Published online Jul 19, 2021. doi: 10.5498/wjp.v11.i7.297
Peer-review started: March 7, 2021
First decision: March 30, 2021
Revised: April 13, 2021
Accepted: May 10, 2021
Article in press: May 10, 2021
Published online: July 19, 2021
The versatility of glutamate as the brain’s foremost excitatory neurotransmitter and modulator of neurotransmission and function is considered common knowledge. Years of research have continued to uncover glutamate’s effects and roles in several neurological and neuropsychiatric disorders, including depression. It had been considered that a deeper understanding of the roles of glutamate in depression might open a new door to understanding the patholo
Core Tip: The versatility of glutamate as the brain’s foremost excitatory neurotransmitter, and modulator of intermediary metabolism in the gastrointestinal tract is considered common knowledge. Years of research suggest glutamate has a role to play in depression. Also, there is increasing evidence of a possible relationship between glutamate and the pathophysiology and/or treatment of depression. The complexity of depression suggests dysregulation of glutamate in sites such as the gastrointestinal tract and brain. The communication link involving dietary glutamate, the gut, endogenous glutamate, and the brain is a multidirectional pathway; the understanding of which is necessary to fully account for glutamate’s role in depression.
- Citation: Onaolapo AY, Onaolapo OJ. Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World J Psychiatr 2021; 11(7): 297-315
- URL: https://www.wjgnet.com/2220-3206/full/v11/i7/297.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i7.297
Clinical depression or major depressive disorder (MDD) is a chronic, debilitating, and disabling mental health disorder that affects over 300 million people (across all age groups) globally[1-4]. It contributes significantly to the global burden of disease and escalating incidence of suicides amongst teenagers and young adults worldwide. Globally, the prevalence of depression continues to increase[4,5]; with factors such as worsening poverty, increasing unemployment, adverse life events and genetics[4] being recognized as important risk factors for its development.
The discovery of monoamine oxidase inhibitors and tricyclic antidepressants opened opportunities for the treatment of depression and provided insights into the role of neurotransmitters such as dopamine, serotonin and norepinephrine in the pathophysiology of depression[6,7]. However, the shortcomings of the currently-approved pharmacotherapies such as the lag time between the effect of drugs on monoamine availability and their therapeutic effect, inadequate response, and the increasing incidence of treatment-resistant depression[7-9] means that there is still a critical need to better understand the pathophysiology of depression; and develop more-effective and efficient therapeutic interventions for depressive disorders.
The monoamine hypothesis supports the notion that the pathology in depression is primarily depletion in the levels of brain monoamine neurotransmitters including serotonin, norepinephrine, and dopamine[10-12]. In the almost seven decades since its formulation, it has largely explained the symptoms and response to currently available antidepressant therapy. However, inconsistencies in the hypothesis have resulted in further research to better understand depression pathophysiology and management. In the last three decades, there has been compelling clinical[13,14] and preclinical[15,16] evidence demonstrating the involvement of the glutamatergic system in the pathophysiology of depression.
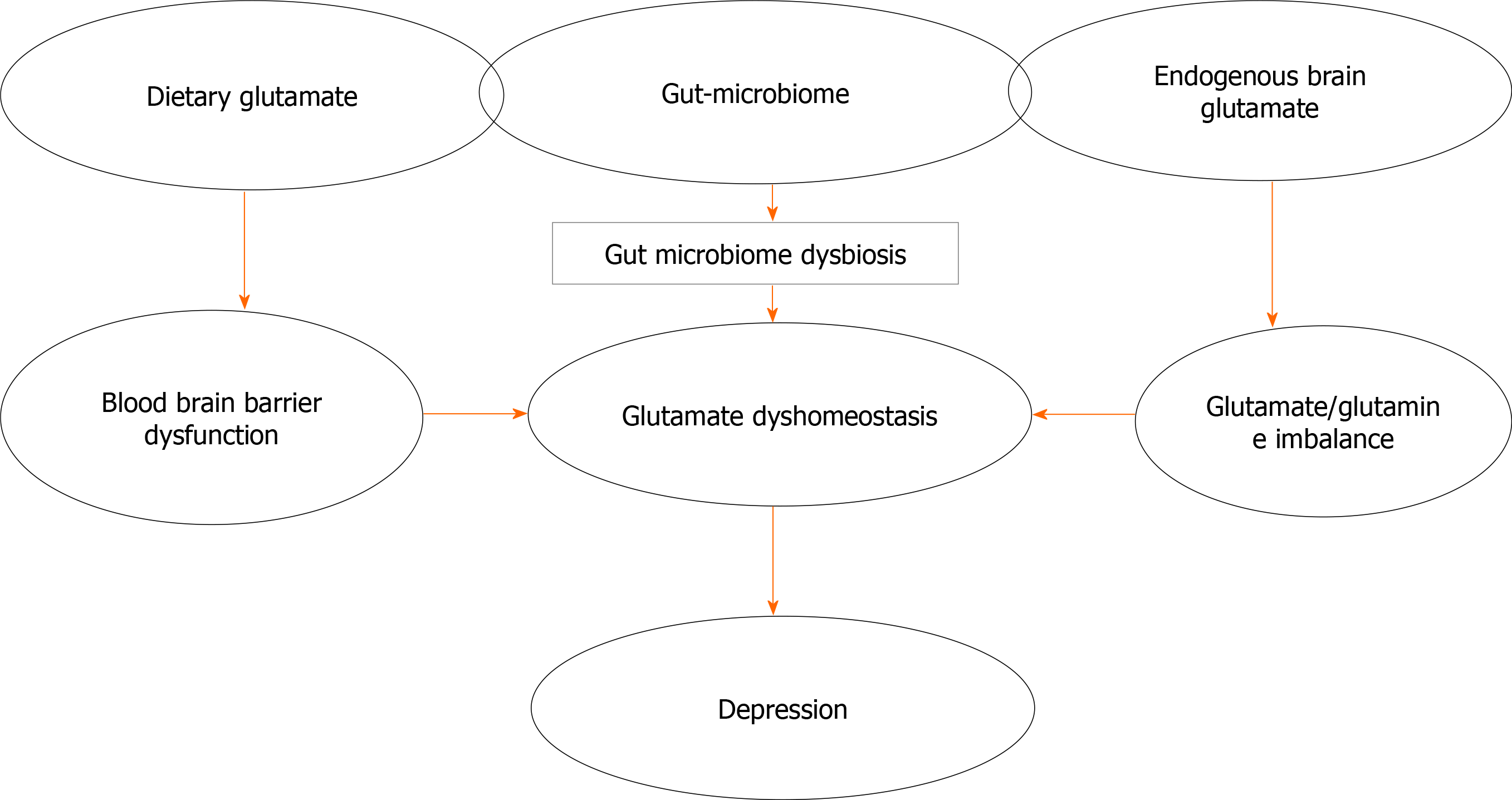
Since the first mention of the glutamate hypothesis of depression in the 1990s[15], our understanding of the versatility of glutamate as the brain’s foremost excitatory neurotransmitter, and modulator of neurotransmission and function has increased considerably. Years of research have continued to uncover glutamate’s effects and roles in several neurological and neuropsychiatric disorders, including depression. More recently, the antidepressant actions of ketamine, an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist especially in treatment-resistant depression[17,18] was reported; suggesting a deeper understanding of the roles of glutamate in depression could open new doors to understanding the pathological basis of the disorder, improve the approach to patient management, and lead to the development of newer drugs that may benefit more patients. Also, the roles of the gut-brain axis in glutamate signalling have been investigated, with reports that these could also impact the pathophysiology and treatment options in depression[19,20]. This review examines current understanding of the roles of endogenous and exogenous sources of glutamate and the glutamatergic system in the aetiology, progression and management of depression. It also examines the relationships that link the gut-brain axis, depression and glutamate (Figure 1); as it emphasizes how the gut-brain axis could impact depression pathogenesis and management via changes in glutamate homeostasis. Finally, we consider what the likely future of glutamate-based therapies and glutamate-based therapeutic manipulations in depression are, and if with them, we are now on the final chapter of understanding the neurochemical milieu of depressive disorder.
Glutamate is an amino acid and the major excitatory neurotransmitter in the brain[21]. In the last few decades, there has been increasing insights into the roles played by the glutamatergic system (Figure 1) in the pathophysiology and treatment of mood disorders generally, and depressive disorders specifically[12,22,23]. Although glutamate is ubiquitous in the brain, excessive release of glutamate has been associated with excitotoxicity-induced brain injury[24]. The possible involvement of the glutamatergic system in mood disorders is supported by preclinical evidence of the antidepressant effects of NMDA antagonists[15,25]. Also, the results of early studies showing alterations in the levels of glutamate (peripherally and centrally) in persons with mood disorders confirmed this involvement[13,26]. Alterations in excitatory and/or inhibitory neurotransmitters resulting in the alteration of functional connectivity patterns within large brain networks have also been reported[27]. More recently, there is overwhelming evidence of the anxiolytic and antidepressant response to subanaesthetic-doses of ketamine in clinical[27-30] and preclinical studies[31,32].
The ability of diet to cause depression and depression-like phenotypes has been reported[33,34]. Research has continued to show that the consumption of diets rich in fat, deficient in magnesium, or high in monosodium glutamate can cause depression-like behaviours such as decreased social interaction, anhedonia and behavioural despair in rodents[35-37]. While there have been suggestions that these effects are linked to the ability of dietary factors to alter the composition of the gut microbiota[36,37], emerging evidence of the interactions between the gut microbiota and brain neurotransmitters such as dopamine, serotonin, gamma amino-butyric acid and glutamate[9,38] are opening new vistas into possible novel treatment modalities for depression.
Glutamate is an α-amino acid that is useful in the biosynthesis of proteins and important in intermediary metabolism, through its ability to link carbohydrate and amino acid metabolism via the tricarboxylic acid cycle[39,40]. Glutamate (in addition to being synthesized in the body in humans) is also derived from dietary sources such as cheese, meat, and several food-seasonings including monosodium glutamate[21,41].
While endogenous brain glutamate has been linked to the pathophysiology of psychiatric conditions such as schizophrenia and mood disorders, the possible role of dietary glutamate (Table 1) in the development of neuropsychiatric conditions is still being evaluated[42]. There have been suggestions that the consumption of diets containing high concentrations of monosodium glutamate could increase body levels of glutamic acid, resulting in hyperglutamatergic neurotransmission, which could possibly contribute to the development of depression[43]. Also, a few studies have reported that factors such as chronic stress that reduce brain levels of glutamate and glutamine causing hypoactive glutamatergic signaling in the mouse prefrontal cortex are also associated with the development of depression[44], suggesting that regarding brain glutamatergic transmission a delicate balance always needs to be maintained.
| Subject | Outcome | Ref. |
| Human | In non-obese participants, diets high in levels of glutamic acid were associated with greater depression symptomatology | Kumar et al[43] |
| Adult mice | While chronic immobilization stress decreased sodium-coupled neutral amino acid transporter (SNAT)-1 and 2 in neurons and glutamate transporter (GLT)1, SNAT3, and SNAT5 in astrocytes in the medial prefrontal cortex, glutamine—supplemented diet ameliorated these decrements | Baek et al[44] |
| Neonatal rats | Subcutaneous injection of monosodium glutamate (MSG) increased the immobility time in the forced swim test and the freezing reaction in the contextual fear conditioning. MSG also increased serotonin uptake in the cerebral cortices and caused deregulation of the hypothalamic-pituitary-adrenal axis | Quines et al[35] |
| Mice | Anxiolytic and memory-enhancing effects at low doses of MSG; however, at higher doses, anxiety and memory retardation were observed | Onaolapo et al[45] |
| Mice | Higher doses of dietary glutamate resulted in an increase in plasma glutamate and glutamine but no difference in total brain glutamate or glutamine levels | Onaolapo et al[21,45,46] |
| Mice | Anxiolytic response in females, and anxiogenic response in males following dietary MSG. A decrease in behavioural despair was observed in both sexes (females more than males) | Onaolapo et al[46,47] |
| Mice | Anxiogenic effect was observed following subchronic oral administration of MSG | Onaolapo et al[49] |
In the last few years, reports from preclinical studies have associated the use of monosodium glutamate with the development of behavioural phenotypes such as anxiety and depression; and the ability to influence brain endogenous glutamate and glutamatergic neurotransmission[35,42,44-46]. There have also been reports from studies that monosodium glutamate could directly influence the concentrations of brain neurotransmitters such as serotonin and glutamate[21,35,45,47], although there have also been reports to the contrary[48].
The relationship between dietary monosodium glutamate and depression has been examined by a few studies[35,43,47]. The results of a clinical analysis that examined the relationship between the consumption of a diet high in glutamic acid and the development of depressive symptoms in a group of persons with schizophrenia revealed that in non-obese patients the consumption of high dietary glutamic acid was associated with an increase in depressive symptoms, although this was linked to the susceptibility of persons with one psychiatric condition to develop other co-morbidities[42]. A preclinical study by Quines et al[35] that examined the effects of monosodium glutamate administered parenterally in the neonatal period with exposure to behavioural paradigms on postnatal days 60-64 reported the presence of anxiety and behavioural despair. However, studies from our laboratory revealed that while orally administered monosodium glutamate was associated with the development of anxiety behaviour, especially in male mice[21,45,49], an antidepressant effect was observed in the behavioural-despair paradigms irrespective of sex[47]. The results of these studies from our laboratory suggest that the antidepressant response observed when monosodium glutamate was administered by gavage (compared to the response following parenteral administration) could have been influenced by the gut microbiota or the gut-brain axis, or by the ability of monosodium glutamate (at these doses) to minimally increase brain levels of glutamate which could have antidepressant benefits as previously reported[44].
Glutamate plays an important role in the modulation of synaptic plasticity and transmission. It is also the precursor of the inhibitory neurotransmitter gamma amino-butyric acid (GABA). Studies have shown that dysregulation of glutamatergic transmission or alterations in brain concentrations of glutamate is associated with derangement of brain function, development of excitotoxic brain injury, and cell death[24,47]. There have also been reports showing that alterations in glutamatergic neurotransmission contribute significantly to the development of peripheral and central nervous system disorders[50]. In the last few years, the possible relationships that exist between glutamate/glutamatergic system and the development of neuropsychiatric disorders such as depression have continued to be examined[22,24,51].
Several studies have linked dysregulation of glutamate neurotransmission with the development and progression of neurodevelopmental, neurodegenerative and psychiatric disorders such as autism, epilepsy and schizophrenia[52,53]. There is also emerging evidence (Table 2) linking the pathogenesis of depression to alterations in glutamate and glutamate signalling[12,52]. Levine et al[54], using a proton magnetic resonance spectroscopy (MRS) technique examined the relationship between cerebrospinal fluid (CSF) metabolites, such as glutamate and glutamine on depressive symptoms in hospitalized persons with severe unmedicated depression, and reported that compared to control subjects, glutamine level in the CSF of depressed patients was elevated. Also, using high performance liquid chromatography with fluorometric detection, Mitani et al[55] examined the relationship between plasma levels of glutamate on severity of depression and concluded that plasma levels of glutamate as well as alanine and L-serine were reflective of the severity of depression. The impact of brain glutamate levels on depression and depression phenotypes have been studied extensively[56-60]. Auer et al[56] and Hasler et al[57] using MRS reported region-specific changes in the levels of brain glutamate in patients with depression. The result of a recent meta-analysis of MRS studies also supported the hypothesis that glutamatergic neurotransmission was involved in the pathophysiology of depression[60]. In another meta-analysis, Luykx et al[58] also reported region and state specific alterations in glutamate and glutamine concentrations in depression. The importance of glutamatergic neurotransmission in depression has been further supported by studies that showed altered glutamine concentrations despite normal glutamate levels[59,61].
| Subject | Method | Outcome | Ref. |
| Human | Using a proton magnetic resonance spectroscopy technique | Compared to control subjects, glutamine levels in the cerebrospinal fluid of the depressed patients were elevated | Levine et al[54] |
| Human | High performance liquid chromatography with fluorometric detection | Plasma levels of glutamate as well as alanine and L-serine were reflective of the severity of depression | Mitani et al[55] |
| Human | Single voxel (1)H-Magnetic resonance spectroscopy in 19 patients with major depressive episodes | A significant decrease was observed in the levels of glutamate and glutamine in the anterior cingulate | Auer et al[56] |
| Human | Magnetic resonance spectroscopy | Depressed patients had reduced glutamine and glutamate levels in the dorsomedial/dorsal anterolateral prefrontal cortex | Hasler et al[57] |
| Human | Magnetic resonance spectroscopy | Compared with controls, depressed patients showed an increase in glutamine levels | Godlewska et al[59] |
| Human | Meta-analysis | Decreased levels of glutamatergic metabolites were observed in the medial frontal cortex of depressed subjects | Moriguchi et al[60] |
| Human | Meta-analysis | Glutamate and glutamine concentrations were found to be lower in the anterior cingulate cortex in patients compared to controls | Luykx et al[58] |
| Human | Functional magnetic resonance imaging and magnetic resonance spectroscopy | Patients with anhedonic major depression showed decreased glutamine but normal glutamate and gamma-aminobutyric acid concentrations | Walter et al[61] |
| Human | Resting state functional magnetic resonance imaging | Decreased amplitude of low frequency fluctuation level in right putamen and right middle temporal cortex correlated positively with glutamate concentration in female patients with depression | Zhang et al[66] |
| Mice | Preclinical study | Blockade of glutamate transporter-1 in the central amygdala and prefrontal cortex induced both anhedonia and anxiety | John et al[62,63] |
Abnormalities of the glutamatergic system such as those associated with glutamate clearance at the synaptic cleft and glutamate-related alterations in astrocytic energy modulation have also been observed in depression[61,62]. The results of preclinical studies have also demonstrated that blockade of astrocytic glutamate uptake in the prefrontal cortex and central nucleus of the amygdala was associated with the development of anhedonia and anxiety[62,63]. Furthermore, results from post-mortem and magnetic resonance imaging studies have also revealed the presence of altered expression of glutamate-related genes, elevated levels of glutamate, reduced glutamine/glutamate ratio, and/or reduced levels of glutathione (a reservoir of neuronal glutamate) in brain regions such as the medial prefrontal cortex (which have been linked to depression symptomatology) in persons with depression[64-67].
Finally, the rapid antidepressant effects of drugs such as tianeptine and NMDA receptor antagonist ketamine further validate the importance of glutamate and the glutamatergic transmission in depression[22,68,69]. While reports provide some evidence for the involvement of endogenous glutamate in the pathogenesis and treatment of depression[22,50,69,70], the complexity of the disorder would suggest that the dysregulation of glutamate needs to occur in multiple sites (Figure 1) such as the gastrointestinal tract and brain, as can be seen if there is a communication link involving exogenous glutamate, the gut, endogenous glutamate, and then the brain in a multidirectional pathway which we would call the “GLUTAMATE-GUT-GLUTAMATE-BRAIN AXIS”.
The impact of the gut (microbiota and gut peptides) in times past was not considered significant in brain development and functioning. Previously, it was believed that the commensal bacteria and their genes which constitute the gut microbiome enjoy a symbiotic relationship with man. In this relationship, they reside in a nutritionally enriched and protected habitat of the human gastrointestinal tract, while they in turn protect humans against colonization of the gut by pathogenic bacteria and provide the body with a rich source of indigestible nutrients. In the light of new evidence, it is now clear that they also play a key role in the brain, either in health or disease.
The gut-brain axis or microbiome-gut-brain axis describes the bidirectional, at times multidimensional system of communication that links the gastrointestinal tract with the central nervous system. It ensures that not only does the central nervous system modulate gastrointestinal function; the gut can also regulate or modulate brain signalling and impact brain structure and function[19]. There is now ample evidence supporting the view that the microbiome-gut-brain axis can influence the development or progression of central nervous system disorders. Some of these include observations of psychiatric co-morbidities occurring in several enteric neuropathies such as chronic inflammatory intestinal disorders[38,71,72]. The presence of altered gut microbial flora and concentration in neurodevelopmental disorders such as autism[73,74] as well as the results of microbial challenge using pathogenic bacteria or pharmacological manipulations with pre or probiotics, are also pointers to the possible roles played by the gut microbiota in the development of brain disorders[72,75]. Further reports have also shown that the gut microbiota influences brain function via its ability to modulate endocrine, immunologic and neurocrine signalling pathways; and brain neurotransmitters[38,76].
Also, several possible mechanisms through which microbiome-gut-brain communication could impact the genesis or progression of central nervous system disorders have been proposed. There have been suggestions that communications occur through the activation of neurotransmitters such as serotonin, dopamine, GABA and noradrenaline in the enteric nervous system; these neurotransmitters are secreted by gut microbiota and are akin to the neurotransmitters in the central nervous system[76]. The possible role of gut peptides and metabolites as mediators of the gut-brain crosstalk have also been suggested[77].
Studies have continued to demonstrate the deleterious effects that alterations in gut microbiome composition and microbiome-related metabolites could exert on the development of obesity, autoimmune disorders, inflammatory bowel disease, irritable bowel syndrome, and neuropsychiatric disorders[77-80]. Also, scientific information showing how gut microbes and gut stimuli (such as intragastric infusion of glucose or fatty acid) can directly influence emotional and cognitive functions[81,82] are pointers to the possible involvement of the gut-brain axis in psychiatric disorders. Several preclinical studies have also shown that the gut microbiota modulates brain behaviours such as behavioural despair, anhedonia and anxiety-like behaviours that have construct validity with clinical depression and anxiety[83-86]. Arentsen et al[84] and Kamimura et al[86] showed that compared to specific pathogen-free (SPF) mice, germ-free (GF) mice showed impaired social interactions choosing to spend more time with an object than conventionally raised mice. Huo et al[85] also observed that the exposure of GF mice and SPF mice to chronic restraint stress paradigm was associated with an increase in open field exploration time in GF compared to the SPF mice; with SPF mice exhibiting more anxiety-like behaviours compared to GF mice. Chronic prebiotic administration has also been shown to exhibit anxiolytic and antidepressant behaviours in mice. The ability of the prebiotic to modulate behaviour correlated positively with its effects on hippocampal and hypothalamic gene expression and its ability to ensure a balance in the concentrations of short-chain fatty acids[87].
Although the mechanisms by which the gut microbiota influences mood and mood-related behaviours are still being studied; there have been suggestions that because the gut and brain share peptide and receptor similarities the gut microbiome is able to modulate brain function through the activity of gut peptides[77]. Also, studies evaluating the impact of gut microbiome modulation (using prebiotics and probiotics) on brain function have demonstrated that chronic treatment with prebiotic had both antidepressant and anxiolytic effects, which could be linked to reduction in stress-induced corticosterone and proinflammatory cytokine release, and modification of the expression of specific genes in the hippocampus[86,87]. Also, probiotics such as GABA-producing Lactococcus lactis strain have been shown to possess the capacity to modulate behaviours. In one study, in which Lactococcus lactis was grown in both glutamate and non-glutamate supplemented media, a significant increase in GABA production was observed in the glutamate supplemented medium[87], reinforcing the importance of glutamate in the modulation of mood and mood disorders.
There is now ample evidence suggesting that microbes play the role of signalling components in the gut-brain axis. This emerging concept of a microbiota-gut-brain axis suggests that our ability to modulate the gut microbiota may be a potential tool towards the development of novel therapies for complex brain disorders such as psychiatric disorders[19,71,72] (Table 3).
| Subject | Outcome | Ref. |
| Germ-free mice | Germ-free (GF) mice showed impaired social interactions, anxiety and derangement of brain-derived neurotrophic factor levels | Crumeyrolle-Arias et al[83], Huo et al[85], and Kamimura et al[86] |
| GF and SPF mice | Exposure of GF mice and specific pathogen-free (SPF) mice to chronic restraint stress paradigm revealed an increase in open field exploration time in GF compared to SPF mice. Also, SPF mice exhibited more anxiety-like behavior than GF mice under the same external stress | Arentsen et al[84] |
| C57BL/6J male mice | Chronic administration of prebiotic (fructo-oligosaccharides and galacto-oligosaccharides) have been associated with antidepressant and anxiolytic effects | Kamimura et al[86] |
| Glutamate and non-glutamate supplemented media | Gamma amino-butyric acid (GABA)-producing Lactococcus lactis strain increased GABA production in the glutamate supplemented medium | Burokas et al[87] |
Glutamate is a multifunctional amino acid that is involved in intermediary metabolism in the gastrointestinal tract and is also crucial for the normal functioning and development of the brain. In the gut, it is derived from exogenous sources including dietary proteins and from free glutamate present in food additives; also, a fraction of the free glutamate in the lumen is from bacterial synthesis[88]. In the central and enteric nervous systems, respectively, glutamate is the major excitatory neurotransmitter[50,89].
Glutamate and the glutamatergic pathways are also crucial to microbiota-gut-brain communication. There is increasing evidence that glutamate is both a neurotransmitter and neuromodulator of several functions[77,89]. Glutamate receptors and their transduction molecules have been demonstrated on the epithelial cells of the gut, splanchnic, vagal, and/or pelvic afferents[89-91]. The stimulation of gut glutamate receptors by dietary or luminal glutamate has been associated with the activation of vagal afferents which directly or indirectly influence brain areas such as the cerebral cortex, limbic system, hypothalamus and basal ganglia[90,92]. Also, the activation of glutamate receptors present on splanchnic, vagal, and/or pelvic afferents, allows the communication of sensory inputs to regions of the brain involved in the gut-brain axis, while, also influencing efferent pathways that convey excitatory or inhibitory inputs to the gastrointestinal tract[89,91].
While in health, the enteric ganglia and the brain are impermeable to dietary or luminal glutamate; there have been reports suggesting that glutamate permeability in these systems increases in diseased states such as enteric neuropathies, or conditions that alter the integrity of the blood-brain-barrier. The gut microbiome also influences brain glutamate, with the results of metabolomic studies revealing that alterations in the composition of the gut saprophytic microflora also affected brain concentrations of glutamate[93,94]. Although there is enough scientific evidence in support of the impact of central glutamate and the glutamatergic system in depression, there is however a dearth of information regarding the possible ways luminal glutamate (either from dietary sources or microbial activities) may influence depression pathophysiology.
However, from the foregoing, it is evident that while exogenous glutamate (from dietary sources or secreted by gut microbiota) can influence brain function through activation of the gut glutamatergic pathways, endogenous glutamate can be influenced by gut microbial composition or metabolites to impact brain function. This shows that glutamate may be more important in the bidirectional communication system of the microbiome-gut-brain axis than previously considered.
In the preceding sections, the possible crucial roles that glutamate and glutamatergic neurotransmission play in the pathogenesis of mood disorders were discussed. The results of studies demonstrating the impact of glutamate dyshomeostasis and an imbalance between glutamatergic neurotransmission and synaptic plasticity on mood disorders have been pivotal in the search for novel pharmacotherapeutic strategies[51]. Also, evidence from studies demonstrating the possible antidepressant-like effects of agents acting at the glutamate receptors including NMDA receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and metabotropic glutamate (mGlu) receptors are all pointers to the possible roles of glutamate and glutamate receptors in depression management.
For more than six decades, drugs that modulate biogenic amines, increasing their availability at the synaptic cleft by selectively blocking the uptake of serotonin and/or norepinephrine have been used in the management of depression. While the safety profile of the newer biogenic amine drugs has improved considerably (compared to the older monoamine oxidase inhibitors and tricyclic antidepressants), they do not address the major drawbacks that have been associated with this mechanism[95-97].
Delayed therapeutic onset, low remission rates, and increased treatment refractoriness are among the major limitations or drawbacks of the standard pharmacological agents for depression treatment[95,96]. Again, up to a third of patients are diagnosed with the treatment-resistant phenotype, adding significantly to the global burden of depression[98]. These limitations are evidence of therapeutics that were still based on an incomplete understanding of disease pathogenesis.
Evidence of ketamine’s quick and relatively sustained antidepressant, anti-suicidal, and anti-anhedonic effects in treatment-resistant depression has been documented; and it represents a turning point in our understanding of the possibly crucial role that the glutamatergic system plays in depression. Such findings also prompted further research into developing novel glutamate-based therapeutic targets with better antidepressant effects and without dissociative side-effects, meaning an improvement over ketamine. The results of animal studies evaluating immobility in the forced swim test and tail suspension tests highlighted that both competitive and non-competitive NMDA receptor antagonists had antidepressant-like effects[15]. Decades later, these findings eventually culminated in the approval of esketamine (Spravato®) by the US Food and Drug Administration[99] and the European Medicines Agency[100]; esketamine was selectively approved for use (in addition to a known antidepressant) in adults with treatment-resistant MDD. Apart from this, clinical investigations also continue to affirm that a single intravenous bolus administration of ketamine can evoke a rapid (within 2 h) and lasting (up to 7 d) antidepressant action[101-103].
Ketamine: In humans, ketamine’s ability to alleviate depressive symptoms (Table 4) was first highlighted by a small, randomized, double-blind study demonstrating that a single subanaesthetic (0.5 mg/kg) dose of ketamine administered intravenously improved depressive symptoms within 72 h in seven persons with treatment resistant MDD[68]. A larger, double-blind, placebo-controlled, crossover study also found that a single ketamine infusion (0.5 mg/kg over 40 min) had a rapid, robust and mildly sustained antidepressant effect (1 wk) in treatment-resistant MDD[104]. Adverse effects that included confusion, euphoria, dizziness, perceptual disturbances, blood pressure elevation and increased libido were self-limiting[104].
| Study | Receptor type | Outcome | Ref. |
| Randomized, double-blind study | NMDAR antagonist | A single subanaesthetic (0.5 mg/kg) dose of ketamine administered intravenously improved depressive symptoms within 72 h in seven persons with treatment resistant major depressive disorder (MDD) | Berman et al[68] |
| Double-blind, placebo-controlled, crossover study | NMDAR antagonist | A single ketamine infusion (0.5 mg/kg over 40 min) had a rapid, robust and mildly sustained antidepressant effect (1 wk) in treatment resistant MDD | Zarate et al[104] |
| Open label study | NMDAR antagonist | Rapid anti-depressant effects of a single ketamine infusion in persons with treatment-resistant bipolar depression | DiazGranados et al[105] |
| Preclinical | NMDAR antagonist | Memantine exhibited a dose-dependent antidepressant-like response in the tail-suspension test, with the response observed at a dose of 15 mg/kg persisting with sub-chronic administration | Kitanaka et al[112] |
| Double-blind placebo controlled | NMDAR antagonist | Memantine administered at doses of between 5-20 mg/d, showed no significant effects on depression phenotypes | Parsons et al[110], Kos and Popik[111], and Muhonen et al[114] |
| Preclinical | NMDAR antagonist | The antidepressant effects of amantadine have been observed in situations where it is administered in combination with standard antidepressants such as fluoxetine and imipramine | Czarnecka et al[115] and Maj and Rogóz[116] |
| Preclinical | NMDA (NR2B) receptor blockers | Ro 25-6981 exhibited behavioural antidepressant-like effects in the forced swim test | Mathews et al[118] and Refsgaard et al[119] |
| Preclinical | NR2B-selective NMDA antagonist | CP-101,606 that was well-tolerated and devoid of psychotropic side effects was also used in a clinical trial involving subjects with traumatic brain injury | Refsgaard et al[119] |
| Randomized, placebo-controlled, double-blind study | NR2B-selective NMDA antagonist | CP-101,606 demonstrated efficacy in treatment-refractory MDD subjects | Merchant et al[120] |
| Cross-over pilot study | NR2B-selective NMDA antagonist | Oral formulation of MK-0657 in persons with treatment-resistant MDD showed a significant antidepressant effect compared with placebo while no improvement in symptoms was noted using the primary efficacy measure | Preskorn et al[121] |
| Preclinical | AMPA-antagonist | LY392098 and LY451616 exhibited antidepressant effects in a number of animal models of depression; including the inescapable stressors, learned-helplessness models, and exposure to chronic mild stress models | Li et al[122] and Lauterborn et al[123] |
| Preclinical | mGLu | LY341495, MSG0039, and MPEP exhibited significant antidepressant effects in rodent models of behavioural despair | Jaso et al[7] and Chaki et al[130] |
Since the first clinical study that demonstrated the antidepressant effects of ketamine, several other studies have continued to examine its efficacy across other depression phenotypes[104,105]. Diazgranados et al[105] demonstrated the rapid anti-depressant effects of a single ketamine infusion in persons with treatment-resistant bipolar depression, which was replicated in a double-blind, randomized, crossover, placebo-controlled study[104]. The impact of ketamine on reducing suicides has also been examined. Ketamine was reported to have a rapid and significant anti-suicidal effect in persons with MDD[101]. While the discovery of ketamine’s antidepressant effect was met with enthusiasm, its associated sedative and psychotomimetic effects remain limitations to its use. Therefore, efforts continue to be directed towards blunting these effects. Studies have tried to research the effects of augmenting ketamine with other drugs that are probably better-tolerated; these drugs such as riluzole and lamotrigine have been examined for their ability to either improve ketamine antidepressant effects and/or reduce its psychotomimetic effects[106-108]. However, a number of these studies reported that these add-ons showed no significant ability to improve the course of the antidepressant response (compared to ketamine alone) or reduce the side effects of ketamine[107,108]. Anand et al[106] on the other hand reported lamotrigine’s ability to decrease the perceptual abnormalities induced by ketamine.
Memantine and amantadine: The dampening of the enthusiasm that arose from the rapid and robust antidepressant effects of ketamine due its psychotomimetic side-effects prompted research into the possible antidepressant activities of other non-competitive NMDA receptor antagonists such as memantine, which has been reported to be devoid of these effects, at least at therapeutic doses[109-111]. The anti-depressant effects of memantine, a low-affinity, non-competitive, open-channel NMDA receptor antagonist which is approved for use in the management of Alzheimer’s disease has been extensively studied [104,112]. The results of a preclinical study by Kos and Popik[111] revealed a dose-dependent antidepressant-like response in the tail-suspension test, with the response observed at a dose of 15 mg/kg persisting with sub-chronic administration. However, the results of clinical studies have shown mixed results[110,111]. In one double-blind, placebo-controlled trial in which memantine was administered at doses of between 5-20 mg/d, no significant effects were observed[113]. This result was also supported by another double-blind placebo-controlled study that examined the effect of memantine administered at 10 mg/kg on late-onset depression[113]. However, the result of a large double-blind randomized Finnish study reported a significant antidepressant effect with memantine in persons with co-morbid alcohol dependence[114].
Amantadine is another NMDA receptor antagonist with possible influence on the serotonergic, dopaminergic and monamine-oxidase systems. The antidepressant effects of amantadine have been observed in situations where it is administered in combination with standard antidepressants such as fluoxetine and imipramine[115,116]. However, clinical trials are limited, mostly using amantadine as an augmentation agent (up to 300 mg/daily) in treatment resistant MDD, where it had shown some modest effects[117].
Subtype-selective NMDA (NR2B) receptor blockers: Investigations into subtype-selective blockers of the NMDA receptor (Table 4) have also been undertaken to by-pass the psychotomimetic effects of ketamine. Along this line, agents such as Ro 25-6981 that can block NR2B receptors have been studied. In preclinical experiments, the NR2B antagonist Ro 25-6981 exhibited behavioural antidepressant-like effects in the forced swim test[118]. The NR2B-selective NMDA antagonist (CP-101,606) that was well-tolerated and devoid of psychotropic side effects was also used in a clinical trial involving subjects with traumatic brain injury[119].
Other selective NMDA NR2B antagonists such as MK-0657 have also been examined. Again, in a randomized, placebo-controlled, double-blind study, the anti-depressant efficacy of CP-101,606 was demonstrated in treatment-refractory MDD subjects[120], while the result of a crossover pilot study that evaluated the potential antidepressant efficacy and tolerability of an oral formulation of MK-0657 in persons with treatment-resistant MDD observed a significant antidepressant effect compared with placebo using recognized secondary efficacy scales; with no improvement noted when symptoms were assessed using the primary efficacy measure[121].
The AMPA glutamate receptors are main contributors in excitatory neurotransmission, as they mediate the fast, rapidly desensitizing excitation of many synapses. The potential beneficial role of AMPA receptor modulators (Table 4) in the treatment of mood disorders has been highlighted by studies that have shown that AMPA receptor potentiators such as LY392098 and LY451616 possess antidepressant effects in a number of animal models of depression; including the inescapable stressors, learned-helplessness models, and exposure to chronic mild stress models[122]. Also, they do not seem to affect the extracellular concentration of monoamines[122]; yet they enhance the neurotrophic actions of BDNF mRNA and protein in primary neuronal cultures[122,123]. However, of the AMPA receptor positive allosteric modulators being investigated for MDD treatment, ORG-26576 was amongst the most-promising until it failed Phase II trial. Currently, while the potential roles of AMPA receptor modulators in experimental models of depression are still being researched, the world is still waiting for drugs whose actions are primarily linked to this. Stimulation of the AMPA receptor has also been associated with mediating the antidepressant-like effects of ketamine and group II mGlu receptor antagonist MGS0039[124], with suggestions that increased transmission via glutamatergic AMPA receptors possibly provide a common mechanism of antidepressant response[51].
Kainate receptors are now recognized as important mediators of the pre- and postsynaptic actions of glutamate and GABA, through mechanisms that are still being evaluated[125]. The results of some studies have associated genetic variations in certain kainate receptor subtypes with the therapeutic outcome of antidepressant medications like citalopram and venaflaxine[125-127]. While it is generally accepted that kainate receptors have modulatory effects on synaptic transmission, the paucity of selective kainate receptor subtype agonists or antagonists has hampered research into the possible mechanisms through which kainite receptors modulate brain function and/or impact the pathogenesis and treatment of depressive disorders[125].
mGlu receptors have been shown to regulate glutamate’s neuronal transmission through the ability to alter neurotransmitter release or modulate the post-synaptic responses to glutamate release. There is enough evidence from studies to support the notion that regulation of glutamatergic neurotransmission through mGlu receptors is associated with the development of mood, leading to suggestions that they could serve as novel targets in depression management[128,129]. Modulation of the mGlu receptor has also been reported to increase neurogenesis and neurotransmitter release that has now been associated with therapeutic response in humans[128]. Several mGlu2, mGlu3 and mGlu5 receptors’ (Table 4) negative allosteric modulators (LY341495, MSG0039, MPEP) have been reported to have significant antidepressant effects in rodent models of behavioural despair[7,130]. Also type III mGluRs (4-8) are mostly expressed presynaptically, modulating glutamate release and response; but while a number of preclinical studies have identified possible type III mGluR novel drug targets such as the mGluR7[131,132], there is a dearth of clinical studies evaluating the possible therapeutic benefits of these type III mGluRs in depression. There have however been reports that a positive allosteric modulator of mGluR7 (AMN082) has antidepressant-like properties in rodent models of behavioural despair[7], that can be linked to its ability to modulate glutamate transmission in the hippocampus[133].
There is ample evidence that dietary and endogenous glutamate (directly or indirectly) influences glutamatergic neurotransmission in the gastrointestinal tract and the brain, respectively. The gut microbiome has been shown to be involved in the synthesis and release of neuroactive molecules, including those involved in the pathogenesis of depression. Alterations in glutamate concentration and glutamatergic neurotransmission have also been linked to the pathophysiology of depressive disorders. Scientific evidence has shown that diet influences the gut microbiota composition and density, and that both diet and gut microbiota influence emotional behaviour and neurological processes[134,135]. By direct evidence and inference, we now know there is a complex interplay involving diet, the gut microbiome and depression. Hence, specific dietary patterns that can help prevent or mitigate mood disorders, possibly via shifts in gut microbiota equilibrium can be identified and offered as components of clinical management.
Also, the use of dietary intervention may prove to be attractive and cost-effective as an alternative or adjuvant therapy in the clinical management of depression. However, despite the tantalizing prospect, the directionality and mechanism of the relationship involving diet, the gut microbiome, and depression are still subjects of research. That being said, diets rich in vegetables, fruits, cereals, nuts, seeds, pulses and moderate amounts of dairy, eggs, fish and unsaturated fats have been associated with a lower incidence of depression[136-139], a view not supported by some studies[140,141]. Research has shown that different microbiota profiles may be associated with positive or negative mental health, emphasizing the behavioural impact of the gut microbiome. Also, if we know that behaviour is determined by the brain’s neurotransmitter milieu, then the link involving diet, the gut microbiome, neurotransmitters and depression will be easier to appreciate.
Humans with depression have been shown to harbor variations in gut microbiota which tend towards a general pattern of increases in potentially harmful and inflammation promoting bacteria such as Proteobacteria, and a decrease in commensal bacteria, which are normally more abundant[77,141-144]. However, the lack of a specific depression-associated gut microbiota profile is a major challenge[9]. Despite this, the presence of bacteria that produce neuroactive molecules strengthens the link between gut microbiota and behavioural disorders such as depression. In vitro and in vivo studies have demonstrated the ability of intestinally cultured strains of Lactobacillus brevis and Bifidobacterium dentium to efficiently produce GABA from monosodium glutamate enriched medium or monosodium glutamate supplemented food, respectively[145,146]; leading to suggestions that this could represent a promising therapeutic approach for depression management. Also, Lactobacillus rhamnosus (JB-1) has been shown to reduce stress-induced corticosterone levels in mice and ameliorate depression-like behaviour in the forced swim test; while also increasing brain glutamate and glutamatergic activity[9]. Other specific relationships involving glutamate are still being investigated, and their direct implications for therapy are being considered.
The limitations of the amine theory redirected the research focus to what was supposedly missing in the scientific understanding of the aetiology, course and management of depression. The discovery of the antidepressant effect of ketamine brought attention to the impact that manipulation of the glutamatergic system can have on the management of depression. Also, the role of diet and the gut microbiome in glutamate homeostasis is being continuously examined. As it becomes evident that gut microbes are involved in the synthesis and secretion of molecules that directly impact the brain, including glutamate, we now know that their manipulation through diet can become a cornerstone for the prevention and management of behavioural disorders. However, while glutamate-based therapies for depression are still in their infancy (and much more is dietary manipulation of glutamate balance via the gut microbes), it appears that understanding the diet, gut microbiome, gut-brain axis and glutamate link may be the next frontier in advancing our understanding of depression.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pasquini M, Pavón L, Qu SQ S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
| 1. | Birnbaum HG, Kessler RC, Kelley D, Ben-Hamadi R, Joish VN, Greenberg PE. Employer burden of mild, moderate, and severe major depressive disorder: mental health services utilization and costs, and work performance. Depress Anxiety. 2010;27:78-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1093] [Cited by in F6Publishing: 1060] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7855] [Cited by in F6Publishing: 7041] [Article Influence: 1173.5] [Reference Citation Analysis (1)] |
| 4. | World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. [cited 1 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications/i/item/depression-global-health-estimates. [Cited in This Article: ] |
| 5. | World Health Organization. Depression. [cited 1 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. [Cited in This Article: ] |
| 6. | Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Jaso BA, Niciu MJ, Iadarola ND, Lally N, Richards EM, Park M, Ballard ED, Nugent AC, Machado-Vieira R, Zarate CA. Therapeutic Modulation of Glutamate Receptors in Major Depressive Disorder. Curr Neuropharmacol. 2017;15:57-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Park M, Niciu MJ, Zarate CA Jr. Novel Glutamatergic Treatments for Severe Mood Disorders. Curr Behav Neurosci Rep. 2015;2:198-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Du Y, Gao XR, Peng L, Ge JF. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon. 2020;6:e04097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61 Suppl 6:4-6. [PubMed] [Cited in This Article: ] |
| 12. | Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 664] [Cited by in F6Publishing: 718] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 13. | Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Küçükibrahimoğlu E, Saygin MZ, Calişkan M, Kaplan OK, Unsal C, Gören MZ. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol. 2009;65:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 540] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Dhir A. Investigational drugs for treating major depressive disorder. Expert Opin Investig Drugs. 2017;26:9-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Pérez-Esparza R. Ketamine for Treatment-Resistant Depression: a New Advocate. Rev Invest Clin. 2018;70:65-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Mazzoli R, Pessione E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front Microbiol. 2016;7:1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 20. | Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing Depression from the Microbiota⁻Gut⁻Brain Axis. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 21. | Onaolapo OJ, Onaolapo AY, Akanmu MA, Gbola O. Evidence of alterations in brain structure and antioxidant status following 'low-dose' monosodium glutamate ingestion. Pathophysiology. 2016;23:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Jun C, Choi Y, Lim SM, Bae S, Hong YS, Kim JE, Lyoo IK. Disturbance of the glutamatergic system in mood disorders. Exp Neurobiol. 2014;23:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S-1015S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1042] [Cited by in F6Publishing: 1011] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 25. | Skolnick P, Legutko B, Li X, Bymaster FP. Current perspectives on the development of non-biogenic amine-based antidepressants. Pharmacol Res. 2001;43:411-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA Jr. Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr Pharm Des. 2009;15:1595-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA Jr. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol Psychiatry. 2017;81:886-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 28. | Harraz MM, Tyagi R, Cortés P, Snyder SH. Antidepressant action of ketamine via mTOR is mediated by inhibition of nitrergic Rheb degradation. Mol Psychiatry. 2016;21:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl). 2014;231:3663-3676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 30. | Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172:950-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 399] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 31. | Onaolapo OJ, Ademakinwa OQ, Olalekan TO, Onaolapo AY. Ketamine-induced behavioural and brain oxidative changes in mice: an assessment of possible beneficial effects of zinc as mono- or adjunct therapy. Psychopharmacology (Berl). 2017;234:2707-2725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Onaolapo AY, Ayeni OJ, Ogundeji MO, Ajao A, Onaolapo OJ, Owolabi AR. Subchronic ketamine alters behaviour, metabolic indices and brain morphology in adolescent rats: Involvement of oxidative stress, glutamate toxicity and caspase-3-mediated apoptosis. J Chem Neuroanat. 2019;96:22-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Rao TS, Asha MR, Ramesh BN, Rao KS. Understanding nutrition, depression and mental illnesses. Indian J Psychiatry. 2008;50:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond). 2013;37:382-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 35. | Quines CB, Rosa SG, Da Rocha JT, Gai BM, Bortolatto CF, Duarte MM, Nogueira CW. Monosodium glutamate, a food additive, induces depressive-like and anxiogenic-like behaviors in young rats. Life Sci. 2014;107:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Winther G, Pyndt Jørgensen BM, Elfving B, Nielsen DS, Kihl P, Lund S, Sørensen DB, Wegener G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015;27:168-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Hassan AM, Mancano G, Kashofer K, Fröhlich EE, Matak A, Mayerhofer R, Reichmann F, Olivares M, Neyrinck AM, Delzenne NM, Claus SP, Holzer P. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr Neurosci. 2019;22:877-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Onaolapo OJ, Onaolapo AY, Olowe AO. The neurobehavioral implications of the brain and microbiota interaction. Front Biosci (Landmark Ed). 2020;25:363-397. [PubMed] [Cited in This Article: ] |
| 39. | Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 40. | Onaolapo AY, Onaolapo OJ. Peripheral and central glutamate dyshomeostasis in neurodegenerative disorders. Curr Neuropharmacol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Onaolapo AY, Odetunde I, Akintola AS, Ogundeji MO, Ajao A, Obelawo AY, Onaolapo OJ. Dietary composition modulates impact of food-added monosodium glutamate on behaviour, metabolic status and cerebral cortical morphology in mice. Biomed Pharmacother. 2019;109:417-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Kraal AZ, Arvanitis NR, Jaeger AP, Ellingrod VL. Could Dietary Glutamate Play a Role in Psychiatric Distress? Neuropsychobiology. 2020;79:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Kumar P, Kraal AZ, Prawdzik AM, Ringold AE, Ellingrod V. Dietary Glutamic Acid, Obesity, and Depressive Symptoms in Patients With Schizophrenia. Front Psychiatry. 2020;11:620097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Baek JH, Vignesh A, Son H, Lee DH, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Glutamine Supplementation Ameliorates Chronic Stress-induced Reductions in Glutamate and Glutamine Transporters in the Mouse Prefrontal Cortex. Exp Neurobiol. 2019;28:270-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G. Changes in Spontaneous Working-memory, Memory-recall and Approach-avoidance following “Low Dose” Monosodium Glutamate in Mice. AIMS Neuroscience. 2016;3:317-337. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Onaolapo AY, Onaolapo OJ. Dietary glutamate and the brain: In the footprints of a Jekyll and Hyde molecule. Neurotoxicology. 2020;80:93-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Onaolapo AY, Olawore OI, Yusuf FO, Adeyemo AM, Adewole IO, Onaolapo OJ. Oral Monosodium Glutamate Differentially Affects Open-Field Behaviours, Behavioural Despair and Place Preference in Male and Female Mice. Curr Psychopharmacolo. 2019;8:1-16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Fernstrom JD. Monosodium Glutamate in the Diet Does Not Raise Brain Glutamate Concentrations or Disrupt Brain Functions. Ann Nutr Metab. 2018;73 Suppl 5:43-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. |
Onaolapo OJ, Onaolapo AY, Mosaku TJ, Onigbinde OA, Oyedele RA.
Elevated plus maze and Y-maze behavioral effects of subchronic, oral low dose monosodium glutamate in Swiss albino mice |
| 50. | Miladinovic T, Nashed MG, Singh G. Overview of Glutamatergic Dysregulation in Central Pathologies. Biomolecules. 2015;5:3112-3141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 642] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 52. | Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984-997, 979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 53. | Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 54. | Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 56. | Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 400] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 57. | Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 58. | Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 59. | Godlewska BR, Masaki C, Sharpley AL, Cowen PJ, Emir UE. Brain glutamate in medication-free depressed patients: a proton MRS study at 7 Tesla. Psychol Med. 2018;48:1731-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, Plitman E, Sano Y, Tarumi R, ElSalhy M, Katayama N, Ogyu K, Miyazaki T, Kishimoto T, Graff-Guerrero A, Meyer JH, Blumberger DM, Daskalakis ZJ, Mimura M, Nakajima S. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 61. | Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 62. | John CS, Smith KL, Van't Veer A, Gompf HS, Carlezon WA Jr, Cohen BM, Öngür D, Bechtholt-Gompf AJ. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467-2475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | John CS, Sypek EI, Carlezon WA, Cohen BM, Öngür D, Bechtholt AJ. Blockade of the GLT-1 Transporter in the Central Nucleus of the Amygdala Induces both Anxiety and Depressive-Like Symptoms. Neuropsychopharmacology. 2015;40:1700-1708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 345] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 65. | Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 66. | Zhang X, Tang Y, Maletic-Savatic M, Sheng J, Zhang X, Zhu Y, Zhang T, Wang J, Tong S, Li Y. Altered neuronal spontaneous activity correlates with glutamate concentration in medial prefrontal cortex of major depressed females: An fMRI-MRS study. J Affect Disord. 2016;201:153-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Rosa CE, Soares JC, Figueiredo FP, Cavalli RC, Barbieri MA, Schaufelberger MS, Salmon CEG, Del-Ben CM, Santos AC. Glutamatergic and neural dysfunction in postpartum depression using magnetic resonance spectroscopy. Psychiatry Res Neuroimaging. 2017;265:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2441] [Cited by in F6Publishing: 2529] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 69. | McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 70. | Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson's disease: The role of glial cells. J Pharmacol Sci. 2020;144:151-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 71. | Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 72. | Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2403] [Cited by in F6Publishing: 2574] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 73. | Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 608] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 74. | Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 75. | Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 867] [Cited by in F6Publishing: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 76. | Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 77. | Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics. 2018;15:36-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 297] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 78. | De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5:419-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 79. | Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23:5486-5498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 241] [Cited by in F6Publishing: 222] [Article Influence: 31.7] [Reference Citation Analysis (3)] |
| 80. | Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 81. | Cani PD, Knauf C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol Metab. 2016;5:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 82. | Van Oudenhove L, McKie S, Lassman D, Uddin B, Paine P, Coen S, Gregory L, Tack J, Aziz Q. Fatty acid-induced gut-brain signaling attenuates neural and behavioral effects of sad emotion in humans. J Clin Invest. 2011;121:3094-3099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 84. | Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis. 2015;26:29719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 85. | Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, Niu R, Wei H, Xie P. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front Cell Infect Microbiol. 2017;7:489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 86. | Kamimura I, Kaneko R, Morita H, Mogi K, Kikusui T. Microbial colonization history modulates anxiety-like and complex social behavior in mice. Neurosci Res. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82:472-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 537] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 88. | Mazzoli R, Pessione E, Dufour M, Laroute V, Giuffrida MG, Giunta C, Cocaign-Bousquet M, Loubière P. Glutamate-induced metabolic changes in Lactococcus lactis NCDO 2118 during GABA production: combined transcriptomic and proteomic analysis. Amino Acids. 2010;39:727-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Filpa V, Moro E, Protasoni M, Crema F, Frigo G, Giaroni C. Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology. 2016;111:14-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr. 2009;90:832S-837S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 465] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 92. | Kondoh T, Torii K. Brain activation by umami substances via gustatory and visceral signaling pathways, and physiological significance. Biol Pharm Bull. 2008;31:1827-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 94. | Kawase T, Nagasawa M, Ikeda H, Yasuo S, Koga Y, Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr. 2017;117:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 95. | Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2618] [Cited by in F6Publishing: 3031] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 96. | McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 279] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 97. | Malhi GS, Byrow Y. Is treatment-resistant depression a useful concept? Evid Based Ment Health. 2016;19:1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 98. | Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 99. | Bahr R, Lopez A, Rey JA. Intranasal Esketamine (SpravatoTM) for Use in Treatment-Resistant Depression In Conjunction With an Oral Antidepressant. P T. 2019;44:340-375. [PubMed] [Cited in This Article: ] |
| 100. | European Medicines Agency. Esketamine nasal spray Summary of Product Characteristics. [cited 1 March 2021]. In: European Medicines Agency [Internet]. Available from: https://www.ema.europa.eu/en/medicines. [Cited in This Article: ] |
| 101. | Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 102. | Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry. 2016;173:816-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 103. | Adell A. Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules. 2020;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 104. | Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2275] [Cited by in F6Publishing: 2404] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 105. | DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 440] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 106. | Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 107. | Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 108. | Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526-1533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 109. | Hesselink MB, De Boer AG, Breimer DD, Danysz W. Dopamine release in the prefrontal cortex in response to memantine following sub-chronic NMDA receptor blockade with memantine: a microdialysis study in rats. J Neural Transm (Vienna). 1999;106:803-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 674] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 111. | Kos T, Popik P. A comparison of the predictive therapeutic and undesired side-effects of the NMDA receptor antagonist, memantine, in mice. Behav Pharmacol. 2005;16:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Kitanaka N, Kitanaka J, Hall FS, Kubota Y, Mimura Y, Ogura S, Okada Y, Uhl GR, Takemura M. Psychotomimetic-like behavioral effects of memantine in the mouse. Biomed Pharmacother. 2018;100:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012;27:974-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | Muhonen LH, Lönnqvist J, Juva K, Alho H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 115. | Czarnecka K, Chuchmacz J, Wójtowicz P, Szymański P. Memantine in neurological disorders - schizophrenia and depression. J Mol Med (Berl). 2021;99:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 116. | Maj J, Rogóz Z. Synergistic effect of amantadine and imipramine in the forced swimming test. Pol J Pharmacol. 2000;52:111-114. [PubMed] [Cited in This Article: ] |
| 117. | Rogóz Z, Kubera M, Rogóz K, Basta-Kaim A, Budziszewska B. Effect of co-administration of fluoxetine and amantadine on immunoendocrine parameters in rats subjected to a forced swimming test. Pharmacol Rep. 2009;61:1050-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72:1313-1333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 119. | Refsgaard LK, Pickering DS, Andreasen JT. Investigation of antidepressant-like and anxiolytic-like actions and cognitive and motor side effects of four N-methyl-D-aspartate receptor antagonists in mice. Behav Pharmacol. 2017;28:37-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci. 1999;890:42-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 122. | Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology. 2001;40:1028-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 123. | Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 124. | Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 125. | Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 127. | Laje G, Perlis RH, Rush AJ, McMahon FJ. Pharmacogenetics studies in STAR*D: strengths, limitations, and results. Psychiatr Serv. 2009;60:1446-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Sun Q, Yuan F, Yuan R, Ren D, Zhu Y, Bi Y, Hu J, Guo Z, Xu F, Niu W, Ma G, Wu X, Yang F, Wang L, Li X, Yu T, He L, He G. GRIK4 and GRM7 gene may be potential indicator of venlafaxine treatment reponses in Chinese of Han ethnicity. Medicine (Baltimore). 2019;98:e15456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 129. | Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 130. | Chaki S, Ago Y, Palucha-Paniewiera A, Matrisciano F, Pilc A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology. 2013;66:40-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 131. | Chaki S. [The potential of group II metabotropic glutamate receptor antagonists as a novel antidepressant]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2012;32:219-226. [PubMed] [Cited in This Article: ] |
| 132. | Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409-2417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 133. | O'Mahony CM, Bravo JA, Dinan TG, Cryan JF. Comparison of hippocampal metabotropic glutamate receptor 7 (mGlu7) mRNA levels in two animal models of depression. Neurosci Lett. 2010;482:137-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Bradley SR, Uslaner JM, Flick RB, Lee A, Groover KM, Hutson PH. The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacol Biochem Behav. 2012;101:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Oriach CS, Robertson RC, Stanton C, Cryan JF, Dinan TG. Food for thought: the role of nutrition in the microbiota-gut–brain axis. Clin Nutr Exp. 2016;6:25-38. [Cited in This Article: ] |
| 136. | Bear TLK, Dalziel JE, Coad J, Roy NC, Butts CA, Gopal PK. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv Nutr. 2020;11:890-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 137. | Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010;64:832-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 138. | Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med. 2011;73:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 207] [Article Influence: 15.9] [Reference Citation Analysis (6)] |
| 139. | Ford PA, Jaceldo-Siegl K, Lee JW, Youngberg W, Tonstad S. Intake of Mediterranean foods associated with positive affect and low negative affect. J Psychosom Res. 2013;74:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 140. | Suzuki T, Miyaki K, Tsutsumi A, Hashimoto H, Kawakami N, Takahashi M, Shimazu A, Inoue A, Kurioka S, Kakehashi M, Sasaki Y, Shimbo T; J-HOPE study group (the Japanese study of Health; Occupation, and Psychosocial factors related Equity). Japanese dietary pattern consistently relates to low depressive symptoms and it is modified by job strain and worksite supports. J Affect Disord. 2013;150:490-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 141. | Sugawara N, Yasui-Furukori N, Tsuchimine S, Kaneda A, Tsuruga K, Iwane K, Okubo N, Takahashi I, Kaneko S. No association between dietary patterns and depressive symptoms among a community-dwelling population in Japan. Ann Gen Psychiatry. 2012;11:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 142. | Chocano-Bedoya PO, O'Reilly EJ, Lucas M, Mirzaei F, Okereke OI, Fung TT, Hu FB, Ascherio A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am J Clin Nutr. 2013;98:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 143. | Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1201] [Cited by in F6Publishing: 1339] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 144. | Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 849] [Cited by in F6Publishing: 923] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 145. | Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 665] [Article Influence: 55.4] [Reference Citation Analysis (2)] |
| 146. | Lim HS, Cha IT, Roh SW, Shin HH, Seo MJ. Enhanced Production of Gamma-Aminobutyric Acid by Optimizing Culture Conditions of Lactobacillus brevis HYE1 Isolated from Kimchi, a Korean Fermented Food. J Microbiol Biotechnol. 2017;27:450-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |