixture of brown and black rice on lipid profiles and antioxidant status in rats. Ann Nutr Metab 2006;50:347-353.
- Kim JY, Kim JH, Lee DH, Kim SH, Lee SS. Meal replacement with mixed rice is more effective than white rice in weight control, while improving antioxidant enzyme activity in obese women. Nutr Res 2008;28:66-71.
- Yan BC, Park JH, Lee CH, Yoo KY, Choi JH, Lee YJ, Cho JH, Baek YY, Kim YM, Won MH. Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res 2011;1425:142-154.
- Kim YO, Kim HJ, Kim GS, Park HG, Lim SJ, Seong NS, Ham YW, Lee SD, Jang KH, Jung KH, Chung JH, Kang SA. Panax ginseng protects against global ischemia injury in rat hippocampus. J Med Food 2009;12:71-76.
- Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009;80:297-305.
- Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim YS, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med 2014;20:886-896.
- Lin YH, Liang HY, Xu K, Ni HY, Dong J, Xiao H, Chang L, Wu HY, Li F, Zhu DY, Luo CX. Dissociation of nNOS from PSD-95 promotes functional recovery after cerebral ischaemia in mice through reducing excessive tonic GABA release from reactive astrocytes. J Pathol 2018;244:176-188.



