Revised: September 9, 2014
Accepted: October 1, 2014
Published online: December 9, 2014
Artemisinin from the plant Artemisia annua (A. annua) L., and used as artemisinin combination therapy (ACT), is the current best therapeutic for treating malaria, a disease that hits children and adults especially in developing countries. Traditionally, A. annua was used by the Chinese as a tea to treat “fever”. More recently, investigators have shown that tea infusions and oral consumption of the dried leaves of the plant have prophylactic and therapeutic efficacy. The presence of a complex matrix of chemicals within the leaves seems to enhance both the bioavailability and efficacy of artemisinin. Although about 1000-fold less potent than artemisinin in their antiplasmodial activity, these plant chemicals are mainly small molecules that include other artemisinic compounds, terpenes (mainly mono and sesqui), flavonoids, and polyphenolic acids. In addition, polysaccharide constituents of A. annua may enhance bioavailability of artemisinin. Rodent pharmacokinetics showed longer T½ and Tmax and greater Cmax and AUC in Plasmodium chabaudi-infected mice treated with A. annua dried leaves than in healthy mice. Pharmacokinetics of deoxyartemisinin, a liver metabolite of artemisinin, was more inhibited in infected than in healthy mice. In healthy mice, artemisinin serum levels were > 40-fold greater in dried leaf fed mice than those fed with pure artemisinin. Human trial data showed that when delivered as dried leaves, 40-fold less artemisinin was required to obtain a therapeutic response compared to pure artemisinin. ACTs are still unaffordable for many malaria patients, and cost estimates for A. annua dried leaf tablet production are orders of magnitude less than for ACT, despite improvements in the production capacity. Considering that for > 2000 years this plant was used in traditional Chinese medicine for treatment of fever with no apparent appearance of artemisinin drug resistance, the evidence argues for inclusion of affordable A. annua dried leaf tablets into the arsenal of drugs to combat malaria and other artemisinin-susceptible diseases.
Core tip: Artemisinin, extracted from the plant Artemisia annua (A. annua) L., and artemisinin derivatives are the current best antimalarial therapeutics and are delivered as artemisinin combination therapy (ACT). Availability and cost are problematic for the developing world where malaria is endemic. Oral consumption of A. annua dried leaves is more effective than the pure drug. A tea infusion of the leaves has prophylactic effects. Cost of producing and delivering the tea and A. annua dried leaf tablets is much more affordable than ACT.
-
Citation: Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO. Dried-leaf
Artemisia annua : A practical malaria therapeutic for developing countries? World J Pharmacol 2014; 3(4): 39-55 - URL: https://www.wjgnet.com/2220-3192/full/v3/i4/39.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.39
Nearly three billion people are affected by malaria with almost a million deaths annually, especially in Africa and amongst children[1]. Currently extracted from Artemisia annua (A. annua) L., artemisinin (Figure 1) is delivered in concert with another antimalarial drug [artemisinin combination therapy (ACT)] as the preferred treatment to slow emergence of drug resistance. Despite these efforts, artemisinin resistance is appearing[2] and persistent and/or asymptomatic malaria may also be playing a role in disease transmission[3-5]. Moreover, for developing countries ACT is costly and the supply is inadequate[6-9].
Artemisinin is a sesquiterpene lactone that is produced and stored in the glandular trichomes that are mainly on the leaves and floral buds of A. annua, a GRAS medicinal herb[10-12]. The plant also produces > 40 flavonoids[13], many polyphenols, and a variety of other terpenes including mono-, sesqui-, di-, and triterpenes[14]. As discussed later, many of these have weak antimalarial activity, and, based on transcriptome analyses, many also seem to be produced and/or stored in the glandular trichomes that also contain artemisinin[15].
We and others proposed direct consumption of A. annua either as a tea infusion[16-19] or by oral consumption of the leaves[20-24]. In contrast to the oral consumption of pure artemisinin, we showed that the presence of plant material significantly enhanced appearance of artemisinin in the serum of healthy and Plasmodium chabaudi-infected mice[22]. Because of the plethora of mild antimalarial compounds naturally present in the dried leaves of the plant, we have termed this orally consumed dried leaf therapeutic plant-based artemisinin combination therapy, or pACT. These whole plant approaches are similar to the more than 2000 year traditional use of the plant by the Chinese[25].
To produce a therapeutically effective drug using a complex material like a medicinal plant requires that a number of key factors be met: the medicinal herbal product must be therapeutically effective; levels of key chemical components in the herb must be verifiably consistent; production must also be cost effective. Here we summarize and update our recent review[26] on the effects of A. annua on malaria and further discuss the bioavailability and therapeutic efficacy of pACT and how such an herbal drug could inexpensively be produced with a consistent dose.
Until recently, there have been, to our knowledge, few well-controlled studies examining extraction, recovery, and stability of artemisinin and other compounds in A. annua tea infusion. A systematic study of preparations of A. annua therapeutic tea infusion was performed by van der Kooy et al[27] and showed that nearly 93% of available artemisinin was extracted from dried A. annua leaves, but only under certain conditions. Best preparation method was: 9 g DW leaves/L, for 5 min at 100 °C. Subsequent storage of the tea infusion at room temperature showed that artemisinin concentration was stable for > 24 h, important for malaria-endemic locations where there is no refrigeration. Artemisinin water solubility is approximately 50 mg/L[27], so the amount of artemisinin recovered from hot water tea infusions is reasonable. Other studies using the same extraction protocol also measured extraction and stability of artemisinin and some key flavonoids in the tea. Artemisinin was found to be stable at room temperature for up to 48 h[28]; however, some flavonoids were poorly extracted and not stable at room temperature[29].
Carbonara et al[28] detected an assortment of phenolics, including 0.06 mg/g DW cirsilineol, in an A. annua tea infusion prepared at about a 4-10 fold higher proportion (approximately 38 g DW/L) than that proposed as optimal (9 g DW/L) by van der Kooy et al[27]. Most of the measured phenolics in the tea remained constant at room temperature for 48 h post-infusion. More recently, Suberu et al[19] identified milligram amounts of phenolic acids, flavonoids, and sesquiterpenes in a liter of A. annua tea, all of which demonstrated IC50 values in the micromolar or less range (Table 1). Indeed, the IC50 of the tea infusion itself was 7.6 and 2.9 nmol/L for the chloroquine (CQ)-sensitive HB3 and CQ-insensitive Dd2 strains of P. falciparum, respectively, and better than artemisinin alone suggesting synergism of constituents in the tea mixture. Clearly if a tea infusion is to be a therapeutic option, it must be consistently and reliably prepared and ingested. As suggested by van der Kooy et al[27], ideally a liter of tea infusion would be prepared daily and consumed in equal aliquots of about 250 mL over 24 h for several days.
| Compound | Compound IC50 (μmol/L) | Compound + artemisinin IC50 (nmol/L) | Ref. |
| Terpenes | |||
| Artemisinin | 0.033 0.022, 0.0231 | Not applicable | Liu et al[52] |
| Artemisinic acid | 77.8, 61.61 | No numerical value provided; response depended on concentration of compound tested with artemisinin | Suberu et al[19] |
| Arteannuin B | 3.2, 4.81 | ||
| Dihydroartemisinic acid | 21.1, 17.71 | ||
| Nerolidol | 94 | Interaction with artemisinin not yet tested | van Zyl et al[55] |
| α-pinene | 14 | ||
| 1,8-cineole (eucalyptol) | 704 | ||
| Limonene | 5334 | ||
| Phenolic acids | |||
| Chlorogenic acid | 69.4, 61.41 | No numerical value provided; response depended on concentration of compound tested with artemisinin | Suberu et al[19] |
| Rosmarinic acid | 65.1, 65.01 | ||
| Flavonoids | |||
| Artemetin | 26 | 26 | Liu et al[52] |
| Casticin | 24 | 26 | |
| Cirsilineol | 23 | 22.5 | |
| Chrysoplenol-D | 32 | 15 | |
| Chrysoplenetin | 36 | 16 | |
| Eupatorin | 65 | 30 | |
| Isovitexin | 72.5, 48.11 | Interaction with artemisinin not yet tested | Suberu et al[19] |
| Luteolin | 11, 122 | Lehane et al[54] | |
| Kaempferol | 33, 252 | ||
| Myricetin | 40, 762 | ||
| Quercetin | 15, 142, 14.7, 4.11, 2.943 | ||
| Ganesh et al[58] | |||
| Rutin | 7.1, 3.5, 10.383 |
Ogwang et al[30,31] tested Artemisia tea as a prophylaxis against malaria in 132 adult farm workers, aged 18-60 years, for 12 mo in a randomized clinical trial in Uganda. Tea infusion was consumed once a week at 2.5 g dried leaves per adult infusion dose with 55-100 mg artemisinin/L. Malaria was tracked for 9 mo while adverse clinical effects were tracked for 12 mo. Among those who used Artemisia tea there were 80% fewer fever-related hospital visits. Indeed, some patients reported using A. annua tea for > 7 years with no incidence of malaria and no serious adverse events. Although this study suggested that once weekly consumption of A. annua tea infusion may offer prophylactic protection, there were no children or elderly in the study, so additional clinical trials need to be conducted with different populations and age groups. Authors argued that since a single weekly dose was effective, compounds other than artemisinin may have played the prophylactic role since artemisinin itself has short plasma half-life.
Reports on the efficacy of A. annua (cv. Artemis) tea on human malaria patients by Mueller et al[17,32] and Blanke et al[33] yielded at times conflicting results. Their tea infusions contained 47-94 mg artemisinin/L, but recrudescence was much lower in the quinine-treated control group, so parasite reappearance in the tea-treated patients was ascribed to recrudescence and not re-infection[17]. In the Blanke et al[33] trial that included a placebo tea, recrudescence was consistently lower in the tea patients than in those treated with 500 mg pure artemisinin. More recently, however, De Donno et al[34] showed that 5 g dried leaves in one liter of A. annua tea infusion was effective against both CQ-resistant (W2) and CQ-sensitive (D10) strains of P. falciparum with IC50 values of 5.60 nmol/L and 7.08 nmol/L, respectively, results also consistent with those of Suberu et al[19] as already highlighted. These latter in vitro studies suggested that tea should be efficacious, so why the discrepancy with the earlier human trials? Preparation methodology is crucial for preserving as much biochemical integrity of the plant as possible[27]. The more recent in vitro studies likely used more consistently prepared tea infusions than the earlier human trials, so variations in chemical composition of the infusions and in the plant source material could explain the different responses.
The argument that tea is a monotherapy is unsubstantiated considering the now well-established chemical complexity and related antiplasmodial activity of tea infusions of A. annua and its components. Although data from therapeutic tea trials in animals and in humans correlate well, unfortunately, they do not support use of A. annua tea for treating malaria because animal and human data are comparably negative, the artemisinin dose is not easily controlled, and other potentially synergistic components in the tea are not readily controlled or extracted. Nevertheless, use of the tea could play a role in malaria prophylaxis to reduce incidence of malaria in different communities, or in temporary relief from malaria, mainly in prevention of coma or “to buy time” to enable an infected person from a rural area to travel to a hospital or clinic stocked with ACT.
Recently, Elfawal et al[23] measured parasitemia in mice infected with P. chabaudi that were fed two different doses (0.6 or 3.0 mg artemisinin; 24 and 120 mg/kg) of either pure artemisinin in mouse chow or as pACT. Artemisinin delivered via pACT was at least five times more effective, and with a longer lasting response, than pure artemisinin in reducing parasitemia. Excluding artemisinin there are > 600 phytochemicals that have been identified in Artemisia annua[35], but there is currently a lack of information on the chemistry, effect of the preparation method (harvesting, drying, storage, etc.), and overall bioavailability of these chemicals[36].
Clinical trials using dried leaf A. annua are scarce in the scientific literature and few, other than those in Democratic Republic of Congo by Mueller et al[17,32], are published. Despite the fact that WHO does not encourage either whole plant or tea infusion clinical trials[37], some African universities have been conducting their own trials, many of which have not been published nor results assessed by polymerase chain reaction (PCR) as later done for clinical trials with ACTs (personal comm from C. Kasongo to P. Lutgen). Many of these trials used A. annua infusions, and compared to controls or even other antimalarial drugs, e.g., artesunate-amodiaquine, showed significantly greater sensitivity of the infusion with fewer late therapeutic failures. For example, in Democratic Republic of Congo, 54 malaria-infected volunteers were treated for 10 d with capsules containing powdered leaves of A. annua. Each patient was given 15 g dried leaves containing 15 mg of artemisinin (artemisinin content in leaves = 0.1%[38]). After 2 d all were free of fever and 51 (or 94%) were parasite free after 10 d.
In a study aimed at preventing severe post-operative malaria at Bangui, Central Africa, powdered leaves of A. annua were administered in capsules to 25 patients, 22 of them children aged 1-16 years[24]. Treatment duration ranged from 3-4 d with a dose of 0.4-0.5 g/d of A. annua dried leaves (0.1% artemisinin leaf content) delivering 0.4-0.5 mg/d artemisinin. In spite of the very low administered daily dose of artemisinin, average parasitemia dropped by 62% in the patients with an added benefit of a strong antinociceptive response, especially beneficial to post-operative patients.
The most clinically definitive study to date of pACT efficacy was conducted at the International Centre of Insect Physiology and Ecology (ICIPE) Mbita Field campus, Suba District, in Western Kenya. This was a collaborative project between ICIPE and Kenya Medical Research Institute[20] (Table 2[39]) and was an open-label, non-randomized clinical trial mainly targeted to assess efficacy, safety, and tolerance of increasing doses of pACT delivered as tablets. The tablets were made by a Tanzania-based NGO, Natural Uwemba System for Health, from a hybrid of A. annua grown in the Tanzania highlands (2000-2200 m altitude). Leaves were harvested just before flowering, dried for approximately 3 wk under shade, then crushed, powdered, homogenized, and pressed into 500 mg tablets under ambient temperature. Tablets were robust with no excipient required. Using HPLC with diode array detector, analysis of hexane extracts of randomly selected batches of 100 tablets showed artemisinin content of the tablets was consistent at 0.74% ± 0.06% (i.e., approximately 3.7 mg per tablet).
| pACT (dried leaf A. annua tablets, ea 500 mg, 3.7 mg artemisinin/tablet) | |||||
| Artemisinin dose (mg) | No. ofpatients | Leaf DW (g) | %Recrudescence | ||
| Day 1 | Days 2-6 | Day 1 | Days 2-6 | ||
| 7.4 × 2 | 3.7 × 2 | 12 | 2 | 1 | 25 |
| 11.1 × 2 | 7.4 × 2 | 12 | 3 | 2 | 9.1 |
| 14.8 × 2 | 11.1 × 2 | 12 | 4 | 3 | 16.7 |
| 18.5 × 2 | 14.8 × 2 | 12 | 5 | 4 | 9.1 |
| Compare to orally delivered pure artemisinin[39] | |||||
| Day 1 | Day 2-7 | ||||
| 500 × 2 | 500 | 227 | NA | 24 | |
The four cohorts of the trial each had 12 consenting patients aged 15-56 years (average 23.42) with P. falciparum malaria. Based on Giemsa-stained blood smears counted against 200 wbc, parasitemia was 0.02%-4% and hemoglobin levels > 8 mg/dL. Each cohort received one of four increasing numbers of A. annua tablets, ranging from 2-5 tablets twice on day 1, followed by 1-4 tablets twice daily for the next 5 d (Table 2). A week following the treatments, three patients scattered throughout different cohorts showed re-appearance of parasites in blood smears; however, all doses were effective in clinical and parasitological regression of malaria, with 9%-20% recrudescence at day 28 and no measurable toxicity.
Compared to the usual large pure artemisinin doses of 1000 mg on day 1 followed by 500 mg on each of days 2-7 that were administered to 227 malaria patients[39], patients treated with pACT had generally better therapeutic outcomes (Table 2). The measured pACT cure rate also was comparable to or exceeded other results using pure artemisinin[40,41], and similar levels of artemisinin (artesunate, artemether, etc.[42]). Furthermore, the positive therapeutic response using pACT appeared somewhat independent of dose beyond the second level of dose tested (Table 2[20]). Although oral doses used in the ICIPE[20] trials were far less than any tea studies, levels of recrudescence were much lower than tea and often better than in studies using pure artemisinin[39] (Table 2). Indeed, about 100 total mg of total artemisinin delivered via pACT for a full malaria treatment yielded a better recrudescence rate than the 4000 mg of pure artemisinin used by Giao et al[39] (Table 2). This 40-fold difference correlates well with the early pharmacokinetic studies by Weathers et al[21] that showed 45-fold enhanced bioavailability of the drug when delivered as pACT.
These results suggest that the natural phytochemical blend in pACT is important especially when orally administered as tablets. The results are also consistent with a study in China on mice infected with P. berghei, which compared the effects of pure artemisinin with crude A. annua extracts[43], and the studies by Elfawal et al[23] and Weathers et al[22]. In all three studies the administered products had comparable levels of artemisinin, but crude preparations and pACT were at least 3.5 times more effective in reducing parasitemia than pure artemisinin, suggesting a synergistic role for non-artemisinin constituents in the extracts and orally consumed dried leaves.
When given orally or rectally, dihydroartemisinin showed higher bioavailability in humans than artemisinin in an early pharmacokinetic study by Zhao et al[44]. The Cmax, Tmax, and T1/2 for orally delivered dihydroartemisinin were 0.13-0.71 mg/L, 1.33 h, approximately 1.6 h, respectively; for pure artemisinin they were 0.09 mg/L, 1.5 h, and 2.27 h, respectively. Alin et al[45] compared orally delivered artemisinin and artemisinin-mefloquine combination therapy for treatment of P. falciparum malaria. Infected and uninfected patients had similar pharmacokinetic parameters. After a single dose, bioavailability of artemisinin was not altered. Interestingly, pharmacokinetics were similar when comparing treatment failures with successes, suggesting that studies that only measure artemisinin pharmacokinetics were inadequate for predicting therapeutic success[45]. Ilet et al[46] also reviewed artemisinin pharmacokinetics in patients with falciparum malaria and reported a dose of 9.1 mg/kg, which was comparable to that of Alin et al[45]. Cmax and Tmax values did not differ much from those reported by Alin et al[45].
In the Ilet et al[46] review of pharmacokinetic parameters of artemisinin and its derivatives, oral pure artemisinin doses ranged from about 6-11 mg kg/L in healthy subjects and Cmax was 0.15-0.39 mg/L. Dose seemed to have no major effect. An earlier study by Ashton et al[47] compared increasing artemisinin doses of 250, 500, and 1000 mg per person and both Cmax and T1/2 showed dose-dependent increases of 0.21, 0.45, and 0.79 mg/L, and 1.38, 2.0, and 2.8 h, respectively, but Tmax remained relatively constant at 2.3-2.8 h.
Diet is an important consideration for any orally delivered drug, and when Dien et al[48] compared artemisinin oral doses given with and without food, Cmax values were similar between subjects who fasted and those who did not. Food consumption along with artemisinin did not seem to affect artemisinin absorption. In contrast, a later rodent study by Weathers et al[21] observed that when artemisinin was consumed as part of a complex plant material, pACT, approximately 45-fold more drug entered the serum of mice than orally administered pure drug. Similarly, when pure artemisinin was fed to mice, it was not detectable in the serum after 60 min. However, artemisinin was detected in the serum when consumed in conjunction with mouse chow, which consists of a variety of plant materials including soy, oats, wheat, alfalfa, beet pulp, corn, etc[22].
In a study by Ashton et al[49], artemisinin at 9.1 mg/kg was given daily for 7 d, and measurements taken on days 1, 4, 7, and 21. On day 1 plasma Cmax and T1/2 were similar and comparable to data from other studies using a similar dose. On day 4 and 7, however, Cmax decreased, while T1/2 increased, indicating that although artemisinin was delivered daily for 7 d, it was either not readily absorbed or it degraded after the first dose. After the third dose, Cmax fell from 0.31 to 0.11 mg/L, and T1/2 increased from 3.0 to 4.8 h. These results suggested that either artemisinin was metabolized or accumulated elsewhere in the body.
In the liver, cytochrome P450 (CYP450) enzymes metabolize artemisinin to deoxyartemisinin, deoxydihydroartemisinin, 9,10-dihydrodeoxyartemisinin, and a metabolite named “crystal 7”[50]. Extended artemisinin dosing may not be beneficial as shown by Svensson et al[50] using human liver microsomes where activity of CYP450s, CYP2B6 in particular, correlated with decreasing artemisinin serum levels. In intermittent dosing studied by Ashton et al[49], the P450 levels were allowed to decline for 14 d before delivery of another dose, and Cmax rose from 0.11 to 0.20 mg/L, and T1/2 decreased from 4.8 to 2.7 h. Generally, maximum concentration of artemisinin in the body increased with increasing doses with T1/2 ranging from about 1.4-4.8 h for reported trials using oral pure artemisinin. Thus, increased and extended artemisinin treatment may reduce recrudescence.
Other than Räth et al[16], there are few reports on the pharmacokinetics of tea infusion artemisinin delivered in humans. In the Räth et al[16] study, artemisinin Cmax was 0.24 mg/L at 0.6 h post consumption. Tea infusion containing 94.5 mg artemisinin had a Cmax equivalent to a dose of 250 mg pure artemisinin, but at a significantly shorter Tmax, 0.6 h vs 2.8 h[47]. Compared to pure artemisinin, the shorter half-life of artemisinin in the tea infusion may account for the observed higher recrudescence. Although tea-delivered artemisinin seemed more bioavailable, its shorter T1/2 of 0.9 h compared with about 2 h for pure artemisinin, suggested that more than two doses per day may be more beneficial; indeed, four doses a day were recommended.
The unacceptably high recrudescence rates in clinical tea infusion trials were attributed to low plasma concentrations, almost 40% lower than that for traditional doses (500 mg per person of 60 kg or 8.3 mg artemisinin/kg) of pure artemisinin. Although not specified, tea trial doses have been estimated at about 1.5 mg/kg, close to the 1.1 mg/kg dose of pure artemisinin used by Zhao et al[44], which is far below the 8.3 mg/kg that is traditionally accepted as pharmacologically effective. Nevertheless, the Cmax of 0.24 mg/L artemisinin for the tea dose is nearly twice that for pure artemisinin (Cmax = 0.13 mg/L) as measured by Zhao et al[44]. A. annua tea also showed potent antiplasmodial activity against 40 field isolates of P. falciparum collected in Pikine, Senegal (mean IC50 0.095 µg/mL[51]).
There are as yet no pharmacokinetic studies of pACT in humans. In a small PK study of healthy mice fed artemisinin there was about 45-fold more artemisinin delivered via pACT than when delivered as the pure drug[21]. More recently, pharmacokinetics of artemisinin and one of its liver metabolites, deoxyartemisinin, were compared over 120 min in healthy and P. chabaudi-infected mice treated with dried A. annua leaves at a 100 mg/kg body weight dose of artemisinin[22]. In pACT-treated healthy mice, the first order elimination rate constant for artemisinin was estimated to be 0.80/h, corresponding to a T1/2 of 51.6 min. Cmax and Tmax were 4.33 mg/L and 60 min, respectively. The AUC was 299.5 µg.min/mL. The first order absorption rate constant was estimated at 1.39/h. In contrast, the AUC for pACT-treated infected mice was greater at 435.6 µg·min/mL. Serum levels of artemisinin in the infected mice continued to increase over the 120 min of the study period. As a result, the elimination half-life, T1/2 could not be determined, so Cmax and Tmax could only be estimated at ≥ 6.64 mg/L and ≥ 120 min, respectively. Nevertheless, both Cmax and Tmax of artemisinin were greater in infected than in healthy mice.
Generally, artemisinin concentrations decreased with a concomitant rise in deoxyartemisinin levels only in healthy subjects[22]. In contrast, artemisinin levels in infected mice continued to rise over the study period whilst deoxyartemisinin levels fell and then leveled, so infection seemed to retard the capacity of the mice to process artemisinin into deoxyartemisinin over the two-hour period. Many compounds in A. annua inhibit P. falciparum[52-55] and CYP34A[56]. At the high (100 mg/kg) dose used in the study, nearly equal amounts of artemisinin and deoxyartemisinin were measured in the serum, indicating that an excessive dose of artemisinin was used.
The presence of plant material affected artemisinin pharmacokinetics. At 60 min no artemisinin was detected in serum of mice fed pure artemisinin at 100 mg/kg body weight. When plant material was present, however, as mouse chow or A. annua pACT, artemisinin level in the serum rose to 2.44 and 4.32 µg/mL, respectively, demonstrating that the presence of plant material, even mouse chow, had a major positive impact on the appearance of artemisinin in the blood[22]. To our knowledge, these are the only data available on pharmacokinetics for orally delivered A. annua in animals or humans.
A. annua is rich in essential oils, coumarins, polyphenols, polysaccharides, saponins, terpenes, and flavonoids. The levels of flavonoids and other compounds in A. annua change with developmental growth stage, with some being highest during full bloom[57]. There are > 40 flavonoids[13], and at least 11, including artemetin, casticin, chrysoplenetin, chrysoplenol-D, cirsilineol, eupatorin, kaempferol, luteolin, myricetin, quercetin, and rutin, are reported to have weak therapeutic efficacy against falciparum malaria (Table 1[52-54,58]). Some of these flavonoids were shown to improve the IC50 of artemisinin against P. falciparum in vitro by as much as 50%, suggesting synergy (Table 1[52]). Elford et al[53] also showed that while casticin [5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6,7-trimethoxychromen-4-one] showed synergism with artemisinin, it did not synergize with chloroquine, suggesting a different interactive mechanism. Combining casticin with artemisinin inhibited parasite-mediated transport systems that control influx of myo-inositol and L-glutamine in malaria-infected erythrocytes. These apparent synergistic actions between flavonoids and artemisinin suggest that flavonoids are likely to be important for efficacious use of A. annua consumed either as whole dried leaves or as tea.
Many flavonoids have antiplasmodial effects and inhibit P. falciparum growth in liver cells in vitro as reported for dietary flavonoids[54]. To our knowledge, there are no reports on pharmacokinetics of A. annua delivered flavonoids. Some flavonoids are reported to have long plasma half-lives; e.g., quercetin, found in A. annua and most fruits, has a plasma half-life of 27 h[59]. Quercetin [2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one], also found in garlic, inhibits parasite growth with differential activity against different strains of Plasmodium (Table 1[54,58]). Rutin, which is a rutinose [α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose] glycoside of quercetin, showed similar results, suggesting that the sugar moiety did not significantly affect antimalarial activity (Table 1[58]). Flavonoids are known to persist in the body for > 5 d; this may explain the once a week dose inducing a prophylactic effect from A. annua tea infusion that was reported by Ogwang et al[30,31]. Many dietary flavonoids inhibit Plasmodium growth in vitro, but amounts in the diets are reportedly insufficient to offer protection against malaria[54]. Plants such as A. annua with high concentrations of flavonoids (e.g., up to 0.6%) may, however, work in concert with artemisinin to prevent malaria when consumed regularly.
The flavone luteolin [2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone] comprises up to 0.0023% DW in Artemisia[14] and has been used for a variety of ailments including cough, diarrhea, dysentery, diabetes, cancer, and malaria. Although luteolin has an IC50 value around 11 µmol/L[54] and is one of the more active antiplasmodial flavonoids found in A. annua, one cannot compare its role between studies as indicated by Ganesh et al[58] (see Table 1). The antimalarial response of different flavonoids seems to be affected by the strain of Plasmodium being tested. Luteolin also prevents completion of a full intra-erythrocytic cycle by inhibiting progression of parasite growth beyond the young trophozoite stage. The mechanism of this antiplasmodial activity seems to be related to the inhibition of parasite fatty acid biosynthesis. These lipids are required by the parasite to detoxify heme into hemozoin[60]. Independent of the human host, apicomplexan parasites use a fatty acid biosynthetic pathway. Enzymes in the pathway, like the NADPH-dependent b-ketoacyl-ACP reductase (FabG), are potential antimalarial targets. Among 30 flavonoids studied, luteolin and quercetin had the lowest IC50 values for the inhibition of these enzymes and also showed in vitro activity in the sub-micromolar range against multiple strains of P. falciparum[60].
Isovitexin {5,7-dihydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]chromen-4-one} is another flavone, the 6-C-glucoside of apigenin, that was found in A. annua tea infusion at > 100 mg/L with micromolar antiplasmodial activity (Table 1[19,28]). Isovitexin inhibits lipid peroxidation and xanthine oxidase activity and protects cells from ROS damage with an overall LD50 > 400 µmol/L[61].
Limonene (1-methyl-4-(1-methylethenyl)-cyclohexene) is part of the “cineole cassette” that includes 1,8-cineole (eucalyptol), limonene, myrcene, α-pinene, β-pinene, sabinene, and α-terpineol[62]; many of these affect particular stages of Plasmodium species. For example, limonene is often present at 7 mg/kg in A. annua[14] and inhibits isoprenoid biosynthesis in Plasmodium[63] and development at the ring and trophozoite stages[64]. Eucalyptol affects the trophozoite stage[65]. Limonene also arrests protein isoprenylation in P. falciparum, halting parasite development within 48 h of treatment[64]. The IC50 against in vitro Plasmodium in these trials was 2.27 mmol/L, more than twice the IC50 of 533 µmol/L measured by van Zyl et al[55]. Limonene and its metabolites remain in the plasma for at least 48 h[66], so the pharmacokinetics is favorable, which is important for elimination of gametocytes and malaria transmission.
The volatile monoterpene α-pinene (4,6,6-trimethylb-icyclo[3.1.1]hept-3-ene) is present in the plant at levels up to 0.05% of dry weight[14]; it has an IC50 of 1.2 µmol/L, in the range of quinine at 0.29 µmol/L[55]. Eucalyptol (1,8-cineole) may comprise up to 30% [0.24%-0.42% (V/DW)] of the essential oil in A. annua[67] and is a strong inhibitor of the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8[68]. Both chloroquine-resistant and chloroquine-sensitive Plasmodium strains are affected at the early trophozoite stage[65].
Eucalyptol (1,3,3-trimethyl-2-oxabicyclo[2,2,2]octane) is also volatile and rapidly enters the blood when delivered either as an inhalant or orally[69,70]. At an IC50 of 0.02 mg/mL and low toxicity (LD50 of approximately 25 mg/mL), either oral or inhalation delivery is reasonable[65,71]. Indeed eucalyptol concentrations can reach 15 µg/mL in 60 min[69] suggesting its possible use as an antimalarial inhalant.
Artemisia ketone (3,3,6-trimethyl-1,5-heptadien-4-one), a major constituent of some cultivars of A. annua, has barely been studied. Other ketones like curcumin[72] have been implicated as inhibitors of β-hematin synthesis, so artemisia ketone may play a similar role and affect hemozoin formation. Although hemoglobin is required for Plasmodium survival and multiplication in merozoites inside the red blood cell, it leaves toxic debris like heme. The parasite subsequently oxidizes Fe2+ in heme to Fe3+ forming hematin, a nontoxic insoluble polymeric crystal called β-hematin (also known as hemozoin), which also inhibits cell-mediated immunity against the parasite. Water extracts of A. annua inhibit hemozoin synthesis[73].
Essential oils often contain a large amount of monoterpenes that may enhance the antimalarial effect of artesunate and even reverse the observed resistance of P. berghei against artesunate[74]. Monoterpenes tend to be higher in the pre-flowering phase of A. annua[75], but are drastically reduced by high drying temperatures or drying in the sun[13,76] and, of particular concern, during compression of dried leaves into tablets[77]. Although monoterpenes have some antimalarial potential, most are rather volatile and thus they may be therapeutically less important than the nonvolatile flavonoids, phenolic acids, and higher molecular weight sesquiterpenes.
Unlike α-pinene and eucalyptol, camphor (1,7,7-trimethylbicyclo[2.2.1]heptan-2-one) has no reported antimalarial activity, but it may comprise as much as 43.5% of the essential oil of A. annua[78]. Considering camphor is less volatile than either eucalyptol or α-pinene (melting points of 204 °C, 176 °C, and 155 °C, and flash points of 54 °C, 49 °C, and 33 °C, respectively), it may instead play a role in enhanced transport of hydrophobic molecules like artemisinin from pACT across the intestinal wall into the bloodstream[21,22]. Camphor may also affect thymocyte viability and aid in developing malaria immunity through production of T-cells[79]. At 50 μg/mL, camphor increased viability of cultured thymocytes[80].
The sesquiterpene nerolidol (3,7,11-trimethyl-1,6,10-dodecatrien-3-ol) has an IC50 of 0.99 µmol/L and arrests development of the intraerythrocytic stages of the parasite (Table 1[55]). Indians of the Amazon basin in Brazil treated malaria using the vapors of the leaves of Viola surinamensis; nerolidol was identified as the active constituent leading to 100% growth inhibition at the schizont stage[81]. Nerolidol levels vary with the cultivar tested, with one of the highest values found in plants from Ethiopia[82]. There is a greater concentration of this sesquiterpene in stems than leaves of A. annua[83].
Other sesquiterpenes found in the artemisinin biosynthetic pathway were only recently shown to have antiplasmodial activity at µmol/L levels, similar to that of other compounds found in the plant (Table 1[19]). These artemisinic compounds were extracted into A. annua tea infusions and showed varying interactions with artemisinin depending on their relative concentrations and the target parasite strain. For example, arteannuin B showed an additive interaction with artemisinin against the CQ-sensitive Plasmodium HB3 strain, while against the CQ-insensitive Dd2 strain the interaction was synergistic.
Rosmarinic ((2”R”)-2-[[(2”E”)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]]oxy]-3-(3,4-dihydroxyphenyl) propanoic acid) and chlorogenic ((1S,3R,4R,5R)-3-{[(2Z)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexanecarboxylic acid) acids are strong antioxidants found in a wide variety of A. annua cultivars[56]. In Caco-2 studies, these acids significantly inhibited activity of CYP3A4, one of the hepatic P450s responsible for metabolism of artemisinin to deoxyartemisinin, an inactive form of the drug[50]. These and other phenolic acids are present in A. annua tea infusion[19]. Both phenolic acids have an IC50 of about 65 µmol/L (Table 1) and also significantly reduced secretion of cytokines IL-6 and IL-8, and thus enhanced antimalarial activity while reducing inflammation[56].
Although polysaccharides in other medicinal plants have been more extensively studied, they seem to have been rather overlooked in A. annua, probably because most Artemisia extracts are obtained using organic solvents and polysaccharides are only soluble in water. Polysaccharides extracted from Artemisia iwayomogi showed hydroxyl radical scavenging activity three times stronger than glutathione or caffeic acid, and ROS inhibition was twice as strong as ascorbic acid[84]. In A. iwayomogi, more polysaccharides were found in stems than in leaves and their solubility was also higher from stem than from leaf tissue[84].
The combination of polysaccharides with lipophilic molecules like artemisinin may lead to a higher bioavailability of the antimalarial constituents when delivered via A. annua, which may explain the lower effective therapeutic dose against malaria observed for pACT than for pure artemisinin[20,23,26]. Indeed, Han[85] showed that ginseng polysaccharides had preventive and curative antimalarial activities and synergized with artesunate in malaria-infected mice. Sulfated polysaccharides inhibited the in vitro invasion of merozoites into erythrocytes and interfered with merozoite surface protein[86-88]. Heparin and other sulfated polysaccharides have been shown to inhibit blood-stage growth of plasmodium[89,90]. Some sulfated polysaccharides inhibited the formation of rosettes between infected red blood cells (iRBC) and uninfected RBCs, as well as adhesion of iRBCs to placental chondroitin sulfate A, which is linked to severe disease outcome in pregnancy-associated malaria[91].
Saponins, common in many plants, have an important role in human and animal nutrition and are reportedly present in A. annua, but only as measured in alcoholic extracts using the nonquantitative foaming test[92,93] (Weathers, unpublished). These soap-like amphiphilic (lypo- and hydro-philic) bioactive compounds are mainly produced by plants. Recently, there has been interest in the clinical use of saponins as chemotherapeutic agents[94], and as adjuvants for vaccines[95]. At very low doses saponins are efficient, have hemolytic properties, produce 40-50 Å pores in erythrocyte membranes, and modulate the sodium pump and ATPase[96]. Saponins also have a hypoglycemic effect mainly by inhibiting intestinal permeability and absorption of glucose and may therefore inhibit the growth of P. falciparum, which needs glucose to grow[97]. Better identification, quantification, and investigation into the role of saponins in pACT efficacy are warranted.
The coumarin, scopoletin (7-hydroxy-6-methoxychromen-2-one), also known for its antinociceptive properties[98,99], is commonly found in most Artemisia species at, for example, about 0.2% (w/w) in a Luxembourg cultivar. Known for its anti-oxidant, hepatoprotective, and anti-inflammatory activities, scopoletin scavenging capacity for hydroxyl radical, DPPH, superoxide anion, hydrogen peroxide, and Fe2+ chelating activity is almost at the level of α-tocopherol (Vitamin E)[100].
Although not antiplasmodial, scopoletin inhibits TNF-α, IL-6, and IL-8 at millimolar concentrations, and is thus likely one of the major anti-inflammatory and antipyretic constituents of A. annua[101]. Coumarins can activate lymphocytes, thereby stimulating immunological functions[102]. Indeed, scopoletin induced cell proliferation in normal lymphocytes with an immunomodulatory effect[101]. In uninfected erythrocytes internal Na concentration is much lower than external concentration, but the K concentration is higher; in infected blood cells this situation is drastically reversed[103]. Scopoletin significantly stimulated erythrocyte membrane ATPases at 0.1 µmol/L, in particular Na-K-ATPase vs Ca-ATPase or Mg-ATPase[104], so scopoletin may affect malaria infection. A significant hormetic effect was also noticed; stimulation was higher at scopoletin concentrations of 10 µg/mL than at 1 or at 100 µg/mL. In addition scopoletin also inhibited ADP-platelet aggregation at a range of 0.1 to 5 µmol/L and improved blood rheology[105].
Scopoletin may also affect the interaction between malaria and uric acid. Cyclical fevers and high levels of inflammation characterize malaria and this likely aids parasite clearance. Excessive and persistent inflammation, on the other hand, can lead to severe malaria[106]. In the cytoplasm of their parasitophorous vacuole, Plasmodium-infected erythrocytes contain uric acid precipitates that are released upon erythrocyte rupture. Uric acid precipitates are mediators for inflammatory cytokines IL-6, IL-8, and are considered a danger signal for innate immunity. Uric acid is also the causative agent in gout. These precipitates could offer a novel molecular target for anti-inflammatory therapies in malaria. Scopoletin inhibits the activity of xanthine oxidase in hyperuricemic mice after peritoneal administration, and this hypouremic effect is fast and dose-dependent[107].
Although many of the compounds in A. annua have not been tested for their toxicity in, a survey of available MSDS data showed that the LD50 levels for orally administered compounds in rodents ranged from about 160 mg/kg for quercetin to > 8000 mg/kg for nerolidol. The artemisinin LD50 measured via oral dose in a mouse was 4228 mg/kg. Therefore, at the estimated amounts of dried leaves of pACT that may be orally consumed by a malaria patient, most of the compounds reported thus far in A. annua are at concentrations that are orders of magnitude below their LD50 toxicity values.
Toxicology of the dried leaf tablets used in the Kenyan human trial measured the following components: serum levels of urea, serum proteins, creatinine, γ-glutaryl transferase, serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase, or alkaline phosphatase levels, hemoglobin, and pre- and post-electrocardiograms[20]. Compared to levels prior to treatment with pACT, there was no significant change post-treatment.
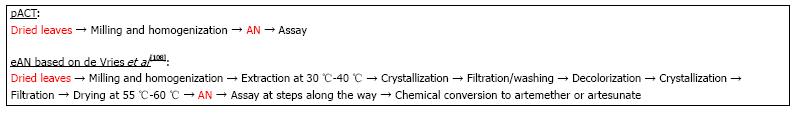
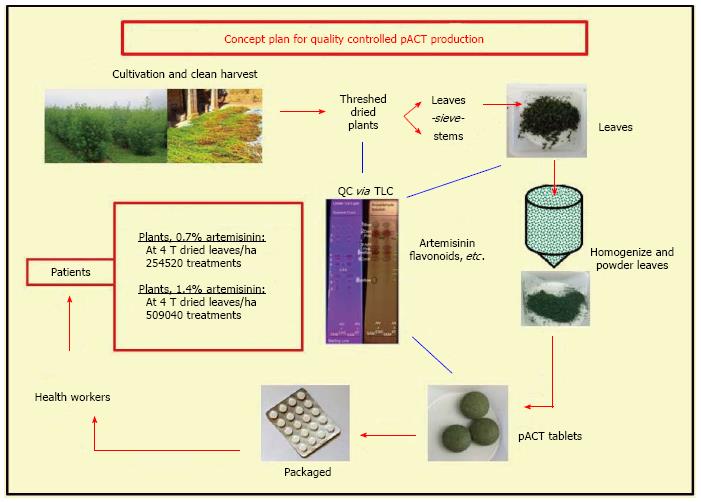
Because production costs are usually closely held secrets, there are few cost estimates that are publicly available to compare pACT production with extracted artemisinin. However, costs can be estimated from a study by de Vries et al[108] where they reported a 1 kg recovery of artemisinin from A. annua containing 0.6% artemisinin. Downstream processing costs and product losses increase with increasing number of unit operations (unit ops), a fact often not generally appreciated[109]. Indeed for biotechnology processes, recovery can be anywhere from 9%-51%[110]. As an example, if each step of a 4 step process is 95% efficient, then the overall process has a final efficiency of about 81%, while a single step process at 95% efficiency has a 95% overall recovery. The described process steps for extracted artemisinin (eAN) vs pACT-AN are shown in Figure 2. From the point of harvested dried leaves to material ready for packaging or conversion to the delivered drug (e.g., artesunate or artemether), pACT has one unit op and eAN has eight[108]. Extraction solvents and other chemicals are clearly no longer part of the cost. Because there is one vs eight unit ops for eAN and at least two of the eAN unit ops involve significant amounts of heat, pACT energy cost is significantly reduced by at least 90%. Costs for labor, interest, depreciation, and maintenance are all also affected by the number of unit ops[109], so we estimated that with seven fewer unit op steps those costs would reduce by approximately 88%. Although better extraction processes may be in play[111], using the de Vries et al[108] analysis our estimate of cost reduction for producing pACT is about 30% less than the cost of producing eAN. Data provided by de Vries et al[108] was based on 0.6% artemisinin content, so if a higher producing cultivar was harvested, costs would drop proportionately. Moreover, cost drops again because with pACT there is no need to convert artemisinin to artesunate or artemether; those conversions were necessary because they have higher bioavailability than pure artemisinin, which is not an issue with pACT[21,22].
The de Vries et al[108] process cost estimation focuses on a production yield of 1 kg of artemisinin from 500 kg dried leaves, so per Giao et al[39] that amount of pure artemisinin would treat only 250 patients. Based on the data shown in Table 3 from Kenyan or WPI A. annua at 0.7 and 1.4% artemisinin, 15 and 7.5 g DW leaves, respectively, are required for a total adult pACT treatment; so from 500 kg leaves, 33300 and 66600 patients could be treated, respectively. This represents more than a 130-fold increase in patients treated compared to pure artemisinin with proportionate reduction in price.
A. annua dry leaf production varies around the globe. “In East Africa yields average 2.5 T/ha (range = 0.75-4.2)...”[112]. Based on our field trials[113], the reported average A. annua leaf production in E. Africa[112], and the doses used in the Kenyan human trial[20], one can estimate the amount of dry leaf production, and depending on the amount of artemisinin in the biomass, estimate possible number of adult patients that could be treated with pACT (Table 3).
Using the dosing information obtained from the Kenyan human malaria trial[20], each adult needs about 100 mg artemisinin total over 6 d for a malaria treatment, so for A. annua leaves with 0.7% artemisinin, 15 g of dried leaves would be needed for a 6 d treatment course. At 2 ton of dried leaves harvested per hectare, 127260 adult patients could be treated for malaria (Table 3). For leaves containing 1.4% artemisinin, only 7.5 g of dried leaves are required, so from a hectare of land producing 2 tons of leaves twice as many patients could be treated (Table 3). Clearly choosing cultivars that have higher levels of artemisinin in their leafy biomass will dramatically increase the number of patients that can be treated from 1 ha.
According to Roll Back Malaria, from one ton of purified artemisinin current ACT therapy can provide 1.76 million adult malaria treatments using artemether/lumefantrine, and 2.5 million adult treatments using artesunate/amodiaquine[114] (Table 4). Using the same one ton artemisinin equivalent, but delivering the drug via pACT with 0.7% artemisinin content, one would have harvested about 142.8 tons of dried A. annua leaves. Assuming 15 g dried leaves per patient from the dosing data in the Kenyan human malaria trial (Table 2[20]), 8.64 million adult patients could be treated, about a four-fold increase over either of the current ACT drugs. The actual cost of pACT, therefore, mainly depends on the cost of the dried leaves and their artemisinin content.
As yet unpublished data from the Rich and Weathers labs demonstrated that pACT prevents emergence of artemisinin drug resistance; the plant itself seems to function as its own ACT (pACT). This would obviate the need for inclusion of a co-drug as used in currently administered ACTs. The co-drug costs at least as much as the artemisinic portion of the drug[6]. Consequently, elimination of the added co-drug could result in at least an additional 50% reduction in cost, so that the final pACT cost reduction is conservatively estimated to be far below that of a current course of ACT therapy.
Considering that A. annua is nontoxic and safe to consume orally, dose may not have to be adjusted for children. On the other hand, the leaves taste bitter, so masking the taste, perhaps with sugar, should help with pediatric treatment. Our recent simulated digestion study showed that adding table sugar (sucrose) to pACT did not significantly alter the amount of artemisinin released after digestion, with the added benefit of doubling the amount of flavonoids released[115].
There are at least three other emerging antimalarial therapeutic technologies: synthetic artemisinin[116], semi-synthetic artemisinin (SSA) production from genetically engineered microbes[117], and a single dose drug, OZ439[118]. In early 2013, Sanofi/PATH Drug Development Programme, announced they would have the capacity to produce up to 60 MT of SSA in 2014 at about $400/kg, depending on quantity; Sanofi now has WHO prequalification for its SSA[119]. Although not much cheaper than the current price of about $550/kg[120], supply would be more or less unlimited. Despite what might seem as an advantage to large amounts of SSA production, there are also some serious disadvantages, and comparison of some advantages and disadvantages for each of these new synthetic antimalarial drugs and pACT is noted in Table 5.
| Technology | Advantages | Disadvantages |
| Synthetic AN[116] | Fully synthetic method giving AN = compound | Requires co-drug to obviate emergence of AN drug resistance |
| Lowers AN cost compared to extraction | Not yet in production | |
| Needs sophisticated process | ||
| Likely all under Western control | ||
| Challenging patient compliance due to multiday dosing | ||
| Semi-synthetic AN[117] | Semi-synthetic method giving authentic AN | Requires co-drug to obviate emergence of AN drug resistance |
| Lowers AN cost compared to extraction | Production began via Sanofi | |
| Needs sophisticated process | ||
| Likely all under Western control | ||
| Challenging patient compliance due to multiday dosing | ||
| OZ439[118] | Single dose cure insures patient compliance | Requires co-drug to obviate emergence of AN drug resistance |
| In successful Phase 2 trials | Not yet in production | |
| Mechanism of action not the same as AN | Needs sophisticated process | |
| Probably low cost due to full synthesis | Likely all under Western control | |
| pACT[20-24] | Has its own in planta co-drug to obviate emergence of AN drug resistance | Not yet in production |
| Very low cost | Likely to meet push back from pharmaceutical industry | |
| Very consistent product | Challenging patient compliance due to multiday dosing | |
| Can be used to treat other diseases | ||
| Can be locally owned, produced, managed, and distributed |
The traditional and least costly method for cultivating A. annua uses seeds and in developing countries farmers prefer to save seeds from one growing season to the next. However, seed generated plants of A. annua will vary widely from generation to generation even with high quality starting stock (see review by Ferreira et al[10]). Stem cuttings of A. annua readily root in about two weeks, so clonal propagation via rooted cutting is recommended to eliminate this variability. Although this method of propagation is not cost effective for large plantations, it would work for a few hectares or for controlled environment agriculture. Given the large numbers of patients that could be treated from growing just a few hectares of A. annua (Table 3), clonal propagation by rooted stem cuttings is recommended. Since pACT therapy involves the direct consumption of the dried leaves of the plant, harvested leaf material must be kept clean, which is easiest to do in controlled environment agriculture and following Good Agricultural Procedures[121], particularly as applied to fresh produce[122]. However, controlled agriculture would probably result in loss of agricultural jobs, a concern to be assessed locally. Alternatively, great care must be taken during field harvest and post-harvest storage, so as not to affect the quality of the product. WHO has established good agricultural practices specifically for A. annua for purposes of artemisinin extraction[123], for general medicinal plants[124], and to minimize contamination of herbal medicines[125].
To deliver a reliable dose of therapeutics to a patient, the dried leaves of harvested A. annua must have a reliable and consistent composition. Clonal propagation provides the required consistency. Recently we showed that of 10 crops harvested from vegetative and early flowering plants grown over three years under diverse conditions in the lab, field, and home garden, the artemisinin content of a single clone of A. annua (SAM) was 1.38% ± 0.26% (w/w)[77]. Thus, despite variations in culture and environmental conditions, a consistent level of the main therapeutic constituent can be achieved. Moreover, the content of harvested leaves is certainly not a guarantee of finished product, e.g., compressed leaf tablets. Analyses by Weathers et al[77] showed that although artemisinin content was very stable after tablet compression, other constituents vaied significantly. For example, although flavonoids increased with tablet compression, the more volatile monoterpenes decreased substantially. Thus, it is critical to monitor the composition profile of both incoming harvested material as well as the final product.
Complex and expensive analytical procedures have been used to analyze the many products found in A. annua, but they are not necessary to measure and assure product quality. Artemisinin is easily extracted and then can be quantified using a variety of thin layer chromatography (TLC) methods and visualized with p-anisaldehyde stain[126,127]. Other key constituents like the flavonoids are also readily separated using TLC and visualized under either UV ± AlCl3 reagent[128]. Total flavonoids also can be quantified using inexpensive visible spectroscopy via the AlCl3 method with quercetin used as an inexpensive standard. To our knowledge no inexpensive, reliable spectrophotometric assay is available to measure artemisinin in complex plant extracts.
Artemisinin and its derivatives are also effective against a number of viruses[129], a variety of human cancer cell lines[130-133], and several neglected tropical diseases including schistosomiasis[134], leishmaniasis[135,136], trypanosomiasis[137], and some livestock diseases[133,138].
Although they rank below malaria in terms of public health importance, schistosomiasis, leishmania, and trypanosomiasis result in estimated annual infections of about 240 million, 1.3 million (0.3 visceral and 1.0 cutaneous), and 30000, respectively[139]. These diseases along with many others respond to treatment with artemisinins. Although the IC50 is about 1000-fold greater than for Plasmodium sp., the greater apparent bioavailability of artemisinin via oral pACT[20-22] would likely reduce the amount of drug required for treatment. At present, pACT has not been tested in vivo for diseases other than malaria.
Malaria treatment is further complicated for Human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) patients. Malaria and HIV co-infection represents a major health burden in Africa mainly because it is now “well established that HIV infection results in a higher incidence and more severe manifestations of malaria”[140]. With a weakened immune system, AIDS patients are more susceptible to malaria and also respond slower to malaria therapy[140-142]. Furthermore, in a meta-analysis by Tusting et al[143], socioeconomic development strongly correlated with better malaria therapeutic outcomes. Recently, A. annua has demonstrated anti HIV activity[126,144] and thus oral consumption of the dried leaves of this herb will not only treat malaria, but should also enhance the well-being of HIV/AIDS patients.
A. annua is grown in more than 75 countries[145]. In 2011 about 163 MT of artemisinin were extracted from plantations and small stakeholder farms mainly located in China, Vietnam, and Eastern Africa including Madagascar; value was about $550/kg[120]. With the advent of the production of semi synthetic artemisinin by Sanofi, 60 MT were projected for 2014 with an anticipated price of about $400/kg[119]. As this new source of artemisinin becomes available, the Netherlands Royal Tropical Institute projected that the market for natural Artemisia will significantly destabilize, undermining the security of farmers. The Tropical Institute was further concerned that “pharmaceutical companies will accumulate control and power over the production process; Artemisia producers will lose a source of income; and local production, extraction and (possibly) manufacturing of ACT in regions where malaria is prevalent will shift to the main production sites of Western pharmaceutical companies”, disrupting the fragile economics of these already impoverished countries[120]. The average small stakeholder crop area is about 0.2 ha in China and Africa[120], so while implementation of pACT may not require as much agricultural land as for extracted artemisinin, it could still help provide small stakeholders with a source of income. We have estimated that localized micro manufacturing plants could be constructed for < $50000 USD, and produce quality-controlled pACT tablets with readily verifiable contents. Our overall approach, schematically illustrated in Figure 3, leads to local control of malaria and possibly other artemisinin susceptible diseases while also improving the socioeconomic status of the populations.
Evidence is mounting for the therapeutic efficacy of the use of dried leaves of A. annua, pACT, to treat malaria and possibly other diseases. The complex mixture of antiparasitic compounds in the plant seems to account for its therapeutic activity with animal and human trials supporting this claim. It is also clear that the cost of using pACT is a fraction of that for any other current or emerging antimalarial therapeutic. Likewise, the recent evidence of persistent and/or asymptomatic malaria suggests that a more prophylactic approach to malaria using pACT or even A. annua tea may be warranted. Considering that for > 2000 years this plant was used in traditional Chinese medicine for treatment of fever with no apparent appearance of artemisinin drug resistance, taken together the cumulative evidence argues for inclusion of pACT into the arsenal of drugs to combat malaria, and very likely, other diseases.
P- Reviewer: Masocha W S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | WHO. World Malaria Report 2013. Available from: http: //www.who.int/malaria/publications/world_malaria_report_2013/en/. [Cited in This Article: ] |
| 2. | Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960-1966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 692] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 3. | Betson M, Sousa-Figueiredo JC, Atuhaire A, Arinaitwe M, Adriko M, Mwesigwa G, Nabonge J, Kabatereine NB, Sutherland CJ, Stothard JR. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology. 2014;16:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, Omar SA, Shekalaghe SA, Kaur H, Ndaro A. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017-2024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 6. | Yeung S, Van Damme W, Socheat D, White NJ, Mills A. Cost of increasing access to artemisinin combination therapy: the Cambodian experience. Malar J. 2008;7:84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | O’Connell KA, Gatakaa H, Poyer S, Njogu J, Evance I, Munroe E, Solomon T, Goodman C, Hanson K, Zinsou C. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malar J. 2011;10:326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Davis B, Ladner J, Sams K, Tekinturhan E, de Korte D, Saba J. Artemisinin-based combination therapy availability and use in the private sector of five AMFm phase 1 countries. Malar J. 2013;12:135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mikkelsen-Lopez I, Shango W, Barrington J, Ziegler R, Smith T, deSavigny D. The challenge to avoid anti-malarial medicine stock-outs in an era of funding partners: the case of Tanzania. Malar J. 2014;13:181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Ferreira JFS, Laughlin JC, Delabays N, de Magalhães PM. Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin. Plant Gen Res. 2005;3:206-229. [DOI] [Cited in This Article: ] |
| 11. | Duke JA. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL: CRC Press LLC; 2001; 70. [Cited in This Article: ] |
| 12. | Duke MV, Paul RN, Elsohly HN, Sturtz G, Duke SO. Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci. 1994;155:365-372. [Cited in This Article: ] |
| 13. | Ferreira JF, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135-3170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Curr Sci. 2001;80:35-48. [Cited in This Article: ] |
| 15. | Wang W, Wang Y, Zhang Q, Qi Y, Guo D. Global characterization of Artemisia annua glandular trichome transcriptome using 454 pyrosequencing. BMC Genomics. 2009;10:465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Räth K, Taxis K, Walz G, Gleiter CH, Li SM, Heide L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (annual wormwood). Am J Trop Med Hyg. 2004;70:128-132. [PubMed] [Cited in This Article: ] |
| 17. | Mueller MS, Runyambo N, Wagner I, Borrmann S, Dietz K, Heide L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans R Soc Trop Med Hyg. 2004;98:318-321. [PubMed] [Cited in This Article: ] |
| 18. | Silva LF, Magalhães PM, Costa MR, Alecrim Md, Chaves FC, Hidalgo Ade F, Pohlit AM, Vieira PP. In vitro susceptibility of Plasmodium falciparum Welch field isolates to infusions prepared from Artemisia annua L. cultivated in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 2012;107:859-866. [PubMed] [Cited in This Article: ] |
| 19. | Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC, Lapkin AA. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract--possible synergistic and resistance mechanisms. PLoS One. 2013;8:e80790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Suberu JO; ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concepts studies. Available from: http: //www.google.com/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=2&ved=0CDgQFjAB&url=http://www.iwerliewen.org/index.php/component/edocman/?task=document.download&id=96&Itemid=181&ei=J2miUbnFNo80QGoi4GACw&usg=AFQjCNHoLJmPt4n0AkKyBlXPSyl5W7rc6w&sig2=ppM08X1tZglQLLiaojZx1w&bvm=bv.47008514,d.dmQ. [Cited in This Article: ] |
| 21. | Weathers PJ, Arsenault PR, Covello P, McMickle A, Reed D, Teoh KH. Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev. 2011;10:173-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Weathers PJ, Elfawal MA, Towler MJ, Acquaah-Mensah GK, Rich SM. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J Ethnopharmacol. 2014;153:732-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS One. 2012;7:e52746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Onimus M, Carteron S, Lutgen P. The surprising efficiency of Artemisia annua powder capsules. Medicin Aromat Plants. 2013;2:3. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Hsu E. The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg. 2006;100:505-508. [PubMed] [Cited in This Article: ] |
| 26. | Weathers PJ, Reed K, Hassanali A, Lutgen P, Engeu PO. Chapter 4: Whole plant approaches to therapeutic use of Artemisia annua L. (Asteraceae). Artemisia annua - Pharmacology and Biotechnology. Heidelberg, GDR: Springer 2014; 51-74. [Cited in This Article: ] |
| 27. | van der Kooy F, Verpoorte R. The content of artemisinin in the Artemisia annua tea infusion. Planta Med. 2011;77:1754-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Carbonara T, Pascale R, Argentieri MP, Papadia P, Fanizzi FP, Villanova L, Avato P. Phytochemical analysis of a herbal tea from Artemisia annua L. J Pharm Biomed Anal. 2012;62:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Weathers PJ, Towler MJ. The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Med. 2012;78:1024-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Ogwang PE, Ogwal-Okeng J, Kasasa S, Ejobi F, Kabasa D, Obua C. Use of Artemisia annua L. infusion for malaria prevention: mode of action and benefits in a Ugandan community. British J Pharm Res. 2011;1:124-132. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ogwang PE, Ogwal JO, Kasasa S, Olila D, Ejobi F, Kabasa D, Obua C. Artemisia annua L. infusion consumed once a week reduces risk of multiple episodes of malaria: a randomised trial in a Ugandan community. Trop J Pharm Res. 2012;13:445-453. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Mueller MS, Karhagomba IB, Hirt HM, Wemakor E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J Ethnopharmacol. 2000;73:487-493. [PubMed] [Cited in This Article: ] |
| 33. | Blanke CH, Naisabha GB, Balema MB, Mbaruku GM, Heide L, Müller MS. Herba Artemisiae annuae tea preparation compared to sulfadoxine-pyrimethamine in the treatment of uncomplicated falciparum malaria in adults: a randomized double-blind clinical trial. Trop Doct. 2008;38:113-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Caccioppola A, Villanova L, Merendino A, Bagordo F, Fanizzi FP. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg. 2012;106:696-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules. 2010;15:7603-7698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | van der Kooy F, Sullivan SE. The complexity of medicinal plants: the traditional Artemisia annua formulation, current status and future perspectives. J Ethnopharmacol. 2013;150:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | WHO Position Statement. Effectiveness of Non-Pharmaceutical Forms of Artemisia annua L. against malaria. Available from: http: //www.who.int/malaria/position_statement_herbal_remedy_artemisia_annua_l.pdf. [Cited in This Article: ] |
| 38. | Tiruneh G, Kebede Y, Yigzaw T. Use of the plant Artemisia annua as a natural anti-malarial herb in Arbaminch town. Ethiop J Health Biomed Sci. 2010;2:76-82. [Cited in This Article: ] |
| 39. | Giao PT, Binh TQ, Kager PA, Long HP, Van Thang N, Van Nam N, de Vries PJ. Artemisinin for treatment of uncomplicated falciparum malaria: is there a place for monotherapy? Am J Trop Med Hyg. 2001;65:690-695. [PubMed] [Cited in This Article: ] |
| 40. | Hien TT. An overview of the clinical use of artemisinin and its derivatives in the treatment of falciparum malaria in Viet Nam. Trans R Soc Trop Med Hyg. 1994;88 Suppl 1:S7-S8. [PubMed] [Cited in This Article: ] |
| 41. | McIntosh HM, Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database Syst Rev. 2000;2:CD000527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818-836. [PubMed] [Cited in This Article: ] |
| 43. | Wan YD, Zang QZ, Wang JS. [Studies on the antimalarial action of gelatin capsule of Artemisia annua]. Zhongguo Jishengchongxue Yu Jishengchongbing Zazhi. 1992;10:290-294. [PubMed] [Cited in This Article: ] |
| 44. | Zhao KC, Song ZY. [Pharmacokinetics of dihydroqinghaosu in human volunteers and comparison with qinghaosu]. Yaoxue Xuebao. 1993;28:342-346. [PubMed] [Cited in This Article: ] |
| 45. | Alin MH, Ashton M, Kihamia CM, Mtey GJ, Björkman A. Clinical efficacy and pharmacokinetics of artemisinin monotherapy and in combination with mefloquine in patients with falciparum malaria. Br J Clin Pharmacol. 1996;41:587-592. [PubMed] [Cited in This Article: ] |
| 46. | Ilet KF, Batty KT. Artemisinin and its derivatives. Antimicrobial Therapy and Vaccines. Pittsburgh USA: ESun Technologies 2005; 981-1002. [Cited in This Article: ] |
| 47. | Ashton M, Gordi T, Trinh NH, Nguyen VH, Nguyen DS, Nguyen TN, Dinh XH, Johansson M, Le DC. Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm Drug Dispos. 1998;19:245-250. [PubMed] [Cited in This Article: ] |
| 48. | Dien TK, de Vries PJ, Khanh NX, Koopmans R, Binh LN, Duc DD, Kager PA, van Boxtel CJ. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob Agents Chemother. 1997;41:1069-1072. [PubMed] [Cited in This Article: ] |
| 49. | Ashton M, Hai TN, Sy ND, Huong DX, Van Huong N, Niêu NT, Công LD. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:25-27. [PubMed] [Cited in This Article: ] |
| 50. | Svensson US, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999;48:528-535. [PubMed] [Cited in This Article: ] |
| 51. | Gueye PEO, Diallo M, deme AB, Badiane A, Dior DM, Ahouidi A, Abdoul AN, Dieng T, Lutgen P, Mbopup S. Tea Artemisia annua inhibits Plasmodium falciparum isolates collected in Pikine, Senegal. Af J Biochem Res. 2013;7:107-113. [DOI] [Cited in This Article: ] |
| 52. | Liu KC, Yang SL, Roberts MF, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992;11:637-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Elford BC, Roberts MF, Phillipson JD, Wilson RJ. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans R Soc Trop Med Hyg. 1987;81:434-436. [PubMed] [Cited in This Article: ] |
| 54. | Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes. 2008;1:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | van Zyl RL, Seatlholo ST, van Vuuren SF, Viljoen AM. The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res. 2006;18:Special Edition 129-133. [Cited in This Article: ] |
| 56. | Melillo de Magalhães P, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider YJ. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012;134:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol. 2008;36:340-348. [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Ganesh D, Fuehrer HP, Starzengrüber P, Swoboda P, Khan WA, Reismann JA, Mueller MS, Chiba P, Noedl H. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitol Res. 2012;110:2289-2295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771-785. [PubMed] [Cited in This Article: ] |
| 60. | Tasdemir D, Lack G, Brun R, Rüedi P, Scapozza L, Perozzo R. Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J Med Chem. 2006;49:3345-3353. [PubMed] [Cited in This Article: ] |
| 61. | Lin CM, Chen CT, Lee HH, Lin JK. Prevention of cellular ROS damage by isovitexin and related flavonoids. Planta Med. 2002;68:365-367. [PubMed] [Cited in This Article: ] |
| 62. | Raguso RA, Schlumpberger BO, Kaczorowski RL, Holtsford TP. Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry. 2006;67:1931-1942. [PubMed] [Cited in This Article: ] |
| 63. | Rodrigues Goulart H, Kimura EA, Peres VJ, Couto AS, Aquino Duarte FA, Katzin AM. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:2502-2509. [PubMed] [Cited in This Article: ] |
| 64. | Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob Agents Chemother. 2001;45:2553-2558. [PubMed] [Cited in This Article: ] |
| 65. | Su V, King D, Woodrow I, McFadden G, Gleadow R. Plasmodium falciparum growth is arrested by monoterpenes from eucalyptus oil. Flavour Frag J. 2008;23:315-318. [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Miller JA, Hakim IA, Chew W, Thompson P, Thomson CA, Chow HH. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr Cancer. 2010;62:783-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Charles DJ, JE Simon, Wood KV, Heinstein P. Germplasm variation in artemisinin content of Artemisia annua using an alternative method of artemisinin analysis from crude plant extracts. J Nat Prod. 1990;53:157–160. [DOI] [Cited in This Article: ] |
| 68. | Juergens UR, Engelen T, Racké K, Stöber M, Gillissen A, Vetter H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm Pharmacol Ther. 2004;17:281-287. [PubMed] [Cited in This Article: ] |
| 69. | Kovar KA, Gropper B, Friess D, Ammon HP. Blood levels of 1,8-cineole and locomotor activity of mice after inhalation and oral administration of rosemary oil. Planta Med. 1987;53:315-318. [PubMed] [Cited in This Article: ] |
| 70. | Stimpfl T, Nasel B, Nasel C, Binder R, Vycudilik W, Buchbauer G. Concentration of 1,8-cineol in human blood during prolonged inhalation. Chem Senses. 1995;20:349-350. [PubMed] [Cited in This Article: ] |
| 71. | Kengne RDC. Caracterisation physico-chimique de Artemisia annua (asteraceae), plante medicinale cultivee au Cameroun. MS thesis in Organic Chemistry. Republic of Cameroon: Univ de Dschang Republic of Cameroun 2010; . [Cited in This Article: ] |
| 72. | Akhtar F, Rizvi MM, Kar SK. Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol Adv. 2012;30:310-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Akkawi M, Jaber S, Abu-Remeleh Q, Ogwang PE, Lutgen P. Investigations of Artemisia annua and Artemisia sieberi water extracts inhibitory effects on β–hematin formation. Medicin Arom Plants. 2014;3:150. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Liu AR, Yu ZY, Lu LL, Sui ZY. [The synergistic action of guanghuoxiang volatile oil and sodium artesunate against Plasmodium berghei and reversal of SA-resistant Plasmodium berghei]. Zhongguo Jishengchongxue Yu Jishengchongbing Zazhi. 2000;18:76-78. [PubMed] [Cited in This Article: ] |
| 75. | Yang ZN, Zhu SQ, Yu ZW. Comparison of terpene components from flowers of Artemisia annua. Bangladesh J Pharmacol. 2012;7:114-119. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Khangholil S, Rezaeinodehi A. Effect of drying temperature on essential oil content and composition of sweet wormwood (Artemisia annua) growing wild in Iran. Pak J Biol Sci. 2008;11:934-937. [PubMed] [Cited in This Article: ] |
| 77. | Weathers PJ, Towler MJ. Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Ind Crop Prod. 2014;In press. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Juteau F, Masotti V, Bessière JM, Dherbomez M, Viano J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 2002;73:532-535. [PubMed] [Cited in This Article: ] |
| 79. | Roberts DW, Rank RG, Weidanz WP, Finerty JF. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977;16:821-826. [PubMed] [Cited in This Article: ] |
| 80. | Cherneva E, Pavlovic V, Smelcerovic A, Yancheva D. The effect of camphor and borneol on rat thymocyte viability and oxidative stress. Molecules. 2012;17:10258-10266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Lopes NP, Kato MJ, Andrade EH, Maia JG, Yoshida M, Planchart AR, Katzin AM. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiãpi Amazon Indians. J Ethnopharmacol. 1999;67:313-319. [PubMed] [Cited in This Article: ] |
| 82. | Muzemil A. Determination of artemisinin and essential oil contents of Artemisia annua L. grown in Ethiopia and in vivo antimalarial activity of its crude extracts against Plasmodium berghei in mice. Ethiopia: MS Thesis in Medicinal Chemistry, Addis Ababa University 2008; . [Cited in This Article: ] |
| 83. | Li Y, Hu HB, Zheng XD, Zhu JH, Liu LP. Composition and antimicrobial activity of essential oil from the aerial part of Artemisia annua. J Medicin Plants Res. 2011;5:3629-3633. [Cited in This Article: ] |
| 84. | Ahn BY, Jung MY. Antioxidant and protective activity of polysaccharide extract from Artemisia iwayomogi Kitamura stems on UVB-damaged mouse epidermis. J Appl Biol Chem. 2011;54:184-189. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Han H. Antimalarial Activity of Ginseng Polysaccharides and Bulgaria Inquinans Polysaccharides. MS Thesis in Biochem & Molec Biol. Changchun PRC: Northeast Normal Univ 2008; . [Cited in This Article: ] |
| 86. | Andrews KT, Klatt N, Adams Y, Mischnick P, Schwartz-Albiez R. Inhibition of chondroitin-4-sulfate-specific adhesion of Plasmodium falciparum-infected erythrocytes by sulfated polysaccharides. Infect Immun. 2005;73:4288-4294. [PubMed] [Cited in This Article: ] |
| 87. | Xiao L, Yang C, Patterson PS, Udhayakumar V, Lal AA. Sulfated polyanions inhibit invasion of erythrocytes by plasmodial merozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect Immun. 1996;64:1373-1378. [PubMed] [Cited in This Article: ] |
| 88. | Clark DL, Su S, Davidson EA. Saccharide anions as inhibitors of the malaria parasite. Glycoconj J. 1997;14:473-479. [PubMed] [Cited in This Article: ] |
| 89. | Munir M, Tjandra H, Rampengan TH, Mustadjab I, Wulur FH. Heparin in the treatment of cerebral malaria. Paediatr Indones. 1980;20:47-50. [PubMed] [Cited in This Article: ] |
| 90. | Rampengan TH. Cerebral malaria in children. Comparative study between heparin, dexamethasone and placebo. Paediatr Indones. 1991;31:59-66. [PubMed] [Cited in This Article: ] |
| 91. | Adams Y, Freeman C, Schwartz-Albiez R, Ferro V, Parish CR, Andrews KT. Inhibition of Plasmodium falciparum growth in vitro and adhesion to chondroitin-4-sulfate by the heparan sulfate mimetic PI-88 and other sulfated oligosaccharides. Antimicrob Agents Chemother. 2006;50:2850-2852. [PubMed] [Cited in This Article: ] |
| 92. | Ashok PK, Upadhyaya K. Preliminary phytochemical screening and physico-chemical parameters of Artemisia absinthium and Artemisia annua. J Pharmacog Phytochem. 2013;1:229-235. [Cited in This Article: ] |
| 93. | Massiha A, Khoshkholgh-Pahlaviani MM, Issazadeh K, Bidarigh S, Zarrabi S. Antibacterial activity of essential oils and plant extracts of Artemisia (Artemisia annua L.) in vitro. Zahedan J Res Med Sci. 2013;15:14-18. [Cited in This Article: ] |
| 94. | Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9:425-474. [PubMed] [Cited in This Article: ] |
| 95. | Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787-1796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 96. | Haruna M, Tanaka M, Sugimoto T, Kojima R, Suzuki Y, Konoshima T, Kozuka M, Ito K. Alteration of Na permeability in human erythrocytes as studied by 23Na-NMR and inhibition of the Na ,K -ATPase activities with saponins: Interactions of Gleditsia saponins with human erythrocyte membranes. Bioorg Med Chem Lett. 1995;5:827-830. [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587-605. [PubMed] [Cited in This Article: ] |
| 98. | Meotti FC, Ardenghi JV, Pretto JB, Souza MM, d’ Avila Moura J, Junior AC, Soldi C, Pizzolatti MG, Santos AR. Antinociceptive properties of coumarins, steroid and dihydrostyryl-2-pyrones from Polygala sabulosa (Polygalaceae) in mice. J Pharm Pharmacol. 2006;58:107-112. [PubMed] [Cited in This Article: ] |
| 99. | Chang TN, Deng JS, Chang YC, Lee CY, Jung-Chun L, Lee MM, Peng WH, Huang SS, Huang GJ. Ameliorative Effects of Scopoletin from Crossostephium chinensis against Inflammation Pain and Its Mechanisms in Mice. Evid Based Complement Alternat Med. 2012;2012:595603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Malik A, Kushnoor A, Saini V, Singhal1S , Kumar S, Yadav YC. In vitro antioxidant properties of scopoletin. J Chem Pharm Res. 2011;3:659-665. [Cited in This Article: ] |
| 101. | Moon PD, Lee BH, Jeong HJ, An HJ, Park SJ, Kim HR, Ko SG, Um JY, Hong SH, Kim HM. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IkappaB/NF-kappaB signal cascade in the human mast cell line HMC-1. Eur J Pharmacol. 2007;555:218-225. [PubMed] [Cited in This Article: ] |
| 102. | Cherng JM, Chiang W, Chiang LC. Immunomodulatory activities of common vegetables and spices of Umbelliferae and its related coumarins and flavonoids. Food Chem. 2008;106:944-950. [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Surono IS, Nishigaki T, Endaryanto A, Waspodo P. Indonesian biodiversities, from microbes to herbal plants as potential functional foods. J Fac Agric Shinshu U. 2008;44:23-27. [Cited in This Article: ] |
| 104. | Ezeokonkwo CA, Obidoa O. Effect of scopoletin on erythrocyte membrane ion motive ATPases. Nigerian J Nat Prod Med. 2001;5:37-40. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 105. | Dunn MJ. Alterations of red blood cell sodium transport during malarial infection. J Clin Invest. 1969;48:674-684. [PubMed] [Cited in This Article: ] |
| 106. | Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509-539, table of contents. [PubMed] [Cited in This Article: ] |
| 107. | Ding Z, Dai Y, Wang Z. Hypouricemic action of scopoletin arising from xanthine oxidase inhibition and uricosuric activity. Planta Med. 2005;71:183-185. [PubMed] [Cited in This Article: ] |
| 108. | de Vries PJ, de Vries PJ, Nguyen GC, de Goeje P, de Goeje P. Production and Application of Artemisinin in Vietnam. Institute of Materia Medica, Viet Nam and the University of Amsterdam: Gioi Publishers 1999; . [Cited in This Article: ] |
| 109. | Atkinson B, Mavituna F. Biochemical Engineering and Biotechnology Handbook. 2nd ed. USA: Stockton Press 1991; 1059-1110. [Cited in This Article: ] |
| 110. | Lim JAC, Patkar A, McDonagh G, Sinclair A, Lucy P. Modeling bioprocess cost: the economic benefits of expression technology based on Pseudomonas fluorescens. Bioprocess Intnl. 2010;8:62-70. [Cited in This Article: ] |
| 111. | Lapkin AA, Plucinski PK, Cutler M. Comparative assessment of technologies for extraction of artemisinin. J Nat Prod. 2006;69:1653-1664. [PubMed] [Cited in This Article: ] |
| 112. | Griffee P, Diemer P. Artemisia annua; the plant, production, processing and medicinal applications. Food and Agriculture Organization of the United Nations. Available from: http: //ecoport.org/ep?SearchType=earticleView&earticleId=727&page=2#section5675. [Cited in This Article: ] |
| 113. | Sipler D, Weathers PJ. Artemisia annua as a high value crop and weed control. Available from: http: //small-farm.org/SARE FNE12-766.html. [Cited in This Article: ] |
| 114. | Available from: http: //www.rollbackmalaria.org/partnership/wg/wg_procurementsupply/docs/psmwg_ppACT-API.pdf. [Cited in This Article: ] |
| 115. | Weathers PJ, Jordan NJ, Lasin P, Towler MJ. Simulated digestion of dried leaves of Artemisia annua consumed as a treatment (pACT) for malaria. J Ethnopharmacol. 2014;151:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Zhu C, Cook SP. A concise synthesis of (+)-artemisinin. J Am Chem Soc. 2012;134:13577-13579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 117. | Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1305] [Cited by in F6Publishing: 1251] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 118. | Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FC, Chollet J, Craft JC, Creek DJ. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci USA. 2011;108:4400-4405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 119. | Charman SA; A2s2. [cited 2014 May 25]. Available from: http: //www.a2s2.org. [Cited in This Article: ] |
| 120. | ETC Group. Synthetic Biology: Livelihoods and Biodiversity. Artemisinin. Cited: 2014-05-25. Available from: http: //www.etcgroup.org/files/CBD_Artemisinin_case_study_TA.pdf. [Cited in This Article: ] |
| 121. | Kentucky Cooperative Extension Service. GAP Good Agricultural Practices (GAP). Cited: 2014-05-25. Available from: http: //www.uky.edu/Ag/CCD/introsheets/gap.pdf. [Cited in This Article: ] |
| 122. | Charman SA; Pewtrusts. GAP Comparison of gaps governing the growing and harvesting of fresh produce. Cited: 2014-05-27. Available from: http://www.pewhealth.org/uploadedFiles/PHG/Content_Level_Pages/Reports/PSP-RPT-GAP-Governing-Fresh-Produce.pdf. [Cited in This Article: ] |
| 123. | World Health Organization. WHO monograph on good agricultural and collection practices (GACP) for Artemisia annua L. Available from: http: //www.who.int/malaria/publications/atoz/9241594438/en/. [Cited in This Article: ] |
| 124. | World Health Organization. WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants. Available from: http: //whqlibdoc.who.int/publications/2003/9241546271.pdf. [Cited in This Article: ] |
| 125. | World Health Organization. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Available from: http: //apps.who.int/medicinedocs/documents/s14878e/s14878e.pdf. [Cited in This Article: ] |
| 126. | Marchand E, Atemnkeng MA, Vanermen S, Plaizier-Vercammen J. Development and validation of a simple thin layer chromatographic method for the analysis of artemisinin in Artemisia annua L. plant extracts. Biomed Chromatogr. 2008;22:454-459. [PubMed] [Cited in This Article: ] |
| 127. | Koobkokkruad T, Chochai A, Kerdmanee C, De-Eknamkul W. TLC-densitometric analysis of artemisinin for the rapid screening of high-producing plantlets of Artemisia annua L. Phytochem Anal. 2007;18:229-234. [PubMed] [Cited in This Article: ] |
| 128. | Arvouet-Grand A, Vennat B, Pourrat A, Legret P. [Standardization of propolis extract and identification of principal constituents]. J Pharm Belg. 1994;49:462-468. [PubMed] [Cited in This Article: ] |
| 129. | Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin Infect Dis. 2008;47:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 130. | Efferth T. Artemisinin: a versatile weapon from traditional Chinese medicine. Herbal drugs: ethnomedicine to modern medicine. Heidelberg: Springer Verlag 2009; 179-194. [Cited in This Article: ] |
| 131. | Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med. 2009;11:e32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 133. | Brisibe EA, Umoren UE, Brisibe F, Magalhäes PM, Ferreira JFS, Luthria D, Wu X, Prior RL. Nutritional characterization and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009;115:1240-1246. [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 134. | Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8:1841-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Avery MA, Muraleedharan KM, Desai PV, Bandyopadhyaya AK, Furtado MM, Tekwani BL. Structure-activity relationships of the antimalarial agent artemisinin. 8. design, synthesis, and CoMFA studies toward the development of artemisinin-based drugs against leishmaniasis and malaria. J Med Chem. 2003;46:4244-4258. [PubMed] [Cited in This Article: ] |
| 136. | Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007;56:1213-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 137. | Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51:1852-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 138. | Ferreira JF, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis: trematocidal plant alcoholic extracts. Parasitol Res. 2011;109:1585-1592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 139. | World Health Organization. The 17 neglected tropical diseases. Available from: http: //www.who.int/neglected_diseases/diseases/en/. [Cited in This Article: ] |
| 140. | Marconi VC. Commentary: Malaria and HIV transmission: old meets new in a deadly partnership or an opportunity for healthcare synergism? Int J Epidemiol. 2011;40:940-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 141. | Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9-15. [PubMed] [Cited in This Article: ] |
| 142. | Ezeamama AE, Spiegelman D, Hertzmark E, Bosch RJ, Manji KP, Duggan C, Kupka R, Lo MW, Okuma JO, Kisenge R. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis. 2012;205:1486-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 143. | Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, Lindsay SW. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382:963-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 144. | Lubbe A, Seibert I, Klimkait T, van der Kooy F. Ethnopharmacology in overdrive: the remarkable anti-HIV activity of Artemisia annua. J Ethnopharmacol. 2012;141:854-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Willcox ML, Burton S, Oyweka R, Namyalo R, Challand S, Lindsey K. Evaluation and pharmacovigilance of projects promoting cultivation and local use of Artemisia annua for malaria. Malar J. 2011;10:84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |