Published online Feb 4, 2018. doi: 10.5492/wjccm.v7.i1.1
Peer-review started: September 17, 2017
First decision: November 27, 2017
Revised: December 3, 2017
Accepted: December 14, 2017
Article in press: December 14, 2017
Published online: February 4, 2018
To evaluate the effects of mineralocorticoid receptor (MR) antagonists on mortality and inflammatory responses after hemorrhagic shock (HS) in rats.
One hundred and two male Sprague–Dawley rats were randomly assigned to one of the following three groups: Control, spironolactone (SPL), and eplerenone (EP) groups. HS was induced by the removal of blood. One half of rats were evaluated to determine mortality, hemodynamics, plasma tumor necrosis factor-alpha (TNF-α) concentrations, and arterial blood gas at 8 h after HS recovery. In the remainder of rats, the expression levels of genes encoding cytokines were evaluated in liver tissue samples at 1 h after HS recovery.
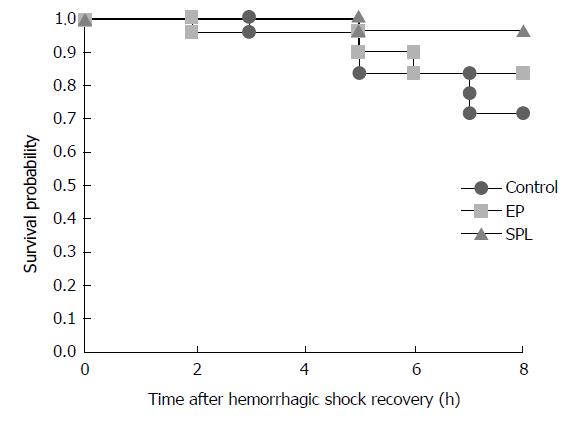
The survival rates 8 h after HS recovery were 71%, 94%, and 82% in the control, SPL, and EP groups, respectively. There were no significant differences in survival rates among the three groups (P = 0.219). Furthermore, there were no significant differences in gene expression levels in the liver or plasma TNF-α concentrations among the three groups (P = 0.888).
Pretreatment with MR antagonists did not improve mortality or cytokine responses in the liver after HS recovery in rats.
Core tip: Mineralocorticoid receptor (MR) antagonists have anti-inflammatory effects in models of ischemic and reperfusion injury, suggesting potential clinical value in patients with hemorrhagic shock. However, our findings indicate that pretreatment with MR antagonists does not improve mortality rates or cytokine responses in the liver after recovery from hemorrhagic shock in rats.
- Citation: Yamamoto K, Yamamoto T, Takamura M, Usui S, Murai H, Kaneko S, Taniguchi T. Effects of mineralocorticoid receptor antagonists on responses to hemorrhagic shock in rats. World J Crit Care Med 2018; 7(1): 1-8
- URL: https://www.wjgnet.com/2220-3141/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v7.i1.1
Hemorrhagic shock (HS), a frequent and dangerous complication of trauma and massive intraoperative bleeding, is associated with high mortality and morbidity. HS causes ischemic injury in vital organs and tissues, and resuscitation for HS causes reperfusion injury in these tissues. Ischemic and reperfusion injury (IRI) causes the release of numerous pro-inflammatory mediators, such as cytokines and nitric oxide, and results in multiple organ dysfunction (MOD), a leading cause of death in HS patients[1-5]. Mineralocorticoid receptor (MR) antagonists, such as spironolactone (SPL) and eplerenone (EP), have anti-inflammatory effects in vitro[6-8]. In particular, SPL inhibits inflammatory responses, such as the attenuation of cytokine and NF-kappa B responses, in vitro[6-8]. Moreover, in several animal models, MR antagonists protect against IRI in various organs, including the kidney, liver, intestine, heart, and brain[9-13]. These observations suggest that MR antagonists have beneficial effects during HS and after recovery from HS. In clinical settings, MR antagonists are often administered to hypertensive patients to control blood pressure (BP)[14,15]. However, it is not clear whether MR antagonists have beneficial effects when administered before reaching the HS state caused by trauma and massive intraoperative bleeding. We hypothesized that pretreatment with MR antagonists has beneficial effects on MOD after HS. Accordingly, we evaluated the effects of pretreatment with SPL and EP on mortality and inflammatory responses after HS in rats.
All procedures involving animals were reviewed and approved by the Committee on Animal Experimentation of Kanazawa University (AP-153421).
Effect of MR antagonists on mortality and inflammatory responses in HS rats: Fifty-one male Sprague–Dawley (SD) rats (body weight, 350–400 g) were randomly divided into the following 3 groups (n = 17 per group): Control (no medication), SPL (oral administration of SPL at 10 mg/kg per day for 5 d), and EP (oral administration of EP at 100 mg/kg per day for 5 d). The rats received SPL and EP with food. Rats in the SPL and EP groups received powder medicine with powder feed for 5 d. The doses of EP and SPL were selected based on previous studies in rats[16,17] .
After medication for 5 d, all rats were anesthetized with pentobarbital sodium (intraperitoneal injection of 50 mg/kg)[18]. Rats underwent tracheostomy, and a cannula was inserted into the trachea. The tracheal cannula was attached to a respirator after a cannula was inserted into the carotid artery. Ventilation was performed by administering oxygen (100%, 1 L/min) at a frequency of 32 breaths/min with an inspiratory/expiratory ratio of 1:1 using a small animal respirator. Then, the femoral artery and vein were cannulated. After the operation, lactate Ringer’s solution containing muscle relaxant (rocuronium bromide 0.75 mg/mL) and an anesthetic (pentobarbital sodium 0.98 mg/mL) were continuously infused through the cannula of the femoral vein at 10 mL/kg per hour. The femoral artery catheter was connected to a pressure transducer to monitor the arterial blood pressure and heart rate (HR). Rats were placed on a warming pad and maintained at 36-38 °C, as measured using a rectal thermometer.
After the stabilization of rats for 30 min, their blood was drawn via the carotid artery cannula to induce HS. Systolic arterial pressure (SAP) was maintained at less than 40 mmHg for 40 min. Removal blood volume were 13 ± 0.4 mL, 13 ± 0.5 mL, and 13 ± 0.4 mL in the control group, SPL group, and EP group, respectively. The removed blood was diluted two-fold in lactate Ringer's solution and an equal volume was returned through the femoral vein cannula. The methods for this experiment were described in a previous study[19].
The survival rate, SAP, and HR were observed for up to 8 h after HS recovery. The arterial blood sample (0.25 mL) was obtained before HS and at 0, 1, 2, 3, 4, and 5 h after HS recovery. And then PH, Lactate, BE and Hb were measured immediately by The ABL800 FLEX blood gas analyzer. Furthermore, arterial blood samples (1.5 mL) were obtained before HS and at 2, 4, and 5 h after HS recovery to measure plasma tumor necrosis factor (TNF)-α. The TNF-α concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (Boster Biological Technology, Pleasanton, CA, United States).
Effects of MR antagonists on gene expression in the liver after recovery from hemorrhagic shock: An additional 51 male SD rats were randomly divided into the following 3 groups (n = 17 per group): Control group (no medication), SPL group (oral administration of SPL at 10 mg/kg per day for 5 d), and EP group (oral administration of EP at 100 mg/kg per day for 5 d).
As described above, all animals were anesthetized and HS was induced. At 1 h after recovery from HS, the organization of the liver was examined in the three groups. Liver tissue samples were obtained at 1 h after recovery from HS; the time was established based on previous studies[20]. Each sample was placed in a container, frozen in liquid nitrogen, and stored at -80 °C.
Total RNA was isolated from liver tissues using the High Pure RNA Tissue Kit (Roche Diagnostics, Mannheim, Germany). The quality and quantity of RNA was determined using a NanoDrop (NanoDrop Technologies, Wilmington, DE, United States). The RNA was reverse-transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States). Quantitative real-time detection polymerase chain reaction (RTD-PCR) was performed using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The following primers and TaqMan probes (Applied Biosystems) were used: interleukin (IL)-6 (Rn01410330_m1), TNF (Rn01525859_g1), IL-1β (Rn00580432_m1), intercellular adhesion molecule (ICAM) 1 (Rn00564227_m1), and 18S rRNA (18S,4319413E). The following standard protocol was followed for all reactions: 30 s at 95 °C (initial denaturation), 40 cycles of 5 s at 95 °C and 30 s at 60 °C. mRNA levels were standardized against 18S rRNA expression levels[21] .
The protocol was designed to minimize pain or discomfort. Before operation all rats were anesthetized with pentobarbital sodium (intraperitoneal injection of 50 mg/kg). Ventilation was performed by administering oxygen (100%, 1 L/min) using a small animal respirator during experiment. Lactate Ringer’s solution containing muscle relaxant (rocuronium bromide 0.75 mg/mL) and an anesthetic (pentobarbital sodium 0.98 mg/mL) were continuously infused through the cannula of the femoral vein at 10 mL/kg per hour during experiment. All rats were euthanized by intravenous injection KCl under general anesthesia for tissue collection.
At a sample size analysis, one-sided Fisher’s exact tests with a significance level of 5% and a power of 85% showed that a minimum of 17 rats per group was needed to detect a difference in the survival ratio of at least 40% between control and treatment groups. It was based on the result of the preliminary experiment. All data are expressed as means ± standard error (SE). At survival rate analysis, the death was defined as an event. The observation period was defined as eight hours since HS recovery. If an event did not occur at the time of the end during an observation period, it was censored. Survival rates for the three groups were compared using the Kaplan–Meier and Log Rank (Mantel–Cox) tests. Significance was defined as P < 0.01. Differences between groups in hemodynamic properties, including blood gas analysis (BGA) and plasma TNF-α, were analyzed using two-way repeated measure ANOVA, followed by post-hoc tests (Bonferroni’s method). Differences in gene expression levels between groups were analyzed by one-way ANOVA. Significance was defined as P < 0.01. Data analyses were implemented in SPSS v.23 (SPSS Inc., Chicago, IL, United States).
The survival rates 8 h after recovery from HS were 71%, 94%, and 82% in the control group, SPL group, and EP group, respectively. There were no significant differences in survival rate among the three groups (Figure 1).
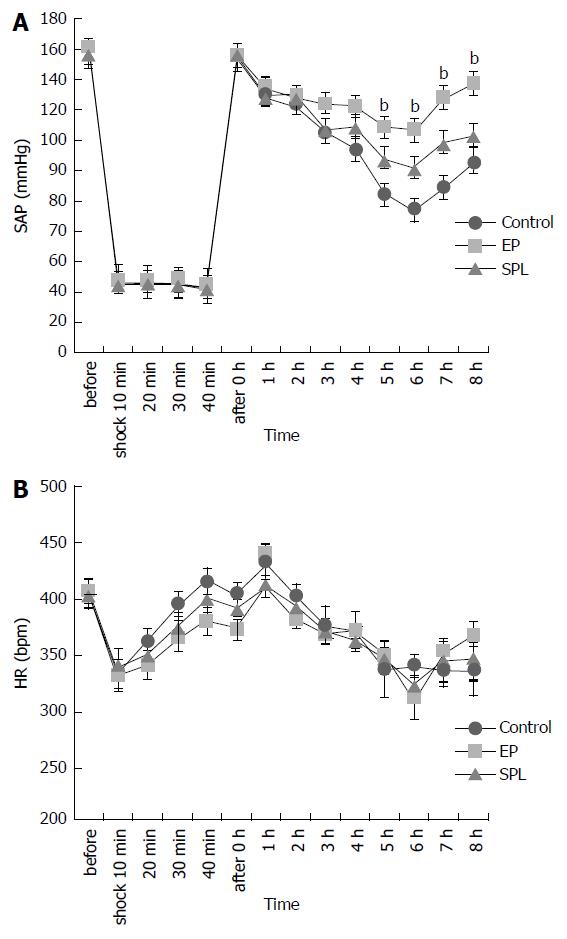
SAPs gradually decreased after HS recovery in all groups. SAPs of the control group decreased more in comparison with SAPs of the MR antagonists treatment group. SAPs of the EP treatment group did not decrease very much in comparison with the SAP of the control group. There were significant differences among three groups in SAP (P < 0.01). There were significant differences between EP group and control group in SAP at 5-8 h after the HS recovery (EP vs control; P < 0.01, Figure 2).
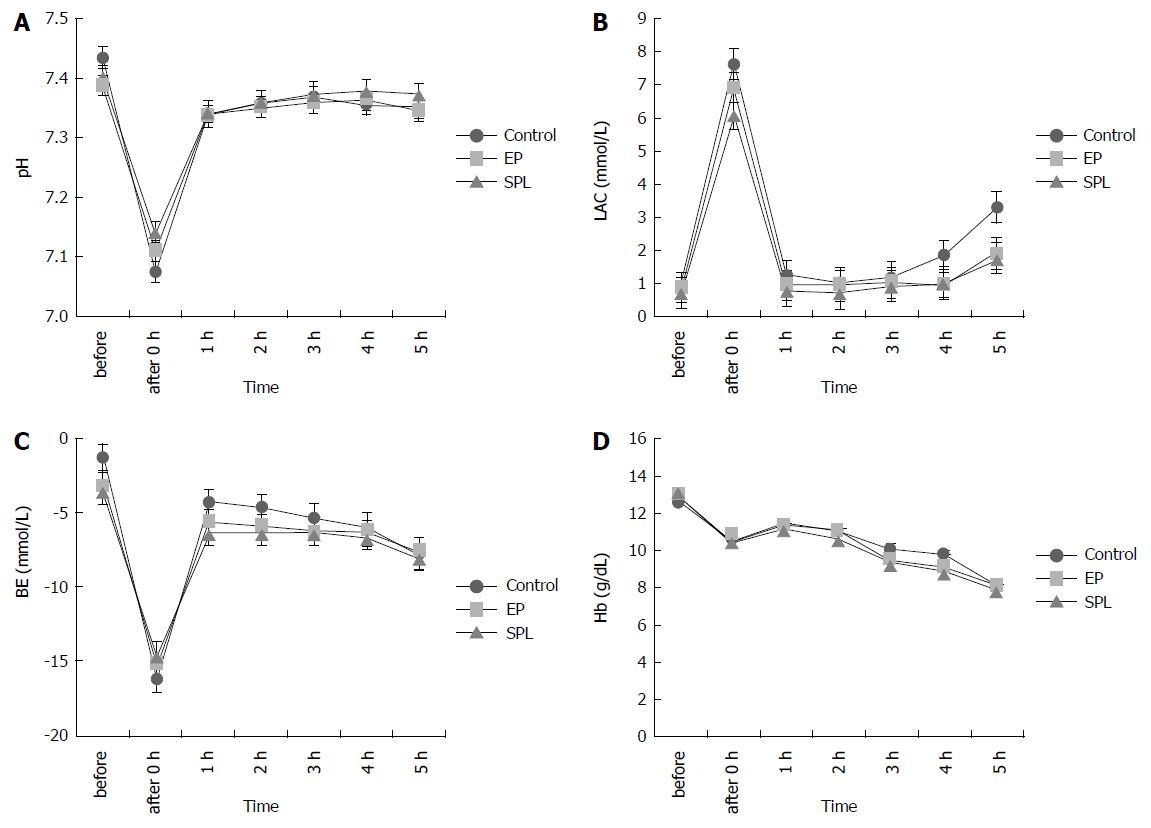
The lactate concentrations increased immediately after HS, but recovered in all groups. There were no significant differences among the three groups in lactate concentration, Hb, PH, and BE (Figure 3).
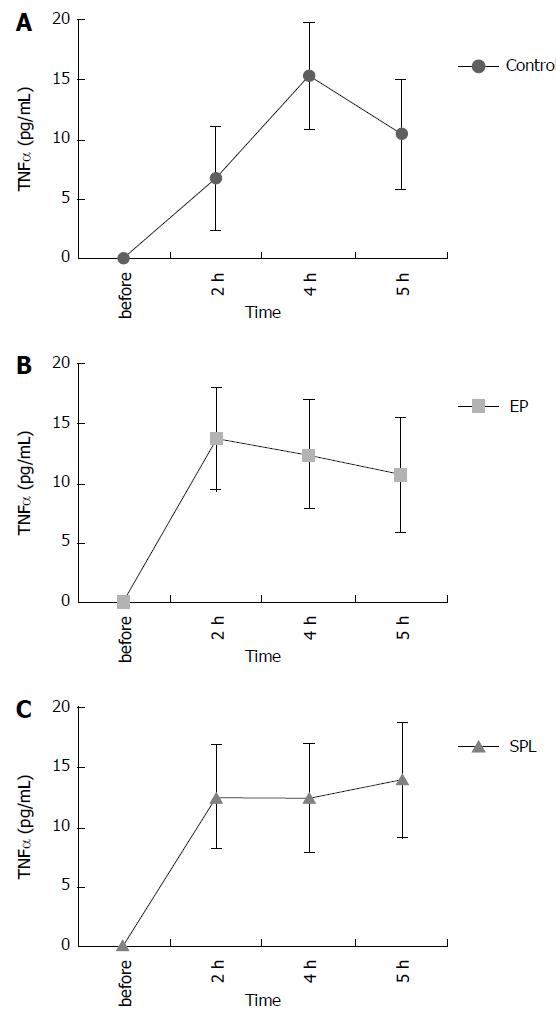
The plasma TNF-α concentration in all groups increased slightly after the recovery from HS. There were no significant differences in plasma TNF-α concentrations among the three groups (Figure 4).
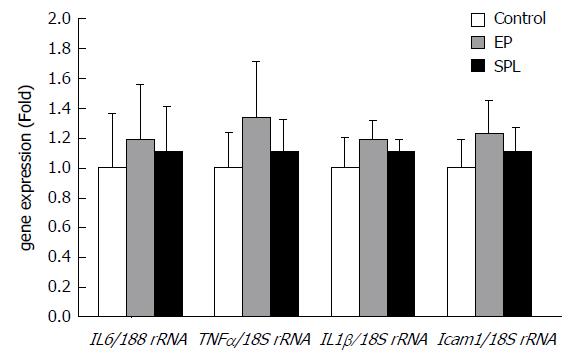
The mRNA expression levels of TNF-α, IL-6, IL-1β, and ICAM-1 did not exhibit significant differences in the liver at 1 h after HS recovery among the three groups (Figure 5).
HS induced by partial exsanguination in rats caused metabolic acidosis and increased TNF-α concentrations immediately after the return of blood. Metabolic acidosis deteriorated immediately after the recovery from HS, but was gradually re-aggravated. As a result, the survival rate was low. HS with the oral administration of SPL and EP, MR antagonists, also resulted in similar to HS of the control. These were no significant differences between the control and MR antagonist-treated groups in the responses after HS recovery. Cytokine gene expression in the liver tissue 1 h after HS recovery did not differ among groups. Thus, our results showed that the oral administration of MR antagonists does not affect the mortality rate and inflammatory responses after HS recovery in rats based on comparisons with control rats after HS recovery.
MR antagonists are often used to treat hypertensive patients and have beneficial effects in patients with chronic heart failure and ischemic heart diseases[14,15,22,23]. Many recent investigations have demonstrated the beneficial effects of MR antagonists with respect to inflammatory responses and IRI in vitro and in vivo[6-13]. Kato et al[6] showed that SPL inhibits the production of pro-inflammatory mediators, such as TNF-α and nitric oxide, in response to lipopolysaccharides in vitro. Ozacmak et al[11] showed that pretreatment with SPL reduces intestinal injury induced by IR, including the inhibition of inflammatory responses, in rats. These studies suggest that the early and continuous administration of MR antagonists has beneficial effects in critical patients with MOD after HS. However, the effects of pretreatment with MR antagonists on MOD after HS are unclear. Therefore, we evaluated the effects of the pretreatment of MR antagonists on mortality and inflammatory responses after HS in rats. Contrary to our expectations, pretreatment with MR antagonists did not have beneficial effects after HS in rats.
Many investigations have demonstrated that activated Kupffer cells release inflammatory cytokines, such as TNF-α or IL-1, soon after liver IRI. TNF-α increases the expression of ICAM-1 and promotes the adhesion of neutrophils[24-26]. Liver failure is recognized after liver IRI, and many other organs (e.g., the myocardium, pancreas, small intestine, kidney, adrenal gland, and lungs) seem to be damaged by inflammatory reactions and oxidation[27]. Therefore, it is important to evaluate cytokine production in the liver at an early stage after HS recovery. Several reports have suggested that MR antagonists modulate the production of cytokines, such as TNF-α, IL-6, and IL-1β, in various organs after IRI[9-13]. Pérez et al[10] showed that SPL reduces liver damage caused by IRI induced by increases in catalase activity in the liver. Therefore, we hypothesized that pretreatment with MR antagonists has beneficial effects on the expression of genes encoding cytokines, such as TNF-α, IL-6, IL-1β, and ICAM-1, in the liver after recovery from HS. However, we did not detect beneficial effects of MR antagonist pretreatment on cytokine gene expression after HS in rats.
There are several potential explanations for the differences between our results and those of previous studies. First, it is possible that these differences are explained by the relatively short period of ischemia in this study. Suzuki et al[28] reported that the activation of Kupffer cell depends on the duration of liver ischemia. The short duration of ischemia may explain lower plasma TNF-α concentrations in this study than in past reports. Further studies are needed to examine long-term ischemic models. Second, the differences among studies may be explained by differences in the dose and duration of the administration of MR antagonists. Ikeda et al[9] used a high SPL dose and long period of administration (100 mg/kg per day and 12 wk) in rats. Ozacmak et al[11] used a high SPL dose and short period of administration (20 mg/kg/day and 3 d) in rats. Further investigations are needed to clarify the dose- and time-dependent effects of MR antagonists. Finally, the present study used normal SD rats. In clinical settings, MR antagonists are generally administered to hypertensive patients. Further investigations of hypertensive rats are needed to establish the general relevance of the results.
In this study, SAPs of the EP treatment group did not decrease in comparison with the SAP of the control group, however it did not affect the survival rate. In a previous study, Kajihara et al[29] showed that blood aldosterone and cortisol levels were rapidly increased after hemorrhage in dog. After recovery of hemorrhagic shock, arterial blood pressure and blood cortisol decreased, however the blood aldosterone level remained relatively high. Rushing et al[30] showed that serum corticosterone was stimulated in hemorrhagic shock model of rats. The blood corticosterone and aldosterone in rat can be related to the blood pressure change after the hemorrhagic shock. However, we did not evaluate the change of the blood pressure and the relations of blood aldosterone and corticosterone, in this study. The difference between EP and SPL is selectivity to MR. So further studies are needed to estimate blood corticosterone and aldosterone in hemorrhagic shock by using rats which MR antagonists were administered.
Clinically, it is important to determine whether MR antagonists have beneficial effects when they are administered before reaching the HS state after trauma and massive intraoperative bleeding. We previously showed that the oral administration of a beta antagonist, carvedilol, increases the mortality rate and worsens inflammatory responses to severe HS in rats[19]. Our previous findings suggest that beta antagonists worsen the recovery from severe HS. In the present study, MR antagonists did not worsen HS recovery.
The present study was limited by the number of rats and the method for inducing HS. It may be necessary to evaluate a larger sample of rats to increase power in the analysis of survival rate, but this is difficult in animal experiments. Blood was removed by the carotid artery rapidly and a low blood pressure was maintained. This HS model may not be representative of clinical situations. Accordingly, additional studies should examine the MR antagonists using different methods for HS induction.
In conclusion, the present study showed that pretreatment with MR antagonists, such as SPL and EP, does not decrease the mortality rate and does not attenuate inflammatory responses to HS in rats. These findings suggest that MR antagonists did not worsen HS recovery.
Clinically, mineralocorticoid receptor (MR) antagonists such as spironolactone (SPL) and eplerenone (EP) are often administered to hypertensive patients to control blood pressure. However, it is not clear whether MR antagonists have beneficial effects when patients administered MR antagonists become hemorrhagic shock (HS) state caused by trauma and intraoperative bleeding.
It is very important for perioperative management to clarify the influence of MR antagonist administration before HS state caused by trauma and intraoperative bleeding.
The effects of pretreatment of MR antagonists on mortality and inflammatory responses after HS were evaluated in rats.
HS was induced by the removal of blood by using rats which MR antagonists were administered or were not administered. The effects of pretreatment of MR antagonists were evaluated by mortality, hemodynamics, plasma TNF-α concentrations, arterial blood gas and liver TNF-α, IL-6, IL-1β and ICAM-1 mRNA expression after HS recovery.
There were no significant differences among the three groups in survival rate, plasma TNF-α concentrations, arterial blood gas and liver TNF-α, IL-6, IL-1β and ICAM-1 mRNA expression. Systolic arterial pressure (SAP) after HS recovery did not decrease in rats of EP group in comparison with control groups. After HS recovery, the reason why blood pressure was maintained in rats of EP group is the problems that remain to be solved, in this research.
Pretreatment with MR antagonists did not improve mortality or cytokine responses in the liver after HS recovery in rats. The HS model in the present study was made during general anesthesia after pretreatment of MR antagonists. This model is similar to the clinical situation when patients administered MR antagonists become HS state during operation. The present study suggested that MR antagonists may not be worsen the recovery of HS state and may not need to be withdrawn before the operations.
The present study used normal SD rats. In clinical settings, MR antagonists are generally administered to hypertensive patients. Further investigations by using hypertensive rats which MR antagonists were administered will be needed. The present study, SAPs of the EP treatment group did not decrease in comparison with the SAP of the control group, so further studies are needed to evaluate relations of blood corticosterone or aldosterone and blood pressure in hemorrhagic shock by using rats which MR antagonists were administered.
We thank Hisayo Masaki and Ayano Nomura for support during the experiment. We would like to thank Editage (http://www.editage.jp) for English language editing.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltowski J, Chello M, Spasojevi SD S- Editor: Kong JX L- Editor: A E- Editor: Li RF
| 1. | Hardaway RM. Traumatic shock alias posttrauma critical illness. Am Surg. 2000;66:284-290. [PubMed] [Cited in This Article: ] |
| 2. | Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Yao YM, Redl H, Bahrami S, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res. 1998;47:201-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2004;286:G225-G233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery. 1995;118:358-364; discussion 364-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 178] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Kato Y, Kamiya H, Koide N, Odkhuu E, Komatsu T, Dagvadorj J, Watarai A, Kondo M, Kato K, Nakamura J. Spironolactone inhibits production of proinflammatory mediators in response to lipopolysaccharide via inactivation of nuclear factor-κB. Immunopharmacol Immunotoxicol. 2014;36:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Sønder SU, Woetmann A, Odum N, Bendtzen K. Spironolactone induces apoptosis and inhibits NF-kappaB independent of the mineralocorticoid receptor. Apoptosis. 2006;11:2159-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Miura R, Nakamura K, Miura D, Miura A, Hisamatsu K, Kajiya M, Nagase S, Morita H, Fukushima Kusano K, Ohe T. Anti-inflammatory effect of spironolactone on human peripheral blood mononuclear cells. J Pharmacol Sci. 2006;101:256-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ikeda H, Tsuruya K, Toyonaga J, Masutani K, Hayashida H, Hirakata H, Iida M. Spironolactone suppresses inflammation and prevents L-NAME-induced renal injury in rats. Kidney Int. 2009;75:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Pérez JC, Ramírez AC, González LT, Espinosa LE, Quintana MM, Galván GA, Chavira HZ, de la Garza FJ, Lemarroy CR, Garza NE. Spironolactone Effect in Hepatic Ischemia/Reperfusion Injury in Wistar Rats. Oxid Med Cell Longev. 2016;2016:3196431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ozacmak HS, Ozacmak VH, Barut F, Araslı M, Ucan BH. Pretreatment with mineralocorticoid receptor blocker reduces intestinal injury induced by ischemia and reperfusion: involvement of inhibition of inflammatory response, oxidative stress, nuclear factor κB, and inducible nitric oxide synthase. J Surg Res. 2014;191:350-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Dorrance AM, Osborn HL, Grekin R, Webb RC. Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R944-R950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Jansen PM, Frenkel WJ, van den Born BJ, de Bruijne EL, Deinum J, Kerstens MN, Arnoldus JH, Woittiez AJ, Wijbenga JA, Zietse R. Determinants of blood pressure reduction by eplerenone in uncontrolled hypertension. J Hypertens. 2013;31:404-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Guo H, Xiao Q. Clinical efficacy of spironolactone for resistant hypertension: a meta analysis from randomized controlled clinical trials. Int J Clin Exp Med. 2015;8:7270-7278. [PubMed] [Cited in This Article: ] |
| 16. | Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802-H1810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Tanaka H, Watanabe K, Harima M, Thanikachalam PV, Yamaguchi K, Tachikawa H, Kodama M, Aizawa Y. Effects of various diuretics on cardiac function in rats with heart failure. Yakugaku Zasshi. 2009;129:871-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Yamashita M, Taniyama M, Tamai M. Cellular localization of tumor necrosis factor-alpha mRNA and interleukin-6 mRNA in the rat liver after hemorrhagic shock. Surg Today. 2002;32:701-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Taniguchi T, Kurita A, Yamamoto K, Inaba H. Effects of carvedilol on mortality and inflammatory responses to severe hemorrhagic shock in rats. Shock. 2009;32:272-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Liu LM, Dubick MA. Hemorrhagic shock-induced vascular hyporeactivity in the rat: relationship to gene expression of nitric oxide synthase, endothelin-1, and select cytokines in corresponding organs. J Surg Res. 2005;125:128-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kitano K, Usui S, Ootsuji H, Takashima S, Kobayashi D, Murai H, Furusho H, Nomura A, Kaneko S, Takamura M. Rho-kinase activation in leukocytes plays a pivotal role in myocardial ischemia/reperfusion injury. PLoS One. 2014;9:e92242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6409] [Cited by in F6Publishing: 5877] [Article Influence: 235.1] [Reference Citation Analysis (0)] |
| 23. | Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3634] [Cited by in F6Publishing: 3288] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 24. | Wanner GA, Ertel W, Müller P, Höfer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 200] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Mendes-Braz M, Elias-Miró M, Jiménez-Castro MB, Casillas-Ramírez A, Ramalho FS, Peralta C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J Biomed Biotechnol. 2012;2012:298657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 195] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Nastos C, Kalimeris K, Papoutsidakis N, Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V, Arkadopoulos N. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Suzuki S, Nakamura S, Sakaguchi T, Ochiai H, Konno H, Baba S, Baba S. Alteration of reticuloendothelial phagocytic function and tumor necrosis factor-alpha production after total hepatic ischemia. Transplantation. 1997;64:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kajihara H, Malliwah JA, Matsumura M, Taguchi K, Iijima S. Changes in blood cortisol and aldosterone levels and ultrastructure of the adrenal cortex during hemorrhagic shock. Pathol Res Pract. 1983;176:324-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Rushing GD, Britt RC, Britt LD. Effects of hemorrhagic shock on adrenal response in a rat model. Ann Surg. 2006;243:652-654; discussion 654-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |