Published online Dec 19, 2021. doi: 10.5498/wjp.v11.i12.1301
Peer-review started: March 16, 2021
First decision: May 5, 2021
Revised: May 25, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: December 19, 2021
Suicide is a major public health problem. Worldwide, around 800000 people die by suicide every year. Suicide is a multifactorial disorder, with numerous environmental and genetic risk factors involved. Among the candidate genes, changes in the BDNF locus at the gene, epigenetic, mRNA, and protein expression levels have been implicated in psychiatric disorders, including suicidal behavior and completed suicides.
To investigate changes in BDNF methylation and expression of four alternative BDNF transcripts for association with completed suicide.
This case-control study included 42 unrelated male Caucasian subjects, where 20 were control subjects who died following acute cardiac arrest, and 22 were suicide victims who died by hanging. DNA and RNA were extracted from brain tissue (Brodmann area 9 and hippocampus) and from blood. DNA methylation and mRNA expression levels were determined by targeted bisulfite next-generation sequencing and reverse-transcription quantitative PCR. Statistical analysis was done by use of two-tailed Student’s t tests for two independent samples, and the Benjamini-Hochberg procedure was implemented for correction for multiple comparisons.
In DNA from brain tissue, there were no significant differences in BDNF methylation between the study groups. However, data showed significantly reduced DNA methylation of the BDNF region upstream of exon I in blood samples of suicide victims compared to the controls (5.67 ± 0.57 vs 6.83 ± 0.64, Pcorr = 0.01). In Brodmann area 9 of the brain of the suicide victims but not in their hippocampus, there was higher expression of BDNF transcript I-IX (NM_170731.4) compared to the controls (0.077 ± 0.024 vs 0.05 ± 0.013, P = 0.042). In blood, expression analysis for the BDNF transcripts was not feasible due to extensive RNA degradation.
Despite the limitations of the study, the obtained data further support a role for BDNF in suicidality. However, it should be noted that suicidal behavior is a multifactorial disorder with numerous environmental and genetic risk factors involved.
Core Tip: BDNF methylation analysis of brain tissues did not show differences between control subjects and suicide victims, although there was higher expression of BDNF transcript I-IX in Brodmann area 9 of the suicide victims. Furthermore, the data obtained from blood were interesting, especially in terms of the direction of the effects. Although due to the extensively degraded RNA in the blood, we were not able to confirm these effects on mRNA expression. Although suicide is a multifactorial disorder, our data overall further support the previously implicated role of BDNF in suicidality.
- Citation: Ropret S, Kouter K, Zupanc T, Videtic Paska A. BDNF methylation and mRNA expression in brain and blood of completed suicides in Slovenia. World J Psychiatr 2021; 11(12): 1301-1313
- URL: https://www.wjgnet.com/2220-3206/full/v11/i12/1301.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i12.1301
Suicide is a major public health problem. Globally, around 700000 people die by suicide every year. Indications are that for every completed suicide, there will have been more than 20 ‘unsuccessful’ suicide attempts[1]. Suicide is the final and extreme act in the continuum of intentional self-destruction by a suicidal subject. Suicidal behaviors are complex and heterogeneous, and they result from interactions between numerous environmental and genetic risk factors[2,3]. Indeed, part of the genetic component of suicide risk is determined by epigenetic factors. These are heritable and can be modified under environmental influences, which can result in altered gene expression without any changes to the DNA nucleotide sequence[3-5].
A number of candidate genes have already been shown to have associations with psychiatric disorders, including suicide and other related behaviors. Among these is a member of the neurotrophin family encoding BDNF[6,7]. BDNF is a nerve growth factor that has an important role in the development of the central nervous system as well as in the regulation of structural, synaptic, and morphological plasticity in adults[8]. BDNF acts through its binding to two distinct receptors: Neurotrophic tyrosine receptor kinase 2 and nerve growth factor receptor (also known as p75 neurotrophin receptor)[9].
BDNF is a secreted protein that is synthesized as a pre-proBDNF precursor. After removal of the signal peptide, proBDNF is proteolytically cleaved into the pro-peptide and the mature BDNF. However, not only the mature BDNF but also proBDNF and the released BDNF pro-peptide are functionally active. A role for the pro-peptide itself has only recently been acknowledged[9,10].
The human BDNF locus spans approximately 70 kb of chromosome 11 and has a complex structure. It includes 11 exons (I-V, Vh, VI-VIII, VIIIh, IX), of which 9 have functional promotors and 4 contain alternative splice sites. The sequence that encodes the BDNF precursor form (i.e. pre-proBDNF) is located within exon IX[11], while the other exons are not translated. The complexity of the BDNF locus enables specific and precise temporal and tissue regulation of BDNF expression as well as regulation of its expression in response to the environment[11,12].
Given the roles of BDNF during development of the nervous system and regulation of brain plasticity in adults, it is not surprising that BDNF is emerging as one of the key factors in the development of mental disorders. Indeed, genetic[13] and epigenetic changes in the BDNF locus[14] and changes in BDNF mRNA and/or protein expre
Numerous studies[19-26] have defined links between epigenetic processes and mental disorders, including for nonfatal suicidal behavior, with DNA methylation established as the most studied mammalian epigenetic mechanism. These studies have shown that BDNF methylation in blood of subjects with mental disorders is usually higher than for that of control subjects. However, studies on BDNF methylation specifically in completed suicides are rare. Targeted and whole-genome methylation analyses were used in two studies that showed higher BDNF methylation in the brains of suicide victims compared to controls[14,27]. Interestingly, a recent study from our group where we also used a whole-genome methylation approach did not show the BDNF locus as differentially methylated in the brains of suicide victims when compared to controls[28].
In contrast to these few studies that have explored BDNF methylation in completed suicides, studies that have examined the expression of BDNF in suicide victims are more abundant. Some of these studies involved subjects who had been diagnosed with psychiatric disorders prior to dying by suicide. Nonetheless, the prevailing majority of these studies has shown that BDNF expression at the mRNA and/or protein levels is downregulated in several brain regions of suicide victims[14-18,29-31]. Keller et al[14] demonstrated a correlation between BDNF methylation and its expression at the mRNA level.
In terms of BDNF expression at the mRNA level, the vast majority of studies have examined total BDNF mRNA levels. Two studies, however, explored BDNF expression with regards to alternative mRNAs transcribed from the BDNF locus[30,31]. Wong et al[30] investigated the four most abundant and best characterized BDNF alternate transcripts (i.e. I-IX, II-IX, IV-IX, VI-IX) and showed significant upregulation of BDNF transcript I-IX (5’-exon = exon I) in patients with schizophrenia who died by suicide. They also reported similar trends for BDNF transcripts II-IX and IV-IX[30]. Reinhart et al[31] instead examined total BDNF mRNA levels and the levels of alternative mRNAs transcribed from the BDNF locus in several brain regions of patients with major depression disorder, bipolar disorder, and schizophrenia. Among all of these patients, about 37 % had died by suicide. The total BDNF mRNA levels did not differ from the controls across these brain regions and disease states. However, interestingly, they showed differences in expression of the alternative BDNF mRNAs[31,32].
To date, the studies that have explored methylation and/or expression of the BDNF locus for association with suicide at different levels have been performed with brain tissue only, with no similar approaches applied to blood. However, these data have shown that despite the multifactorial and polygenic nature of suicide and related behaviors, disturbances in the BDNF locus might make important contributions to the development of psychiatric disorders, including suicide and related behaviors. Therefore, further studies are needed to understand its role better.
Slovenia is a small European country that has a disproportionately high suicide rate. According to the latest suicide figures available from the World Health Organization in 2016, Slovenia ranked 13th highest globally and 9th highest in Europe. Despite the previous decline in the suicide rate in Slovenia from 2007, suicide still remains a serious public health problem. Combined with previous studies from our and other research groups, this encouraged us to investigate methylation of the BDNF gene and its expression levels in both brain tissue and blood from suicide victims and control subjects in the Slovenian population.
This study included 42 unrelated male Caucasian subjects, where 20 were control subjects who died following acute cardiac arrest, and 22 were suicide victims who died by hanging. The patient data, brain tissue samples, and blood samples were collected during regular autopsy procedures in 2014 and 2015 at the Institute of Forensic Medicine, Faculty of Medicine, University of Ljubljana (Ljubljana, Slovenia). The samples were stored at –80 °C prior to being processed.
The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (Approval N° 47/12/12).
BDNF methylation: For the samples from both the control subjects and the suicide victims, the differences in methylation levels of five BDNF regions of interest (ROIs) in or near the CpG islands (regions with high frequency of CpG) were studied by next-generation sequencing (NGS) of bisulfite-converted DNA. The ROIs for methylation of BDNF were I1, I2, II, IV, and VI, as shown in Supplementary Figure 1, relative to the positions of the BDNF CpG islands and exons. The chromosomal coordinates and further details for the nucleotide sequences are given in Supplementary Figure 2.
Genomic DNA was extracted from 25 mg to 30 mg liquid nitrogen pulverized brain tissue from Brodmann area 9 (BA9) and hippocampus and from 200 μL blood from the right femoral vein. The DNA was extracted (QIAmp DNA mini kits; Qiagen, United States), according to the manufacturer’s instructions, with the quantities and qualities of the DNA determined spectrophotometrically using a microplate reader (Synergy H4 Hybrid; BioTek, United States).
The DNA (1.0 μg) was subjected to sodium bisulfite conversion (EpiTect bisulfite kits; Qiagen, United States), according to the manufacturer’s instructions. Then 20 ng to 40 ng of the bisulfite-converted DNA was used as templates for amplicon preparation for library construction. Amplicons were prepared by two rounds of PCR, according to the universal tailed experimental design and in such a way that sequencing was bidirectional (Guidelines for Amplicon Experimental Design, Roche, April 2014). In the first round of PCR, the BDNF ROIs were amplified with simultaneous addition of universal sequences. The specific parts of the fusion primers were designed using the Methyl Primer Express v1.0 software (Applied Biosystems, United States) and are listed in Supplementary Table 1. The universal sequences of the products from the first round of PCR were targeted in the second round of PCR by fusion primers that were labeled by the NGS system (454 Junior; Roche, Germany) adaptor and Multiplex Identifier sequences for sample identification. The reaction mixtures and cycling conditions for first and second rounds of PCR are given in Supplementary Tables 2 and 3, respectively. After each round, the samples were checked for the correct length of the amplicons on 2% agarose gels.
The amplicons from the second round of PCR were purified (Agencourt AMPure XP PCR purification system; Beckman Coulter, United States), with the purities determined on 2% agarose gels. The purified amplicons were precisely quantified using dsDNA assay kits (Quant-iT PicoGreen; Thermo Fisher Scientific, United States). The procedures for the purification and quantification of the amplicons was according to the Amplicon Library Preparation Manual (Roche, April 2014). Each amplicon solution was diluted to 109 amplicon molecules/μL. The amplicon library was prepared by pooling equal volumes of these diluted amplicon solutions. Then the library was diluted to the final concentration of 106 amplicon molecules/μL, with RNA/DNA quantification on a bioanalyzer (High Sensitivity DNA kits; Agilent Technologies, United States). The library was prepared and quantified according to Amplicon Library Preparation Manual (Roche, April 2014). The library was clonally amplified by emulsion PCR (emPCR Amplification Manual Lib-A; GS Junior and GS Junior+ Series; Roche, April 2014).
The libraries prepared from brain tissue (i.e. BA9, hippocampus) and from blood were sequenced in two separate runs on an NGS system (454 Junior; Roche, Germany), according to Sequencing Manual (GS Junior Titanium Series; Roche, January 2013).
BDNF mRNA expression: The expression levels of BDNF transcripts NM_170731.4, NM_170732.4, NM_170733.3, and NM_170735 were determined by reverse transcription-quantitative PCR for the samples from the control subjects and the suicide victims.
Total RNA was extracted from 10 mg to 15 mg liquid nitrogen pulverized tissue from the BA9 and hippocampus brain regions and from 4 mL to 7 mL of blood from the right femoral vein using TRI Reagent solution (Sigma-Aldrich, Germany), according to the manufacturer instructions. RNA quantity and quality were determined spectrophotometrically (NanoDrop ND-1000; Thermo Scientific, United States) and by determination of the RNA Integrity Number on a bioanalyzer (Agilent) using kits (RNA 6000 nano kits; Agilent Technologies, United States).
Then, 3 μg total RNA (in 20 μL) from BA9, hippocampus, and blood of each subject were treated with DNase I and then reverse transcribed. The DNase I reaction mixture (total volume, 25 μL) also included 2.5 μL 10× buffer (Cat. #04 716 728 001; Roche, Germany), 0.2 μL DNase I (Cat. #04 716 728 001; Roche, Germany), and 2.3 μL double-distilled water. The treatment (thermocycler: GenAmp 2700; Applied Biosystems, United States) was for 10 min at 30 °C for DNA degradation, followed by 10 min at 75 °C for DNase I inactivation. The reverse transcription (Transcriptor Universal cDNA Master; Roche, Germany) reaction mixture (final volume, 40 μL) contained 25 μL DNase I treated RNA solution, plus 8 μL 5× buffer (Cat. #05 893 151 001; Roche, Germany), 2 μL 20× reverse transcriptase (Cat. #05 893 151 001; Roche, Germany), and 5 μL double-distilled water. The temperature profile for the reverse transcription reaction (thermocycler: GenAmp 2700; Applied Biosystems, United States) was: primer annealing, 5 min at 25 °C; reverse transcription, 10 min at 55 °C; inactivation, 5 min at 85 °C; with cooling to 4 °C.
The primers used for the reverse transcription-quantitative PCR were either designed using an online tool (Primer-BLAST; NCBI) or predesigned primers (Assay Design Centre, Roche, Germany). The primer sequences, efficiencies, and product lengths are given in Supplementary Table 4. Among the tested candidate reference genes, those encoding beclin1 and dynactin subunit 2 showed the greatest expression stability in these samples and were used for normalization. The primer efficiencies were calculated based on validation experiments on two-fold and five-fold serial dilutions of cDNA derived from a mix of RNA from 10 suicide victims and 10 control subjects.
Quantitative PCR reactions were performed in 5 μL volumes in triplicate (ViiA7 real-time PCR system; Applied Biosystems, United States), as 0.75 μL cDNA sample, 1.15 μL double-distilled water, 2.5 μL SYBR Select Master Mix (Applied Biosystems, United States), and 0.6 μL of each forward and reverse primer (2.5 mM stock). The conditions were the same for all of the quantitative PCR cycling, except for annealing and extension. For the reference genes and for the BDNF transcripts, this followed: UDG activation, 2 min at 50 °C; polymerase activation, 10 min at 95 °C; denaturation, 15 s at 95 °C; with 40 cycles of annealing and extension for 1 min at 60 °C (beclin1, dynactin subunit 2, I-IX, IIc-IX) or 59 °C (IV-IX), or 40 cycles of annealing for 15 s at 59 °C with extension for 1 min at 72 °C (IXabcd); with all followed by the melting curve for 15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C; and finally cooling to 4 °C.
BDNF methylation: After processing the raw sequencing data, quality filtering was applied. Due to an abundance of stretches of long homopolymeric regions, which are characteristic of bisulfite-converted DNA, the reads were quality filtered using bisulfite sequencing adjusted filter settings (Customer Support; Roche, Mannheim, Germany). In the read clean-up, adapter sequences were removed using the Cutadapt v1.11 software[33]. The reads were demultiplexed according to the study subjects (i.e. Multiplex Identifier_Forward#_ Multiplex Identifier_Reverse# combination) using the SFF tools and according to the BDNF ROIs, using the Cutadapt v1.11 software[33]. For sequence alignment, the FASTA format read files together with a file containing the bisulfite unconverted reference sequences were loaded into BiQ Analyzer HT[34] with the following settings: Minimal sequence identity, 0.80; minimal conversion rate, 0.85; and maximal fraction of unrecognized sites, 0.15. The results of the alignment were exported to MS Excel 2010, as the files containing numbers of loaded, filtered out, and exported reads. For each of the BDNF ROIs of each study, the following were determined: Subject mean conversion rate, mean methylation rate, and methylation status of individual CpGs in each read. Where the number of exported reads for a BDNF ROI of a subject was ≤ 20, the reads for the particular BDNF ROIs of this subject were excluded from any further analysis.
After testing for normal distributions of the data, the differences in the mean methylation levels of the BDNF ROIs, and the differences in the methylation frequencies of the individual CpGs for the ROIs between the suicide victims and the controls were determined using two-tailed Student’s t tests for two independent samples. Statistical analysis and figure construction were carried out using the GraphPad Prism v6.0 software (GraphPad Software, United States). The Benjamini-Hochberg procedure[35] was implemented for correction for multiple comparisons, using a calculation file obtained online: BenjaminiHochberg.xlsx (https://github.com/abbiepopa/DPTB_RTOverallEG).
BDNF mRNA expression: The expression of the BDNF transcripts was determined by the relative quantification method. The threshold cycle values were transformed into mRNA quantities, taking efficiency into account. For each sample, the means of the triplicate measurements were calculated. Where the standard error within a triplicate was ≥ 20%, the replicate contributing the most to the standard error was excluded, and only the duplicate was considered for further analyses. Samples with standard errors within the triplicates of ≥ 40% were automatically excluded from further analyses. The experimental data were normalized to the geometric means of the quantities of beclin1 and dynactin subunit 2 mRNAs[36].
After testing the values obtained for normality of distribution, differences in BDNF transcript expression between control subjects and suicide victims were determined using two-tailed Student’s t tests for two independent samples. The analyses were carried out using MS Excel 2010 and the GraphPad Prism v6.0 software (GraphPad Software, United States).
The ages and full background clinical data for the individual control subjects and suicide victims are given in Supplementary Table 5. Two-tailed Student t tests for independent samples showed significant differences in mean ages (± standard deviation) between the controls (54.6 ± 7.7 years) and suicide victims (44.0 ± 12.3 years), with the controls about 10 years older on average (P = 0.002). There were no significant differences in post-mortem intervals between these two study groups (28.2 ± 23.0 h vs 27.7 ± 13.8 h, P = 0.935).
The psychiatric diagnosis (where applicable) and post-mortem toxicology were obtained for each subject of both of the study groups. Among the control subjects, one had been previously diagnosed with schizophrenia and tested positive for psychoactive drugs and ethanol. One control subject without psychiatric diagnosis was also positive for psychoactive drugs, and six were positive for ethanol exclusively (Supplementary Table 5). Six suicide victims were previously diagnosed with one or more psychiatric disorders or symptoms, including: Schizophrenia, previous suicide attempt, depression disorder, bipolar disorder, adjustment disorder, anxiety, and alcohol dependence syndrome. Five of these suicide victims tested positive for psychoactive drugs, and two of them were positive for ethanol. The suicide victim with alcohol dependence syndrome was positive for ethanol. One suicide victim without psychiatric diagnosis was positive for psychoactive drugs. Eleven suicide victims were positive for ethanol exclusively.
The DNA methylation levels of the five BDNF regions for BA9, hippocampus, and blood of the control subjects and suicide victims were analyzed by targeted NGS of bisulfite-converted DNA. The mean bisulfite conversion rates and number of study subjects with sufficient numbers of reads in each of the studied tissues for each of the BDNF regions are given in Supplementary Table 6. Additionally, the number of reads for the post-filtration steps of each of the tissues studied are given in SupplementaryTable 7.
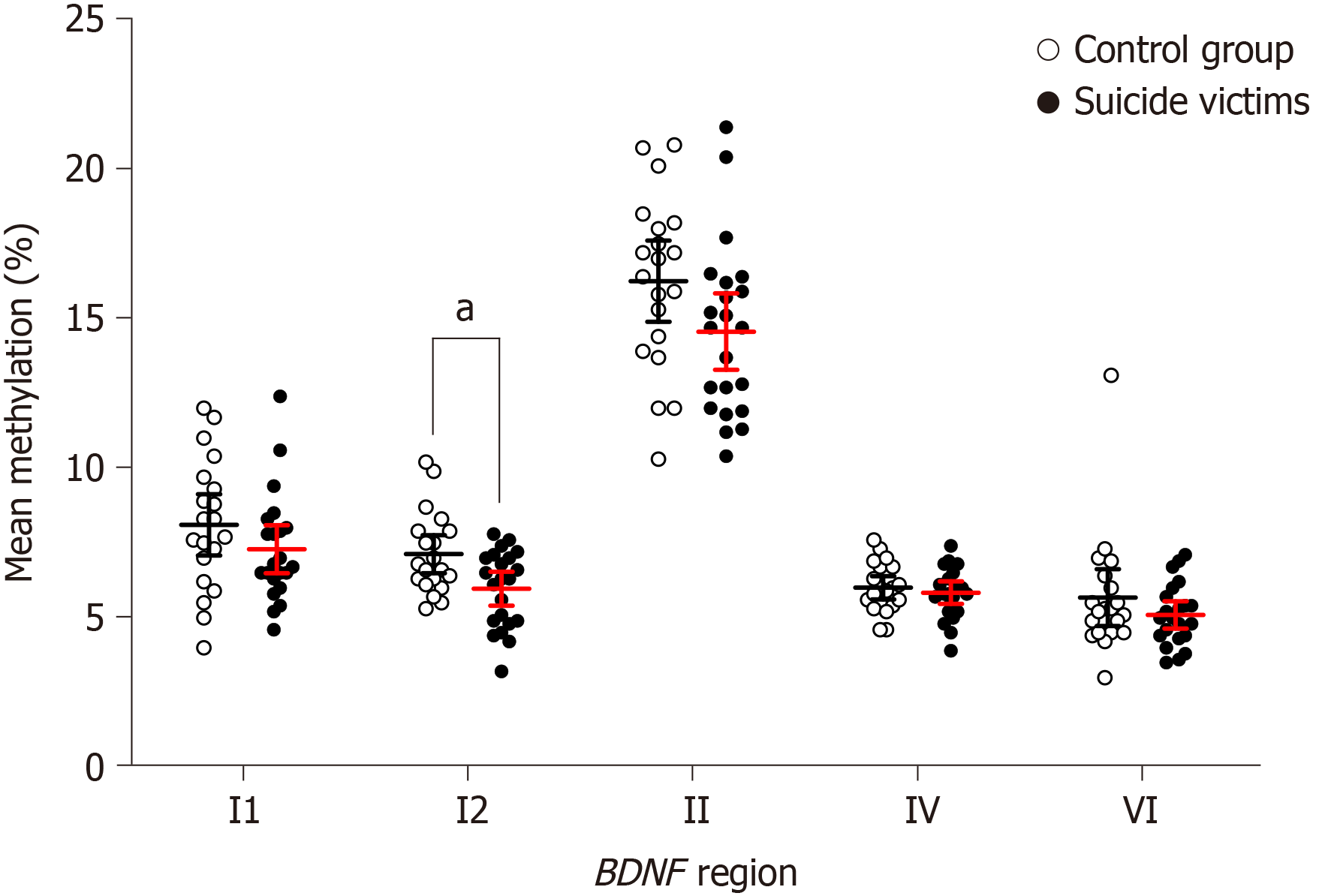
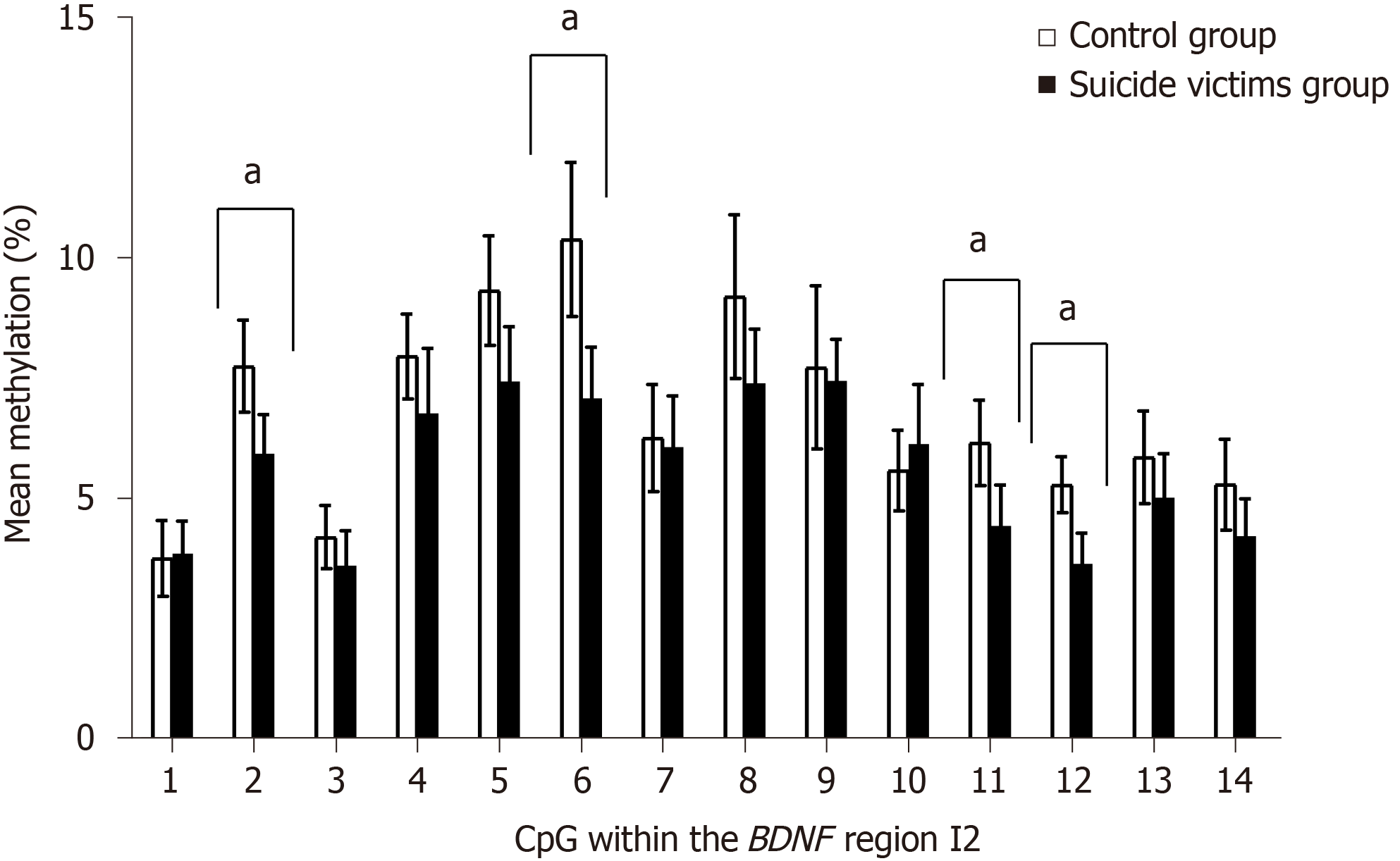
Comparison of the control subjects and the suicide victims showed no significant differences in the methylation levels of the BDNF ROI (i.e. I1, I2, II, IV, VI) for both BA9 (Supplementary Figure 3) and hippocampus (Supplementary Figure 4, Table 1, and Supplementary Tables 8 and 9). In contrast, for blood of suicide victims compared to controls, there were significantly lower mean methylation levels for BDNF region I2 [t(40) = 2.832, Pcorr = 0.01] (Figure 1 and Table 1). Closer inspection of the methylation within BDNF region I2 revealed significant differences for the methylation levels between the study groups for four of the CpGs. Compared to the controls, the suicide victims showed lower methylation of CpG 2 [t(40) = 3.044, Pcorr = 0.011], CpG 6 [t(40) = 3.662, Pcorr = 0.007], CpG 11 [t(40) = 2.923, Pcorr = 0.014], and CpG 12 [t(40) = 3.921, Pcorr = 0.004] (Figure 2 and Supplementary Table 10). However, there were no significant differences in the methylation levels for BDNF regions I1, II, IV, and VI in the blood of the controls and suicide victims (Supplementary Table 10).
| Tissue | Study group | BDNF region (% methylated) | ||||
| I1 | I2 | II | IV | VI | ||
| Brain-BA9 | C | 4.55 ± 0.45 | 4.43 ± 0.39 | 8.48 ± 0.91 | 3.81 ± 0.32 | 3.37 ± 0.30 |
| SV | 4.15 ± 0.56 | 4.28 ± 0.32 | 8.78 ± 1.09 | 4.03 ± 0.46 | 3.05 ± 0.23 | |
| P value | 0.260 | 0.557 | 0.667 | 0.418 | 0.090 | |
| Brain-Hippocampus | C | 4.76 ± 0.48 | 4.16 ± 0.44 | 10.19 ± 0.51 | 3.74 ± 0.46 | 3.46 ± 0.34 |
| SV | 4.40 ± 0.39 | 4.60 ± 0.43 | 10.30 ± 1.10 | 3.66 ± 0.37 | 3.52 ± 0.31 | |
| P value | 0.222 | 0.147 | 0.851 | 0.772 | 0.774 | |
| Blood | C | 7.81 ± 1.03 | 6.83 ± 0.64 | 15.95 ± 1.36 | 5.71 ± 0.39 | 5.38 ± 0.95 |
| SV | 7.00 ± 0.80 | 5.67 ± 0.57 | 14.26 ± 1.2 | 5.54 ± 0.38 | 4.80 ± 0.46 | |
| P value | 0.198 | 0.007a | 0.067 | 0.521 | 0.245 | |
Expression levels of four transcripts from the BDNF locus in BA9, hippocampus, and blood were examined in control subjects and suicide victims by reverse transcription-quantitative PCR. The quality of the isolated RNA and mean quantification cycle values for each condition are given in Supplementary Tables 11 and 12. In blood BDNF transcript expression analysis was not feasible due to extensive degradation of the RNA.
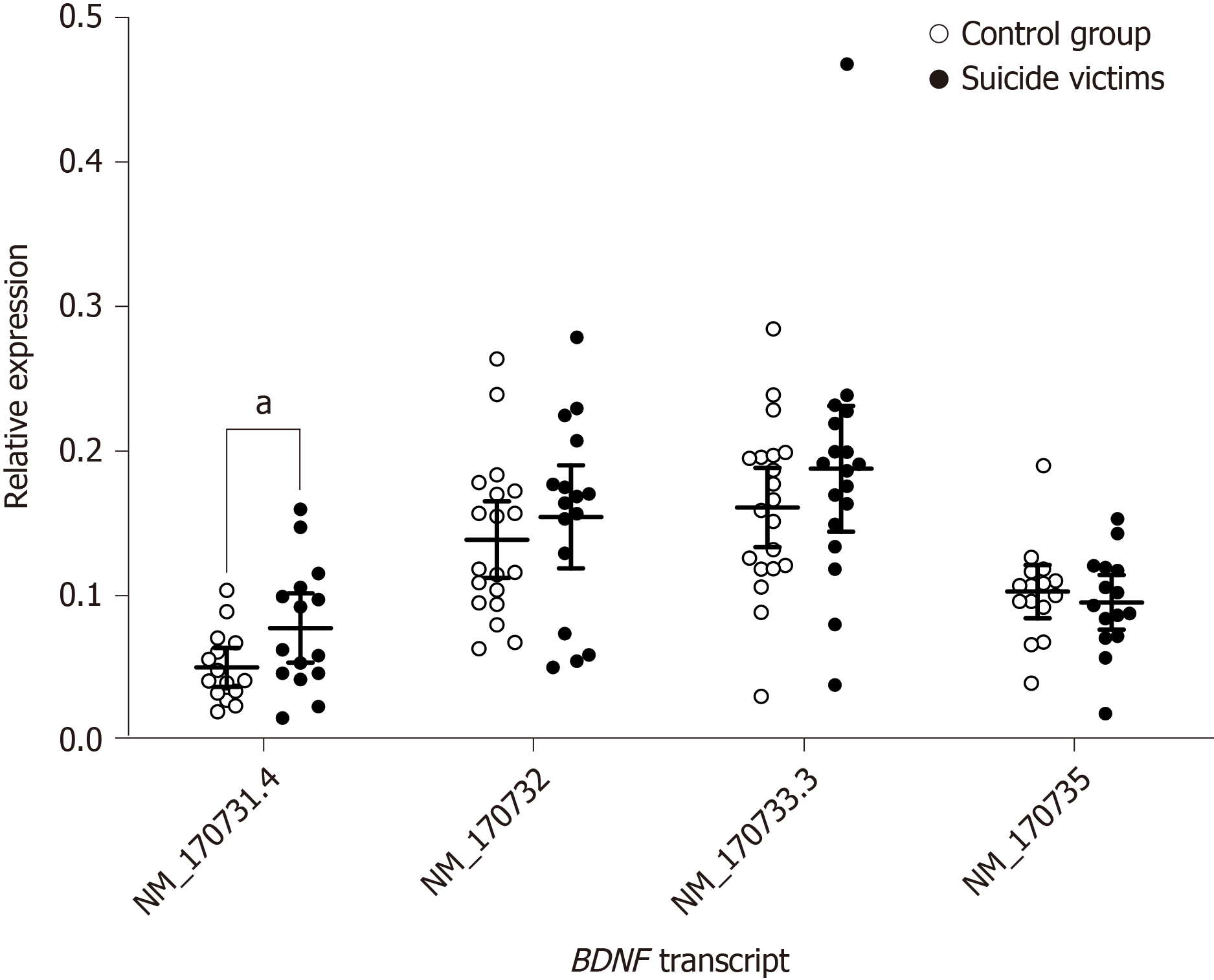
When compared to control subjects, the analysis for the brain region BA9 of the suicide victims showed slightly, but significantly, higher expression of the BDNF transcript NM_170731.4 [t(30) = 2.130, P = 0.042; 95% confidence interval: 0.001–0.054] (Figure 3 and Table 2). The analysis of the expression of other BDNF transcripts (i.e. NM_170732, NM_170733.3, NM_170735) in region BA9 showed no significant differences between study groups (Figure 3 and Table 2). In the hippocampus, none of the BDNF transcripts was significantly differentially expressed between the study groups (Supplementary Figure 5 and Table 2).
| Tissue | BDNF transcript | Total | Subjects | P value | 95%CI | |||
| Analyzed | Controls | Suicide victims | ||||||
| n (%) | n (%) | Relative expression | n (%) | Relative expression | ||||
| BA9 | NM_170731.4 | 30 | 15 | 0.050 ± 0.013 | 15 | 0.077 ± 0.024 | 0.042a | 0.001–0.054 |
| NM_170732.4 | 35 | 19 | 0.139 ± 0.026 | 16 | 0.154 ± 0.036 | 0.451 | -0.026–0.058 | |
| NM_170733.3 | 38 | 20 | 0.162 ± 0.027 | 18 | 0.188 ± 0.044 | 0.270 | -0.022–0.075 | |
| NM_170735 | 30 | 15 | 0.103 ± 0.019 | 15 | 0.095 ± 0.019 | 0.553 | -0.033–0.018 | |
| Hippocampus | NM_170731.4 | 36 | 16 | 0.342 ± 0.117 | 20 | 0.312 ± 0.069 | 0.630 | -0.154–0.094 |
| NM_170732.4 | 35 | 17 | 0.420 ± 0.120 | 18 | 0.369 ± 0.067 | 0.428 | -0.181–0.079 | |
| NM_170733.3 | 37 | 18 | 0.466 ± 0.111 | 19 | 0.475 ± 0.061 | 0.878 | -0.111–0.129 | |
| NM_170735 | 36 | 18 | 0.399 ± 0.092 | 18 | 0.341 ± 0.073 | 0.308 | -0.171–0.056 | |
In this study, we examined BDNF methylation and the expression levels of BDNF transcripts in brain (i.e. BA9, hippocampus) and blood from control subjects and suicide victims from the Slovenian population, which is known to have a high risk of suicidality. Despite the decrease in Slovenian suicide rates in the past decade (SI-STAT Data Portal; Supplementary Figure 6), the number of deaths due to suicide still remains concerning. In Slovenia, roughly 80% of suicide victims are men, and the most commonly used suicide method is suffocation by hanging[37].
To maximize the homogeneity of our study groups, tissue samples were collected only from male controls who died by acute cardiac arrest (age range: 33-64 years) and from male suicide victims who died by hanging (age range: 29-60 years). The difference in the mean ages between the study groups was not unexpected (controls were 10.6 years older), as the control group was represented by subjects who passed away due to a reason more commonly associated with an older population. Due to the small sample size, the suicide victims were not subgrouped in terms of their comorbidities and/or medications.
NGS of bisulfite-converted DNA was used to determine the methylation rates across the five BDNF regions, plus the methylation rates of individual CpGs within these regions. Methylation was assayed for the BA9 of the prefrontal cortex, the hippocampus, and blood and compared between controls and suicide victims.
For BA9 and hippocampus, the present study did not show any significant differences in BDNF methylation between the controls and suicide victims. Our previous whole-genome methylation study showed several differentially methylated CpGs in BA9 and hippocampus of suicide victims compared to controls[28]. However, none of these differentially methylated CpGs was located within or in the vicinity of the BDNF locus, which is in agreement with the present study. In contrast, other studies have shown increased BDNF locus methylation in brains of suicide victims[14,27], although they explored different brain areas, which would explain these discrepancies.
Comparison of the BDNF locus methylation for blood of the controls and suicide victims instead showed BDNF I2 (upstream of exon I) with significantly reduced methylation for suicide victims. Also, more specifically within BDNF I2, 4 of the 14 CpGs examined were significantly hypomethylated in blood of these suicide victims. We have been unable to find similar studies to date that have the BDNF methylation of DNA from blood in completed suicides. Previous studies on blood from elderly people and patients with psychiatric or other conditions who also showed suicidal behavior (i.e. suicide ideation, suicide attempts) have reported increased BDNF methylation over the controls[21-25]. To the best of our knowledge, there are only two studies to date that have reported decreased BDNF methylation in blood of psychiatric patients compared to controls. However, in these two studies, none of the subjects showed suicidal behavior[19,26].
Quantitative PCR was used to determine the expression levels of four alternative BDNF transcripts in brain tissue (BA9, hippocampus) and blood of these controls and suicide victims: NM_170731.4, NM_170732, NM_170733.3, and NM_170735.
In BA9, relative to the controls, the BDNF transcript NM_170731.4 (5’-exon = exon I) showed slightly, but significantly, higher expression in the suicide victims. With regard to psychiatric disorders, there are only two studies to date that have examined expression of some of the individual BDNF mRNA transcripts[30,31]. Wong et al[30] showed higher expression of BDNF transcript I-IX (5’-exon = exon I) compared to control subjects in dorsolateral prefrontal cortex in patients with schizophrenia who died by suicide. However, as almost half of their schizophrenia suicide victims tested positive for antidepressants, the authors could not rule out effects of the drugs on the experimental outcome[30]. Reinhart et al[31] revealed higher expression over controls for transcript IIc-IX (5’-exon = exon IIc) in striatum of subjects with major depressive disorder. Their patient groups (i.e. schizophrenia, bipolar disorder, major depressive disorder) also included subjects who died by suicide (approximately 40 %). Interestingly, the total BDNF mRNA levels did not differ in any of these disease states compared to controls in any of the brain regions they studied (i.e. dorsolateral prefrontal cortex, striatum, hippocampus)[31].
In the present study, determination of the expression of these BDNF transcripts in blood could not be carried out. As shown in Supplementary Table 11, blood RNA was significantly degraded as reflected in the low RNA integrity numbers. Low quality RNA was further indicated by high quantification cycles for the reference genes in comparison to the brain tissue (Supplementary Table 12). It should be noted that the brain and blood tissue samples were frozen at -80 °C, and the nucleic acids were not immediately extracted from the tissues. Freezing and thawing of blood samples causes extensive cell lysis, which leads to poor RNA recovery, while brain tissue can be frozen and thawed without significant effects on RNA recovery[38].
Nonetheless, a number of past studies that explored total BDNF mRNA levels have shown decreased expression in the postmortem brain of suicide victims and in blood of psychiatric patients that attempted suicide[14,15,17,18,39].
Several limitations of this study can be noted. First, the sample size was relatively small (n = 42), which limits the power of the study. Moreover, only tissue samples from males were collected. Therefore, the results might not be generalizable to the wider (female) population. Further, for the group of suicide victims, the lack of subgrouping, the exclusion of subjects with comorbid psychiatric disorders, and the use of psychoactive drugs might represent confounding factors.
A hypothesis-driven approach was used here, which focused on one candidate gene. Psychiatric disorders, and also suicide and related behaviors, are multifactorial disorders with numerous interacting genetic and environmental risk factors involved. However, the choice to study the BDNF gene was based on previous studies that had implicated its involvement in the pathogenesis of psychiatric disorders, including suicide.
Considering the DNA methylation approach used, despite preservation of the methylation patterns during bisulfite treatment of DNA, this reaction does not discriminate between methylation and hydroxymethylation of cytosines. Indeed, recent studies that have used oxidative bisulfite NGS have shown altered gene hydroxymethylation patterns in the brain of depressed patients who died by suicide[40,41]. DNA methylation and gene expression are not only tissue specific but also cell type specific. Thus, for the brain analysis, the tissue here included several cell types of particular brain regions (i.e. within BA9 and hippocampus). Hence, the data obtained from the brain tissues represent BDNF methylation and BDNF mRNA expression across all cell types from these brain regions. We also did not explore total BDNF mRNA expression in the present study sample. Finally, due to substantial RNA degradation, we were not able to obtain data on BDNF transcript expression in blood of these control subjects and suicide victims.
To the best of our knowledge, this is the first study that aimed at exploring BDNF locus methylation and the expression of four BDNF transcripts in brain and peripheral blood in the same cohorts. This was also carried out in a population with a high suicide rate. Despite this, BDNF methylation analysis of the brain tissues did not show differences between the study groups. The higher observed expression of BDNF transcript I-IX in BA9 of the suicide victims should be taken with caution, as this barely reached statistical significance. However, the data obtained from blood are interesting, especially in terms of the direction of the effects, although due to the extensively degraded blood RNA we were not able to confirm these effects on mRNA expression. Finally, although the present study and data from a number of other studies implicate BDNF in suicidality, it must be kept in mind that suicide is a multifactorial disorder with numerous environmental and genetic risk factors involved.
Suicidal behavior is a complex behavior with multifactorial etiology. Despite the large body of work, the full mechanism of suicidal behavior is not known. There are however strong indicators that changes in epigenetic mechanisms, specifically DNA methylation, can be an important factor.
Brain derived neurotrophic factor, BDNF, plays an important role in brain plasticity, and therefore it could be involved in modulation of suicidal behavior. Molecular-genetic data from a population with a high suicide rate could contribute to deeper understanding of the biological background of suicide.
The objective of our study was to investigate BDNF at two levels: DNA methylation and gene expression. As DNA methylation and gene expression can be highly tissue specific, we included two different brain regions and also blood as a peripheral tissue that is more easily accessible.
Altogether, 42 subjects were included in the study (20 control subjects and 22 suicide victims). Samples of brain (hippocampus and Brodmann area 9) and blood were obtained during routine autopsy. We used targeted bisulfite sequencing to assess the DNA methylation level of five BDNF regions of interest (I1, I2, II, IV, and VI), and quantitative PCR to determine gene expression of four BDNF transcripts.
When comparing suicide victims and control group, we observed no significant changes in BDNF DNA methylation level in the brain. Changes were observed in blood, where suicide victims exhibited lower mean DNA methylation level of BDNF region I2 compared to the control group. In gene expression analysis, one BDNF transcript (NM_170731.4.) was upregulated in Brodmann area 9 of suicide victims compared to the control group.
Due to tissue associated limitation, a complete insight into BDNF changes was not possible, namely inspection of blood BDNF expression level. Still, we observed changes both in DNA methylation level in blood and gene expression in brain, indicating the possible association of BDNF with suicidal behavior.
Data from this study was obtained from a Slovenian population, which has a high suicide risk. The findings are thus an important contribution to a better understanding of the biological basis of suicidal behavior and the involvement of neurotrophic factors such as BDNF.
The authors would like to thank the Institute of Forensic Medicine in the Faculty of Medicine of the University of Ljubljana, Slovenia for long term collaboration, and to Ms. Anka Hotko for assistance in DNA isolation and bisulfite conversion. The authors would like to thank Dr. Christopher Berrie and Dr. John Hancock for critical appraisal and scientific English editing of the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Slovenian Biochemical Society; Slovenian Neuroscience Association.
Specialty type: Psychiatry
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oltra E, Soriano-Ursúa MA S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | WHO. Suicide prevention. [cited 27 October 2021]. Available from: https://www.who.int/health-topics/suicide#tab=tab_1. [Cited in This Article: ] |
| 2. | Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1438] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 3. | Turecki G, Ernst C, Jollant F, Labonté B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Jeltsch A. The application of next generation sequencing in DNA methylation analysis. Genes (Basel). 2010;1:85-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Zheleznyakova GY, Cao H, Schiöth HB. BDNF DNA methylation changes as a biomarker of psychiatric disorders: literature review and open access database analysis. Behav Brain Funct. 2016;12:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Lutz PE, Mechawar N, Turecki G. Neuropathology of suicide: recent findings and future directions. Mol Psychiatry. 2017;22:1395-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Dwivedi Y. Brain-derived neurotrophic factor and suicide pathogenesis. Ann Med. 2010;42:87-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 9. | Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 898] [Cited by in F6Publishing: 941] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 10. | Popova NK, Naumenko VS. Neuronal and behavioral plasticity: the role of serotonin and BDNF systems tandem. Expert Opin Ther Targets. 2019;23:227-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 506] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 12. | Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012;17:584-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | González-Castro TB, Salas-Magaña M, Juárez-Rojop IE, López-Narváez ML, Tovilla-Zárate CA, Hernández-Díaz Y. Exploring the association between BDNF Val66Met polymorphism and suicidal behavior: Meta-analysis and systematic review. J Psychiatr Res. 2017;94:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 592] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 17. | Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, Inoue T, Kusumi I, Koyama T, Tsuchiyama K, Terao T. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. 2011;6:e23881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 20. | Ikegame T, Bundo M, Sunaga F, Asai T, Nishimura F, Yoshikawa A, Kawamura Y, Hibino H, Tochigi M, Kakiuchi C, Sasaki T, Kato T, Kasai K, Iwamoto K. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci Res. 2013;77:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Kim JM, Kang HJ, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. Association of BDNF promoter methylation and genotype with suicidal ideation in elderly Koreans. Am J Geriatr Psychiatry. 2014;22:989-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Kang HJ, Kim JM, Lee JY, Kim SY, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. BDNF promoter methylation and suicidal behavior in depressive patients. J Affect Disord. 2013;151:679-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Roy B, Shelton RC, Dwivedi Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J Psychiatr Res. 2017;89:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Kim JM, Kang HJ, Kim SY, Kim SW, Shin IS, Kim HR, Park MH, Shin MG, Yoon JH, Yoon JS. BDNF promoter methylation associated with suicidal ideation in patients with breast cancer. Int J Psychiatry Med. 2015;49:75-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Kang HJ, Bae KY, Kim SW, Shin IS, Hong YJ, Ahn Y, Jeong MH, Yoon JS, Kim JM. BDNF Methylation and Suicidal Ideation in Patients with Acute Coronary Syndrome. Psychiatry Investig. 2018;15:1094-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Xu X, Ji H, Liu G, Wang Q, Liu H, Shen W, Li L, Xie X, Zhou W, Duan S. A significant association between BDNF promoter methylation and the risk of drug addiction. Gene. 2016;584:54-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Schneider E, El Hajj N, Müller F, Navarro B, Haaf T. Epigenetic Dysregulation in the Prefrontal Cortex of Suicide Completers. Cytogenet Genome Res. 2015;146:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Kouter K, Zupanc T, Videtič Paska A. Genome-wide DNA methylation in suicide victims revealing impact on gene expression. J Affect Disord. 2019;253:419-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Maheu ME, Davoli MA, Turecki G, Mechawar N. Amygdalar expression of proteins associated with neuroplasticity in major depression and suicide. J Psychiatr Res. 2013;47:384-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169:1071-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Reinhart V, Bove SE, Volfson D, Lewis DA, Kleiman RJ, Lanz TA. Evaluation of TrkB and BDNF transcripts in prefrontal cortex, hippocampus, and striatum from subjects with schizophrenia, bipolar disorder, and major depressive disorder. Neurobiol Dis. 2015;77:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | WHO. Mental health. [cited 5 December 2020]. Available from: https://www.who.int/data/gho/data/themes/mental-health. [Cited in This Article: ] |
| 33. | Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13233] [Cited by in F6Publishing: 13582] [Article Influence: 1044.8] [Reference Citation Analysis (0)] |
| 34. | Lutsik P, Feuerbach L, Arand J, Lengauer T, Walter J, Bock C. BiQ Analyzer HT: locus-specific analysis of DNA methylation by high-throughput bisulfite sequencing. Nucleic Acids Res. 2011;39:W551-W556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Benjamini Y, Hochberg Y. Controlling The False Discovery Rate-A Practical And Powerful Approach To Multiple Testing. J R Stat Soc. 1995;57:289-300. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17030] [Cited by in F6Publishing: 17347] [Article Influence: 2891.2] [Reference Citation Analysis (0)] |
| 36. | Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12858] [Cited by in F6Publishing: 13796] [Article Influence: 627.1] [Reference Citation Analysis (0)] |
| 37. | Roškar S, Zorko M, Podlesek A. Suicide in Slovenia Between 1997 and 2010. Crisis. 2015;36:126-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Bauer M, Gramlich I, Polzin S, Patzelt D. Quantification of mRNA degradation as possible indicator of postmortem interval--a pilot study. Leg Med (Tokyo). 2003;5:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Lee BH, Kim YK. BDNF mRNA expression of peripheral blood mononuclear cells was decreased in depressive patients who had or had not recently attempted suicide. J Affect Disord. 2010;125:369-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Gross JA, Pacis A, Chen GG, Drupals M, Lutz PE, Barreiro LB, Turecki G. Gene-body 5-hydroxymethylation is associated with gene expression changes in the prefrontal cortex of depressed individuals. Transl Psychiatry. 2017;7:e1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Lutz PE, Gross JA, Dhir SK, Maussion G, Yang J, Bramoulle A, Meaney MJ, Turecki G. Epigenetic Regulation of the Kappa Opioid Receptor by Child Abuse. Biol Psychiatry. 2018;84:751-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |