Published online Nov 10, 2015. doi: 10.5317/wjog.v4.i4.113
Peer-review started: March 31, 2015
First decision: April 27, 2015
Revised: July 25, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: November 10, 2015
AIM: To appraise critically the published randomised controlled trials (RCTs) reporting on the effectiveness of using hyaluronic acid (HA) for sperm immobilisation and selection before intracytoplasmic sperm injection (ICSI).
METHODS: Two authors used the PICO Method in order to perform a comprehensive literature search of the standard medical databases in June 2015. Data from the included studies was extracted independently by two authors using a predefined pro-forma. Review Manager (RevMan) was used to calculate the combined outcomes where multiple studies contributed with their results. Risk ratio (RR) with a 95%CI using the Mantel-Haenszel method was calculated for binary data variables. Heterogeneity was measured using the χ2 test and quantified using I2. In case of substantial heterogeneity (P < 0.10 for χ2 test or I2 > 50%) the combined outcome was calculated using the random effects model. The results from the meta-analysis were displayed as forest plots. The guideline of the Cochrane Collaboration was used to assess the risk of bias and it was illustrated as a risk of bias graph.
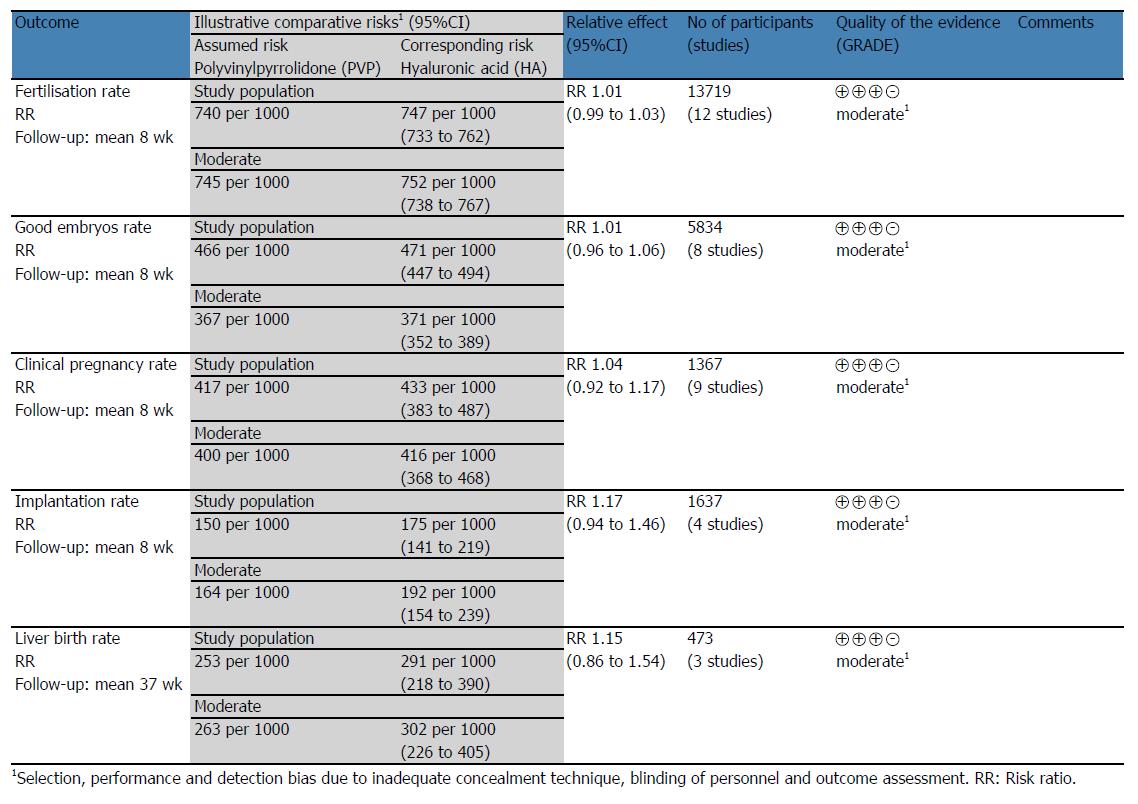
RESULTS: The systematic literature search identified 166 different studies related to sperm immobilisation and selection for ICSI. Eleven RCTs involving 13719 oocyte intracytoplasmatic injections with sperm immobilised and selected using HA or polyvinylpyrrolidone (PVP) were included in this systematic review and meta-analysis. There was low heterogeneity among the included trials (χ2 = 16.86, df = 11, P = 0.11; I2 = 35%). There was no statistical difference between HA and PVP groups in terms of fertilisation rate (RR = 1.01; 95%CI: 0.99-1.03; z = 0.75; P = 0.45), good embryos rate (RR = 1.01; 95%CI: 0.96-1.06; z = 0.30; P = 0.76), live birth rate (RR = 1.15; 95%CI: 0.86-1.54; z = 0.92; P = 0.36), clinical pregnancy rate (RR = 1.04; 95%CI: 0.92-1.17; z = 0.62; P = 0.53) and implantation rate (RR = 1.17; 95%CI: 0.94-1.46; z = 0.40; P = 0.16). The quality of most of the included studies was moderate to poor because of unclear randomisation technique, inadequate allocation concealment and blinding.
CONCLUSION: This systematic review and meta-analysis provides evidence of similar efficiency between using HA or PVP for sperm immobilisation and selection before ICSI.
Core tip: Hyaluronic acid (HA) has been proposed as a physiological alternative to polyvinylpyrrolidone (PVP) for use as a selection medium to reduce sperm motility as a solution for the reported toxicity and unknown long term effects of PVP. We performed a systematic review and meta-analysis of eleven randomised controlled trials involving 13719 oocyte intracytoplasmatic injections with sperm immobilised and selected using HA or PVP. There was no difference between HA and PVP groups in terms of fertilisation, embryo quality, clinical pregnancy, implantation and live birth rates.
- Citation: Craciunas L, Tsampras N, Kollmann M, Stirbu L, Raine-Fenning NJ. Use of hyaluronic acid for sperm immobilisation and selection before intracytoplasmic sperm injection: A systematic review and meta-analysis. World J Obstet Gynecol 2015; 4(4): 113-123
- URL: https://www.wjgnet.com/2218-6220/full/v4/i4/113.htm
- DOI: https://dx.doi.org/10.5317/wjog.v4.i4.113
The success of intracytoplasmic sperm injection (ICSI), as a bypass of the natural selection processes taking place in the female reproductive tract, would be impossible without advances in the laboratory preparation and identification of sperm for use with ICSI. Several methods (ultramorphology, surface electric charge, apoptotic vs nonapoptotic, chromatin structure assay) have been recently proposed for optimising the sperm selection in order to reduce the risk of chromosomal anomalies associated with poor ICSI outcome[1,2].
Hyaluronic acid (HA) is found naturally in the women’s reproductive tract and it forms a component of the cumulus-oocyte complex. It has been proposed as a physiological alternative to polyvinylpyrrolidone (PVP) for use as a selection medium to reduce sperm motility as a solution for the reported toxicity and unknown long term effects of PVP[3,4].
Furthermore, it has been shown that sperm’s capacity to bind HA is a biochemical marker of maturity and function, suggesting the selection of sperm by HA binding to be an alternative to microscopic assessment of motility and morphology[3].
Several descriptive reviews of the current advanced sperm selection methods support the use of HA for sperm immobilisation and selection for ICSI, but none of them report a quantitative measure of the effect it has on the ICSI outcome[5-8].
The objective of this study is to appraise critically the published randomised controlled trials (RCTs) reporting on the use of HA for sperm immobilisation and selection before ICSI.
We used the PICO Method[9] to formulate a specific and answerable clinical question following which we performed a comprehensive literature search based on a predefined protocol. The medical subject headings (MeSH) “sperm injections, intracytoplasmic”, “semen”, “hyaluronic acid”, “infertility”, “fertilization” and “live birth” were combined with free terms “hyaluronan”, “sperm”, “ICSI”, “PICSI”, “SpermCatch”, “SpermSlow”, “polyvinylpyrrolidone”, “PVP”, “embryo quality”, “pregnancy”, “implantation”, “costs”, “adverse events” in order to search Medline/PubMed/PMC, Cochrane Central Register of Controlled Trials (CENTRAL), EBSCOhost, ClinicalTrials.gov and Google Scholar from inception until June 2015. The “Related citations” function and hand search of references were used for all relevant studies in order to identify additional RCTs.
We set our inclusion criteria as RCTs evaluating sperm immobilisation and selection using HA before ICSI with no filter for date, country or hospital of origin, publication language, sample size or blinding. For studies presented in more than one publication, we only included the most extensive and recent version in order to avoid overlapping data.
The primary endpoints of the present meta-analysis were defined as: fertilisation rate, embryo quality and live birth rate. Secondary endpoints were: clinical pregnancy and implantation rates, adverse events and costs.
Two authors extracted the data following the literature search and study selection using predefined tables. Information related to first author, year of publication, country of origin, age of participants, inclusion criteria, the day of embryo transfer, number of embryos transferred, publication type, intervention protocols, number of participants, fertilisation rate, embryo quality, clinical pregnancy rate, implantation rate, live birth rate, adverse events, costs, randomisation technique, allocation concealment, blinding and data reporting was retrieved for each of the included studies. We contacted the study authors in order to obtain more data where it was required.
The software package RevMan 5.2.11[10], provided by the Cochrane Collaboration, was used for statistical analysis. We calculated the risk ratio (RR) with a 95%CI using the Mantel-Haenszel method[11] for binary data variables.
We measured the heterogeneity using the χ2 test and quantified it[12] using I2. In case of substantial heterogeneity (P < 0.10 for χ2 test or I2 > 50%) we reported the combined outcome calculated using the random effects model[13]. Forest plots were used for the visual display of the results from the meta-analysis.
The guideline of the Cochrane Collaboration[14] was used to assess the risk of bias and it was illustrated as a risk of bias graph. GradePro (Version 3.2 for Windows) provided by the Cochrane Collaboration[15] was used to generate the summary of the evidence.
We performed subgroup analysis based on publication type (full text vs abstract) and type of reporting of the results (per women vs per cycle) for each of the variables with summated outcome.
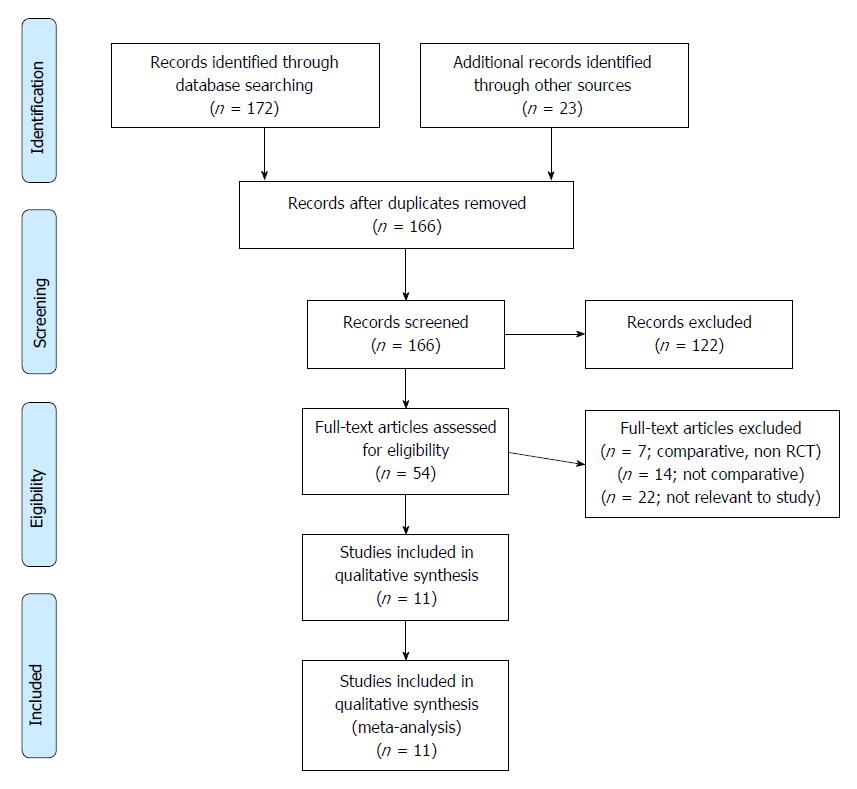
The systemic literature search identified 166 different studies related to sperm immobilisation and selection for ICSI. The PRISMA flow chart to explain the RCTs selection is shown in Figure 1. The summary of the evidence is presented in Figure 2. Eleven RCTs[5,16-25] evaluating 13719 oocyte intracytoplasmatic injections with sperm immobilised and selected using HA or PVP were included in this systematic review and meta-analysis. There were 6926 injections in the HA group and 6793 injections in the PVP group. The characteristics of the included RCTs are shown in Table 1, and the procedure protocols used for the women in all of the RCTs are shown in Table 2. Variables used to achieve a combined outcome are shown in Table 3. One RCT[17] included four arms and we analysed the data as for two studies. Seven RCTs[5,16,18,20,22,23,25] were published as full articles and four RCTs were published as abstracts[17,19,21,24]. Two RCTs[5,22] reported the results per ICSI cycle not per woman randomised. There was complete agreement between authors in terms of included studies and extracted data.
| Trial | Country | Mean age | Inclusion criteria | Day of embryo transfer | Number of embryos transferred | Publication type |
| Balaban et al[16] | ||||||
| HA | Sweden | NA | Male factor | Day 2-3 | 3.1 | Full text |
| PVP | 3.1 | |||||
| Barak et al[5] | ||||||
| HA | Israel | 31.0 | Male factor | Day 2-3 | 3.8 | Full text |
| PVP | 30.9 | 3.8 | ||||
| Castillo-Baso et al[17] | ||||||
| HA | Mexico | 35.1 | NA | Day 2-3 or day 5-6 | NA | Abstract |
| PVP | 35.3 | |||||
| Choe et al[18] | ||||||
| HA | Korea | 35.4 | Previous low fertilization rate | Day 3 or day 5 | NA | Full text |
| PVP | Multiple IVF failures | |||||
| Gandhi et al[19] | ||||||
| HA | Spain | NA | NA | NA | NA | Abstract |
| PVP | ||||||
| Majumdar et al[20] | ||||||
| HA | India | 31.7 | Unexplained infertility, normal semen analysis | Day 2-3 | 2.49 | Full text |
| PVP | 31.5 | 2.39 | ||||
| Moon et al[21] | ||||||
| HA | Korea | NA | NA | Day 3 or day 5 | NA | Abstract |
| PVP | ||||||
| Parmegiani et al[22] | ||||||
| HA | Italy | 37.5 | Sperm number > 106 and sperm motility > 5% | Day 2-3 | 2.25 | Full text |
| PVP | 37.1 | 2.15 | ||||
| Van Den Bergh et al[23] | ||||||
| HA | Switzerland | NA | Women younger than 38 yr with at least four metaphase II oocytes | Day 2-3 | NA | Full text |
| PVP | ||||||
| Worrilow et al[24] | ||||||
| HA | United States | NA | NA | NA | NA | Abstract |
| PVP | ||||||
| Worrilow et al[25] | ||||||
| HA | United States | 33.3 | Women younger than 40 years with at least four metaphase II oocytes; Men with sperm count > 10000/mL | Day 2-3 or day 5-6 | NA | Full text |
| PVP | 33.7 |
| Ref. | HA group | PVP group |
| Balaban et al[16] | Oocytes were injected with sperm exposed to, SpermCatch (NidaCon International, Gothenburg, Sweden), a viscous liquid containing hyaluronate and human serum albumin | Oocytes were injected with sperm exposed to the PVP-containing product |
| Barak et al[5] | Sperm suspension which contained motile spermatozoa was introduced into the center of viscous suspension of hyaluronic acid. Motility of the sperm cells was slowed down. After breaking the sperm tail with the injection pipette, the spermatozoa were injected into the oocytes | MII oocytes were injected with sperm cells which were immobilized and aspirated for injection in 10% PVP solution |
| Castillo-Baso et al[17] | Sperm selected by PICSI (Mid Atlantic Diag. Inc.) | Sperm selected by conventional ICSI |
| Gandhi et al[19] | 2 μL droplet with suspension of spermatozoa was placed to a 5 μL droplet of HA-containing medium (SpermSlow; MediCult, Jyllinge, Denmark) and incubated for 15 min at 37 °C under oil. Spermatozoa bound to HA in the junction of the two droplets were identified and carefully detached by injecting pipette (ICSI Micropipette; TPC, Thebarton, Adelaide, South Australia) and subsequently injected into a MII oocyte | Before injection, 3 μL of sperm suspension was transferred to 7 μL of 7% polyvinylpyrrolidone (PVP; SAGE) solution to remove debris and get better control. Spermatozoa with best morphology were selected for injection into a MII oocytes using inverted microscope equipped with micromanipulators |
| Gandhi et al[19] | Donated oocytes to avoid female infertility as a bias factor, randomly carried out with SpermSlow for sperm selection | Donated oocytes to avoid female infertility as a bias factor randomly carried out with PVP for sperm selection |
| Majumdar et al[20] | Sterile PICSI dishes (Origio MidAtlantic Devices, United States) with three hyaluronan microdots attached to the interior bottom, were used. 10 μL droplets of culture medium (GMOPS, Vitrolife) were placed over the hyaluronan microdots and an elongated 10 μL drop of PVP was made below the drops, before covering the dish with oil. 1-2 μL of sperm suspension was then added to the hyaluronan microdot containing droplets. After 5 min of incubation at 37 °C, HA bound sperm with normal morphology were removed with an injecting micropipette (TPC, Australia) to the adjacent PVP droplet, immobilized and subsequently injected | An elongated 10 μL poly vinyl pyrolidone drop (PVP, Medicult, Denmark) under oil, was used to select spermatozoa with normal morphology for subsequent injection |
| Moon et al[21] | ICSI were performed with husband spermatozoa which immobilized in 2.5 mg/mL hyaluronic acid | ICSI were performed with husband spermatozoa which immobilized in 5% PVP |
| Parmegiani et al[22] | Spermatozoa were selected for their ability to bind to HA: A 2-mL droplet with suspension of spermatozoa was connected with a pipette tip to a 5-mL droplet of HA-containing medium (SpermSlow; MediCult) and allowed to incubate for 15 min at 37 °C under oil (Liquid Paraffin; MediCult). Spermatozoa bound to HA in the junction zone of the two droplets were selected and easily detached by injecting pipette (ICSI Micropipette; Humagen Fertility Diagnostics) and subsequently injected into oocytes | Conventional PVP-ICSI procedure |
| Worrilow et al[24] | At the time of injection, drops were prepared in the lid of a Falcon Petri dish (353004; Becton Dickinson, Franklin Lakes, United States). In the middle of the dish a 10 μL drop of Spermslow (Medicult) and a 10 μL Flushing Medium drop (Medicult) were connected by a 3-4 mm junction bridge of medium and consecutively encircled by five 10 μL drops of Flushing medium. This setup was covered with liquid paraffin (Medicult). A 2 μL volume of prepared semen was added to the medium part of the Spermslow/Flushing medium central mixture. The spermatozoa were allowed to migrate towards the junction for a period of 15-20 min at 37 °C. Spermatozoa were carefully selected near the junction between the sperm droplet and the SpermSlow droplet | Non-bound, forward-moving spermatozoa were taken from the SpermSlow droplet |
| Worrilow et al[24] | PICSI embryos created using Hyaluron Bond-sperm | Standard sperm selection criteria |
| Worrilow et al[25] | The final sperm suspension of HYAL patients was placed upon microdots of hyaluronan in the PICSI Sperm Selection Device (Biocoat, Inc., Horsham, PA) and overlaid with oil. Following a 5-10 min incubation period, HB sperm were selected following the manufacturer’s instructions | The final sperm suspension of patients in the control group was placed into standard ICSI dishes for selection |
| Trial | Transfers (n) | Fertilisation rate | Good embryos | Clinical pregnancy | Implantation rate | Live birth |
| Balaban et al[16] | Women | |||||
| HA | 48 | 360 (72.14) | 226 (50.33) | 20 (41.66) | 27 (18.12) | 19 (39.58) |
| PVP | 44 | 337 (75.05) | 211 (46.99) | 19 (43.18) | 27 (19.14) | 18 (40.90) |
| Barak et al[5] | Cycles | |||||
| HA | 58 | 525 (72.61) | NA | 29 (50.00) | 41 (18.55) | NA |
| PVP | 65 | 484 (74.57) | 25 (38.46) | 35 (14.00) | ||
| Castillo-Baso et al[17] | Women | |||||
| HA | 30 | 143 (49.14) | 87 (29.89) | 16 (53.33) | -34 | NA |
| PVP | 30 | 134 (50.95) | 84 (31.93) | 12 (40.00) | -24 | |
| Castillo-Baso et al[17] | Women | |||||
| HA | 30 | 140 (56.91) | 105 (42.68) | 14 (46.66) | -25 | NA |
| PVP | 30 | 163 (61.97) | 99 (37.64) | 13 (43.33) | -22 | |
| Choe et al[18] | ||||||
| HA | 18 women | 81 (75.70) | 13 (12.14) | NA | NA | NA |
| PVP | 93 (83.03) | 15 (13.39) | ||||
| Gandhi et al[19] | Women | |||||
| HA | 77 | 909 (82.33) | 675 (61.14) | 41 (53.24) | NA | NA |
| PVP | 77 | 923 (82.48) | 698 (62.37) | 47 (61.03) | ||
| Majumdar et al[20] | Women | |||||
| HA | 71 | 353 (64.65) | 154 (43.62) | 25 (35.21) | 39 (22.03) | 22 (30.98) |
| PVP | 80 | 371 (65.66) | 170 (45.82) | 28 (35.00) | 36 (18.84) | 21 (26.25) |
| Moon et al[21] | ||||||
| HA | 1 woman | 18 (81.81) | 11 (50.99) | NA | NA | NA |
| PVP | 22 (78.57) | 10 (35.71) | ||||
| Parmegiani et al[22] | Cycles | |||||
| HA | 125 | 304 (91.56) | 101 (30.42) | 31 (24.80) | 35 (12.41) | 29 (23.20) |
| PVP | 105 | 236 (85.81) | 55 (20.00) | 22 (20.95) | 23 (10.17) | 19 (18.09) |
| Van Den Bergh et al[23] | ||||||
| HA | 44 women | 154 (75.49) | NA | NA | NA | NA |
| PVP | 142 (69.95) | |||||
| Worrilow et al[24] | Women | |||||
| HA | 7 | 77 (61.11) | NA | 4 (57.14) | NA | NA |
| PVP | 8 | 98 (66.66) | 2 (25.00) | |||
| Worrilow et al[24] | Women | |||||
| HA | 237 | 2105 (77.21) | NA | 112 (47.25) | NA | NA |
| PVP | 245 | 2024 (74.41) | 117 (47.75) | |||
| Total | ||||||
| HA | 5169 (74.63) | 1372 (46.44) | 292 (42.75) | 142 (17.12) | 70 (28.68) | |
| PVP | 5027 (74.00) | 1342 (46.59) | 285 (41.66) | 121 (14.97) | 58 (25.32) |
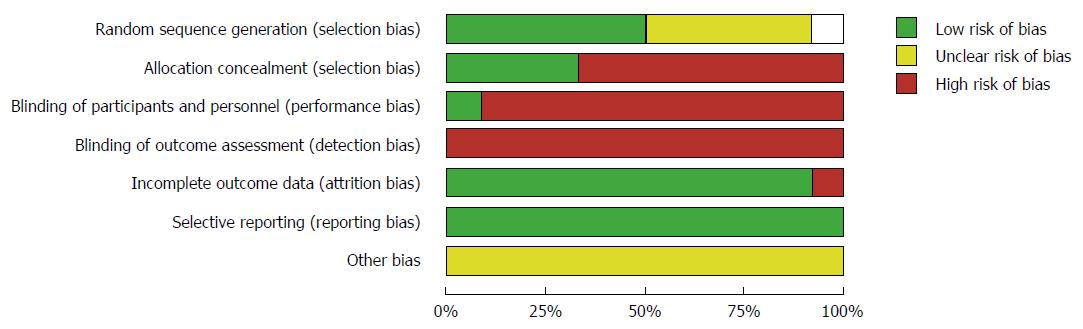
Based upon the guidelines suggested by the Cochrane Collaboration, the quality of most of the included studies was moderate to poor because of unclear randomisation technique, inadequate allocation concealment and blinding (Figure 3).
The combined outcome of all of the variables is given below.
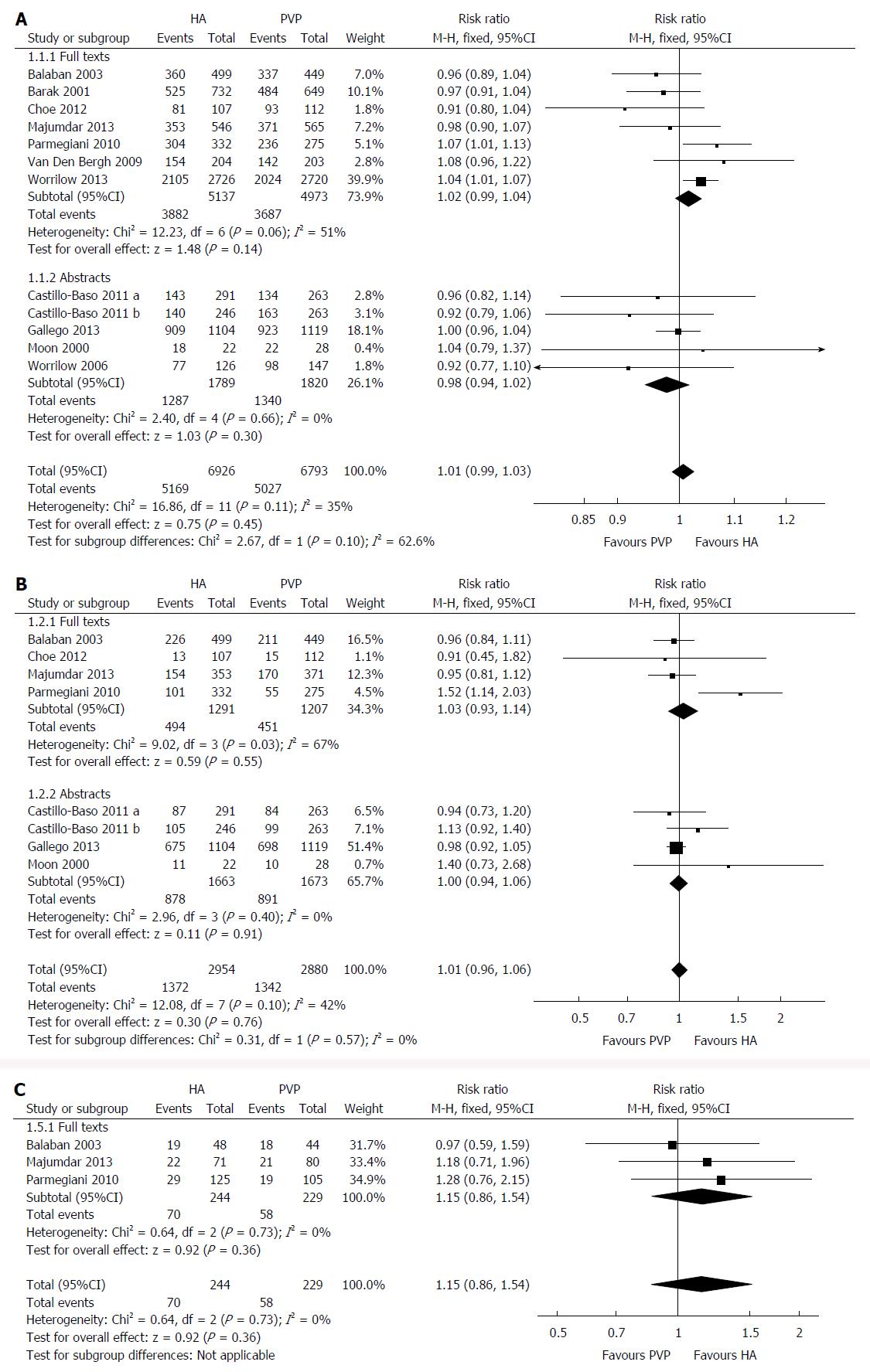
All the included RCTs reported on this outcome with low heterogeneity [χ2 = 16.86, df = 11 (P = 0.11); I2 = 35%] among them. The fertilisation rate was similar (RR = 1.01; 95%CI: 0.99-1.03; z = 0.75; P = 0.45; Figure 4A) in the HA group compared to the PVP group. The result did not change when we excluded the two RCTs[5,22] reporting the outcomes per ICSI cycle (P = 0.47) or when we used the random effects model for calculation (P = 0.83).
Eight RCTs reported on this outcome with low heterogeneity [χ2 = 12.08, df = 7 (P = 0.10); I2 = 42%] among them. There were 2714 good quality embryos obtained from 5835 oocytes injected. No statistically significant difference was found between the HA and PVP groups (RR = 1.01; 95%CI: 0.96-1.06; z = 0.30; P = 0.76; Figure 4B). Similar results were obtained when we excluded the RCT[22] reporting the outcomes per ICSI cycle (P = 0.56) or when we used the random effects model for calculation (P = 0.59).
Three RCTs reported on this outcome with no heterogeneity [χ2 = 0.67, df = 2, (P = 0.73); I2 = 0%] among them. The live birth rate was similar in the HA group compared to the PVP group (RR = 1.15; 95%CI: 0.86-1.54; z = 0.92; P = 0.36; Figure 4C). Same results were obtained after excluding the RCT[22] reporting the outcomes per ICSI cycle (P = 0.68) or when we calculated using the random effects model (P = 0.41).
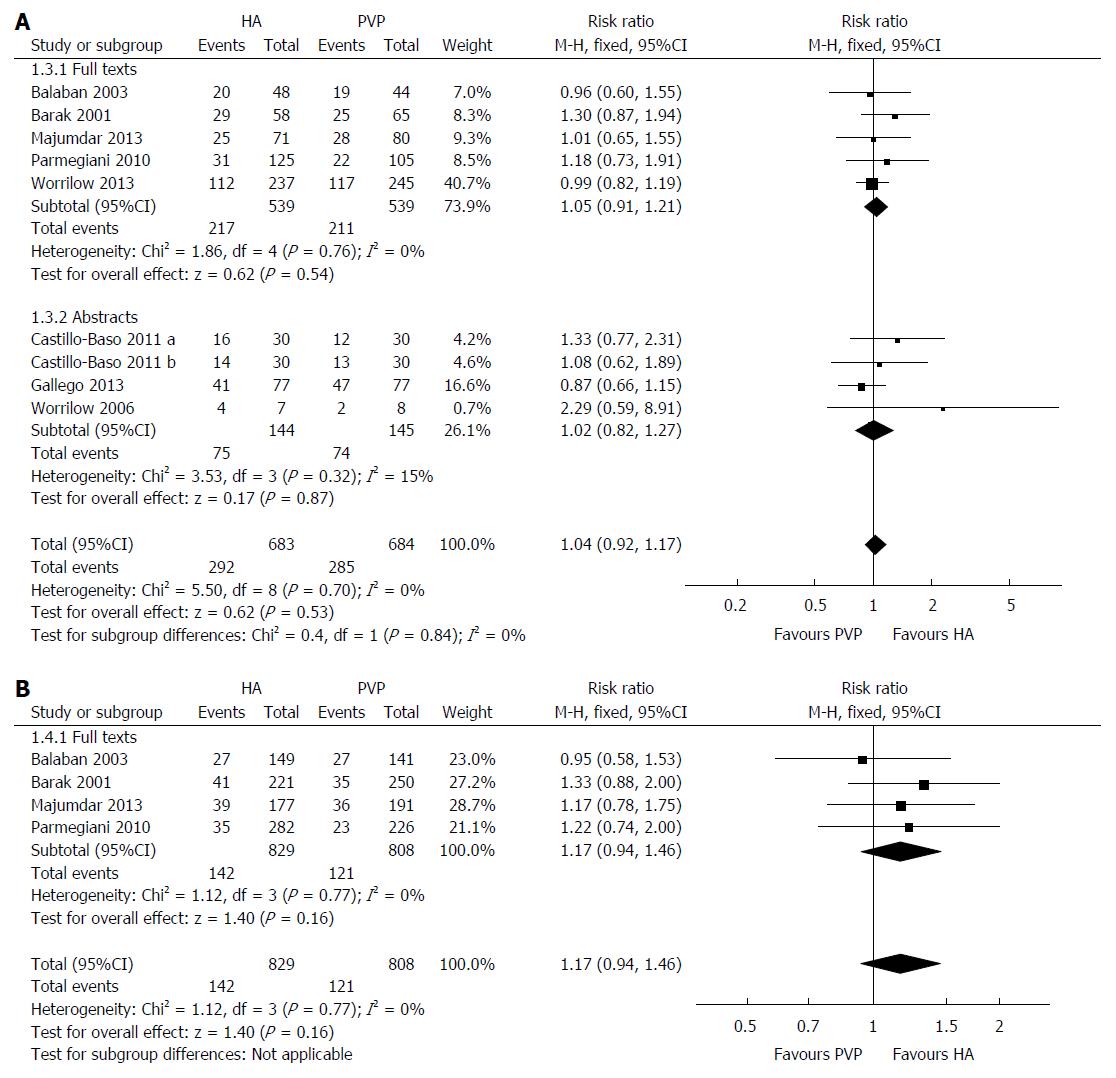
Nine RCTs reported on this outcome with no heterogeneity [χ2 = 5.50, df = 8, (P = 0.70); I2 = 0%] among them. The clinical pregnancy rate was similar between HA group and PVP group (RR = 1.04; 95%CI: 0.92-1.17; z = 0.62; P = 0.53; Figure 5A). No difference was found by excluding the two RCTs[5,22] reporting the outcomes per ICSI cycle (P = 0.98) or by calculating the combined outcome using the random effects model (P = 0.68).
Four RCTs reported on this outcome with no heterogeneity [χ2 = 1.12, df = 3, (P = 0.77); I2 = 0%] among them. Similar implantation rates were obtained in the HA and PVP groups (RR = 1.17; 95%CI: 0.94-1.46; z = 0.40; P = 0.16; Figure 5B). Excluding the two RCTs[5,22] reporting the outcomes per ICSI cycle (P = 0.67) or using the random effects model for calculation (P = 0.17) did not change the result.
None of the studies reported on these outcomes.
This systematic review and meta-analysis based on eleven moderate to low quality RCTs provides evidence of similar efficiency between using HA or PVP for sperm immobilisation and selection before ICSI in terms of fertilisation, embryo quality, clinical pregnancy, implantation and live birth rates. None of the studies reported on costs hence we could not perform a cost-effectiveness analysis.
By performing a comprehensive literature search of the standard medical databases and grey literature with no filters for date, country or hospital of origin, publication language, sample size or blinding we were able to identify eleven RCTs including conference abstracts in order to calculate the combined outcomes. Where the reported data was insufficient we contacted the study authors to gain extended reports.
The heterogeneity was low among the included RCTs and lead to consistent results by using both fixed effect and random effects models for calculations.
Our study is limited by the moderate to poor methodological quality of the included studies because of unclear randomisation technique, inadequate allocation concealment and blinding.
Some might argue in relation to the inclusion of the two RCTs reporting the results per ICSI cycle and not per women randomised, but we performed calculations excluding them for each of the primary and secondary outcomes, without identifying any significant difference.
To our knowledge this is the first meta-analysis comparing HA with PVP for sperm immobilisation and selection before ICSI. By observing the forest plot for each end point one can easily notice the similarities between the RCTs as most of them cross the vertical line meaning there is no statistical difference between the groups.
Future trials should be conducted according to the CONSORT guidelines. Due to the nature of the intervention, it would be difficult to achieve blinding of the embryologist performing the sperm selection, but the risk of bias could be reduced by blinding the outcome assessors and the personnel performing the embryo transfers.
It is important to report on the possible adverse events related to less physiological immobilisation and selection of the sperm and to set live birth rate as a primary outcome for the comparisons.
Sub-group analysis considering the quality of the sperm, previous failed ICSI cycles, infertility cause, number and quality of transferred embryos should be considered in order to eliminate confounding factors.
Until then, this review may provide reassurance of non inferiority of one method over another for embryologists and laboratory staff involved in the acquisition of laboratory materials.
The majority of patients undergoing intracytoplasmic sperm injection (ICSI) treatment will reach the stage of embryo transfer due to important improvements of ovarian stimulation protocols and laboratory technology, but only a small proportion of transferred embryos implant leading to an overall success rate of 10%-40%.
Several methods (ultramorphology, surface electric charge, apoptotic vs nonapoptotic, chromatin structure assay) have been recently proposed for optimising the sperm selection in order to reduce the risk of chromosomal anomalies associated with poor ICSI outcome.
Hyaluronic acid has been proposed as a physiological alternative to polyvinylpyrrolidone (PVP) for use as a selection medium to reduce sperm motility as a solution for the reported toxicity and unknown long term effects of PVP. Several studies investigated this method, but this is the first meta-analysis to assess the effect of using hyaluronic acid compared to PVP.
The results of this meta-analysis combining the outcomes from 11 randomised controlled trials concluded that there is no difference between hyaluronic acid and PVP for sperm immobilisation and selection before ICSI in terms of fertilisation, embryo quality, clinical pregnancy, implantation and live birth rates.
Sperm immobilisation and selection is an important step in the ICSI process and refers to the use of a medium in the laboratory for reducing the speed of sperm in order to allow its manipulation in the ICSI process.
The peer-reviewers appreciated the completeness of this meta-analysis. The manuscript was assessed as being well prepared, interesting, clear and well defined.
P- Reviewer: Cosmi E, Messinis IE, Sandrine MLC S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
| 1. | Sakkas D. Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2013;99:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Henkel R. Sperm preparation: state-of-the-art--physiological aspects and application of advanced sperm preparation methods. Asian J Androl. 2012;14:260-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Huszar G, Jakab A, Sakkas D, Ozenci CC, Cayli S, Delpiano E, Ozkavukcu S. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod Biomed Online. 2007;14:650-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Kato Y, Nagao Y. Effect of polyvinylpyrrolidone on sperm function and early embryonic development following intracytoplasmic sperm injection in human assisted reproduction. Reprod Med Biol. 2012;11:165-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Barak Y, Menezo Y, Veiga A, Elder K. A physiological replacement for polyvinylpyrrolidone (PVP) in assisted reproductive technology. Hum Fertil (Camb). 2001;4:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 2011;17:719-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Yetunde I, Vasiliki M. Effects of advanced selection methods on sperm quality and ART outcome. Minerva Ginecol. 2013;65:487-496. [PubMed] [Cited in This Article: ] |
| 8. | Parmegiani L, Cognigni GE, Filicori M. Sperm selection: effect on sperm DNA quality. Adv Exp Med Biol. 2014;791:151-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12-A13. [PubMed] [Cited in This Article: ] |
| 10. | Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2012; . [Cited in This Article: ] |
| 11. | Egger M, Smith GD, Altman DG. Systematic reviews in healthcare. London: BMJ Publishing 2006; . [Cited in This Article: ] |
| 12. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21630] [Cited by in F6Publishing: 23009] [Article Influence: 1045.9] [Reference Citation Analysis (0)] |
| 13. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26739] [Cited by in F6Publishing: 28514] [Article Influence: 750.4] [Reference Citation Analysis (0)] |
| 14. | Higgins JPT, Green S, editors . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [accessed 2011 Mar]. : The Cochrane Collaboration 2011; . [Cited in This Article: ] |
| 15. | Brozek J, Oxman A, Schünemann HJ. GRADEpro. [Computer program]. Version 3.2 for Windows. [accessed 2010 Oct 21]. Available from: http://mcmaster.flintbox.com/technology.asp?Page=3993. [Cited in This Article: ] |
| 16. | Balaban B, Lundin K, Morrell JM, Tjellström H, Urman B, Holmes PV. An alternative to PVP for slowing sperm prior to ICSI. Hum Reprod. 2003;18:1887-1889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Castillo-Baso J, Garcia-Villafana G, Santos-Haliscak R, Diaz P, Sepluveda-Gonzalez J, Hernandez-Ayup S. Embryo quality and reproductive outcomes of spermatozoa selected by physiologic-ICSI or conventional ICSI in patients with Kruger <4% and >4% normo-morphology. Fertil Steril. 2011;96:S159. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Choe SA, Tae JC, Shin MY, Kim HJ, Kim CH, Lee JY, Hwang D, Kim KC, Suh CS, Jee BC. Application of sperm selection using hyaluronic acid binding in intracytoplasmic sperm injection cycles: a sibling oocyte study. J Korean Med Sci. 2012;27:1569-1573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Gandhi G, Allahbadia G, Kagalwala S, Allahbadia A, Ramesh S, Patel K, Hinduja R, Chipkar V, Madne M, Ramani R. Effect of sperm selection using hyaluronic acid binding on reproductive outcome with donated oocyte cycles. Human reproduction. 2013;28:i149-i206. [Cited in This Article: ] |
| 20. | Majumdar G, Majumdar A. A prospective randomized study to evaluate the effect of hyaluronic acid sperm selection on the intracytoplasmic sperm injection outcome of patients with unexplained infertility having normal semen parameters. J Assist Reprod Genet. 2013;30:1471-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Moon JH, Heo YS, Yoon SH, Jeong JH, Park SP, Lim JH. Effect of Hyaluronic Acid (HA) as a Substitute for Polyvinyl-Pyrrolidone (PVP) on ICSI Outcome. Fertil Steril. 2000;74:S163. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. “Physiologic ICSI”: hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010;93:598-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Van Den Bergh MJ, Fahy-Deshe M, Hohl MK. Pronuclear zygote score following intracytoplasmic injection of hyaluronan-bound spermatozoa: a prospective randomized study. Reprod Biomed Online. 2009;19:796-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Worrilow KC, Huynh HT, Bower J, Peters AJ, Johnston JB. The clinical impact associated with the use of PICSI TM-derived embryos. Fertil Steril. 2006;86:S62. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Worrilow KC, Eid S, Woodhouse D, Perloe M, Smith S, Witmyer J, Ivani K, Khoury C, Ball GD, Elliot T. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes--multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2013;28:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |