Published online Jul 24, 2022. doi: 10.5306/wjco.v13.i7.641
Peer-review started: February 5, 2022
First decision: May 12, 2022
Revised: June 1, 2022
Accepted: June 21, 2022
Article in press: June 21, 2022
Published online: July 24, 2022
Low neutrophil-to-lymphocyte ratio (NLR) has been shown to be associated with a favorable therapeutic response to nivolumab. The activation of immunocompetent cells such as lymphocytes exhibits an antitumor effect; however, the development of excessive immune responses in autologous organs along with the breakdown of self-tolerance causes immune-related adverse events, including hypothyroidism. Therefore, the possibility that NLR is associated with immune response shows that NLR can be not only a predictive factor for good response to nivolumab but also a predictive factor for the development of hypothyroidism.
To evaluate whether continuous NLR monitoring during nivolumab treatment is useful for predicting the incidence and onset period of hypothyroidism.
This retrospective study comprised patients who received nivolumab for treating all types of cancer at our hospital between January 2015 and December 2019. The NLRs of patients were measured before each administration, and the patients were followed up till the administration of 12 doses. NLR at treatment initiation was compared between patients with and without hypothyroidism. Patients who developed hypothyroidism were categorized into three groups: those with NLR < 3.5, 3.5 to < 5, and ≥ 5 according to their maximum NLR from treatment initiation to hypothyroidism development. Further, the onset periods of hypothyroidism were compared between the groups.
Overall, 104 patients were included in the analysis. Twenty-one patients developed hypothyroidism throughout the observation period. NLR at treatment initiation was significantly lower (2.54 ± 1.21 vs 4.58 ± 4.03; P = 0.017) in patients with hypothyroidism than in those without hypothyroidism, and patients with NLR < 5 had a significantly higher incidence of hypothy
Low NLR at treatment initiation increases the incidence of treatment-induced hypothyroidism. Furthermore, its persistence may be a risk factor for the early onset of hypothyroidism.
Core Tip: This study evaluated whether continuous monitoring of neutrophil-to-lymphocyte ratio (NLR) during nivolumab treatment is useful for predicting the incidence and onset period of hypothyroidism. Patients with hypothyroidism had a significantly lower NLR at treatment initiation, and hypothyroidism incidence was higher among those with NLR < 5. Patients with persistently low NLR (< 3.5) developed hypothyroidism earlier than those with an NLR of 3.5 to < 5 and ≥ 5. Low NLR at treatment initiation increases the incidence of treatment-induced hypothyroidism. Furthermore, its persistence may be a risk factor for the early onset of hypothyroidism.
- Citation: Gannichida A, Nakazawa Y, Kageyama A, Utsumi H, Kuwano K, Kawakubo T. Necessity of neutrophil-to-lymphocyte ratio monitoring for hypothyroidism using nivolumab in patients with cancer. World J Clin Oncol 2022; 13(7): 641-651
- URL: https://www.wjgnet.com/2218-4333/full/v13/i7/641.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i7.641
The immune checkpoint inhibitor nivolumab restores and activates antigen-specific T cells that have become unresponsive to cancer cells by inhibiting the binding of programmed death-1 (PD-1) to PD-1 Ligands (PD-L1) and exerts antitumor effects[1]. Nivolumab has been successfully used to treat various types of cancer, including advanced melanoma, non-small-cell lung cancer, renal cell carcinoma, classical Hodgkin lymphoma, head and neck cancer, gastric cancer, and malignant pleural mesothelioma. Although nivolumab exerts a remarkable effect on cancer, it requires a certain period until the manifestation of treatment response[2-10]. Considering that other treatments may be required if nivolumab does not achieve a good treatment response, early identification of predictive factors for its efficacy is highly desired. Treatment with nivolumab is accompanied by immune-related adverse events (irAEs), such as hypothyroidism[11]. A recent study suggested that the development of irAEs was associated with treatment benefit[12-15]. The mechanism by which nivolumab elicits an antitumor and antithyroid immune response has not been fully elucidated. The activation of immunocompetent cells by nivolumab results in an antitumor effect. However, the development of excessive immune responses in autologous organs along with the breakdown of self-tolerance causes irAEs, such as hypothyroidism. Neutrophil-to-lymphocyte ratio (NLR) has gained attention as a predictive factor for the efficacy of nivolumab; particularly, low NLR at treatment initiation has been associated with a favorable therapeutic response[16-20]. Therefore, it is assumed that the association of NLR with an immune response shows that NLR is both a predictive factor for nivolumab efficacy and an indicator of the risk for hypothyroidism. In our previous study with patients who responded to six or more doses of nivolumab, we showed that patients with NLR < 5 at the 6th administration had a significantly higher incidence of hypothyroidism[21]. Although we showed the effect of low NLR on the incidence of hypothyroidism, NLR was evaluated only at a fixed observation point, i.e., at the 6th administration of nivolumab. In this study, we investigated whether continuous monitoring of NLRs during nivolumab treatment is necessary to predict the frequency and onset period of hypothyroidism.
This single-center retrospective study comprised patients who received nivolumab regardless of the type of cancer at the Jikei University Hospital between January 2015 and December 2019. The dosage of nivolumab was 3 mg/kg every 2 wk up to October 2018, and due to the revision in guidelines, the dosage of nivolumab was 240 mg/person every 2 wk thereafter. This study included patients who underwent thyroid-stimulating hormone (TSH) and free thyroxine (FT4) measurements at every or alternate administration of nivolumab to assess fluctuation in NLR. The exclusion criteria were as follows: patients with a history of hypothyroidism, thyroid cancer; those at treatment initiation; and those with TSH levels above the upper limit or FT4 Levels below the lower limit of the reference values. Patients who discontinued nivolumab after single administration were also excluded from the analysis because fluctuations in laboratory data could not be analyzed. The reference values of TSH and FT4 Levels were 0.34-4.04 µIU/mL and 0.88-1.67 ng/dL, respectively, based on the Japanese Committee for Clinical Laboratory Standards. In this study, hypothyroidism was defined as TSH levels exceeding the upper limit or FT4 Levels falling below the lower limit of the reference values twice in a row during the nivolumab observation period, with the follow-up period being up to the 12th administration.
NLR was calculated by dividing the absolute neutrophil and lymphocyte counts measured in peripheral blood samples at each administration. The follow-up period was up to the 12th administration, and each NLR from treatment initiation to the 12th administration was investigated. The decision to discontinue treatment was made by the clinician depending on the progression of disease or the development of severe irAEs. Fluctuations in NLRs were assessed for the following groups of patients: Those who discontinued treatment after administering nivolumab < 6 times, those who discontinued treatment after administering nivolumab 6-11 times, and those who administered nivolumab ≥ 12 times. In particular, we compared NLR fluctuation at treatment initiation and discontinuation among the patients who received nivolumab < 6 times and 6-11 times. Among the patients who received nivolumab ≥ 12 times, we compared NLR fluctuation at treatment initiation and the 12th administration.
Furthermore, we categorized the patients into three groups according to the tertiles of their mean NLR as follows: NLR < 3.5, NLR 3.5 to < 5, and NLR ≥ 5 during the observation points. This analysis compared the differences in treatment continuity between the NLR 3.5 to < 5 and NLR ≥ 5 groups relative to the NLR < 3.5 group.
Patients were classified into two groups according to the presence or absence of hypothyroidism, and the difference in treatment continuity between the two groups was evaluated.
Patients who developed hypothyroidism were categorized into three groups according to the tertiles of their maximum NLR from treatment initiation to development of hypothyroidism as follows: NLR < 3.5, NLR 3.5 to < 5, and NLR ≥ 5. The onset period of hypothyroidism was defined as the number of times nivolumab was administered until the onset. This analysis compared the differences in onset period of hypothyroidism between the NLR < 3.5 and NLR 3.5 to < 5 groups relative to the NLR ≥ 5 group.
The distribution of continuous variables was evaluated using the Shapiro-Wilk test. Based on the distribution of the data, continuous variables were statistically analyzed using the Student t test or Mann-Whitney’s U-test. Categorical variables were statistically analyzed using Fisher’s exact test. For comparing the NLR levels during nivolumab treatment or at discontinuation, we used the Wilcoxon signed-rank test for the following groups: patients who discontinued treatment after administering nivolumab < 6 times, those who discontinued treatment after administering nivolumab 6-11 times, and those who administered nivolumab ≥ 12 times. The differences in nivolumab treatment continuity and onset period of hypothyroidism were calculated using the Kaplan-Meier method and analyzed using the log-rank test and Cox proportional hazards analysis. All statistical data were analyzed using the BellCurve for Excel (Social Survey Research Information Co., Ltd. Tokyo, Japan). The significance level of the tests was set at 0.05.
A total of 104 patients were included in the analysis. Nivolumab was administered primarily at 2-week intervals, but it was temporarily administered at 3-week intervals when the hospital was closed or requested by the patient. Table 1 summarizes the background characteristics of patients who received nivolumab and their types of cancers. Throughout the observation period, 21 of 104 (20%) patients developed hypothyroidism. NLR at treatment initiation in patients with hypothyroidism was significantly lower than that in patients without hypothyroidism (2.54 ± 1.21 vs 4.58 ± 4.03; P = 0.017). Patients with NLR < 5 had a significantly higher incidence of hypothyroidism than those with NLR ≥ 5 (26%: 20 of 78 patients vs 4%: 1 of 26 patients; P = 0.022).
| All patients (n = 104) | Hypothyroidism | |||
| Yes (n = 21) | No (n = 83) | P value | ||
| Male/female | 69/35 | 14/7 | 55/28 | 1.000 |
| Median age (min-max) (years) | 68.5 (32-91) | 70.0 (45-91) | 68.0 (32-88) | 0.382 |
| Body weight (kg) | 52.7 ± 11.9 | 54.0 ± 9.6 | 52.4 ± 12.4 | 0.340 |
| Cancer type | ||||
| Head and neck cancer | 29 | 6 | 23 | 1.000 |
| Non-small-cell lung cancer | 29 | 6 | 23 | 1.000 |
| Malignant melanoma | 16 | 1 | 15 | 0.183 |
| Renal cell cancer | 15 | 4 | 11 | 0.497 |
| Gastric cancer | 15 | 4 | 11 | 0.497 |
| Laboratory data | ||||
| TSH (µIU/mL) | 2.08 ± 0.80 | 2.34 ± 0.78 | 2.02 ± 0.79 | 0.058 |
| FT3 (pg/mL) | 2.21 ± 0.50 | 2.12 ± 0.32 | 2.23 ± 0.54 | 0.584 |
| FT4 (ng/dL) | 1.18 ± 0.20 | 1.11 ± 0.20 | 1.20 ± 0.19 | 0.064 |
| NLR | 4.17 ± 3.73 | 2.54 ± 1.21 | 4.58 ± 4.03 | 0.017 |
| NLR < 3.5 | 60 | 16 | 44 | 0.082 |
| NLR ≥ 3.5 | 44 | 5 | 39 | |
| NLR < 5 | 78 | 20 | 58 | 0.022 |
| NLR ≥ 5 | 26 | 1 | 25 | |
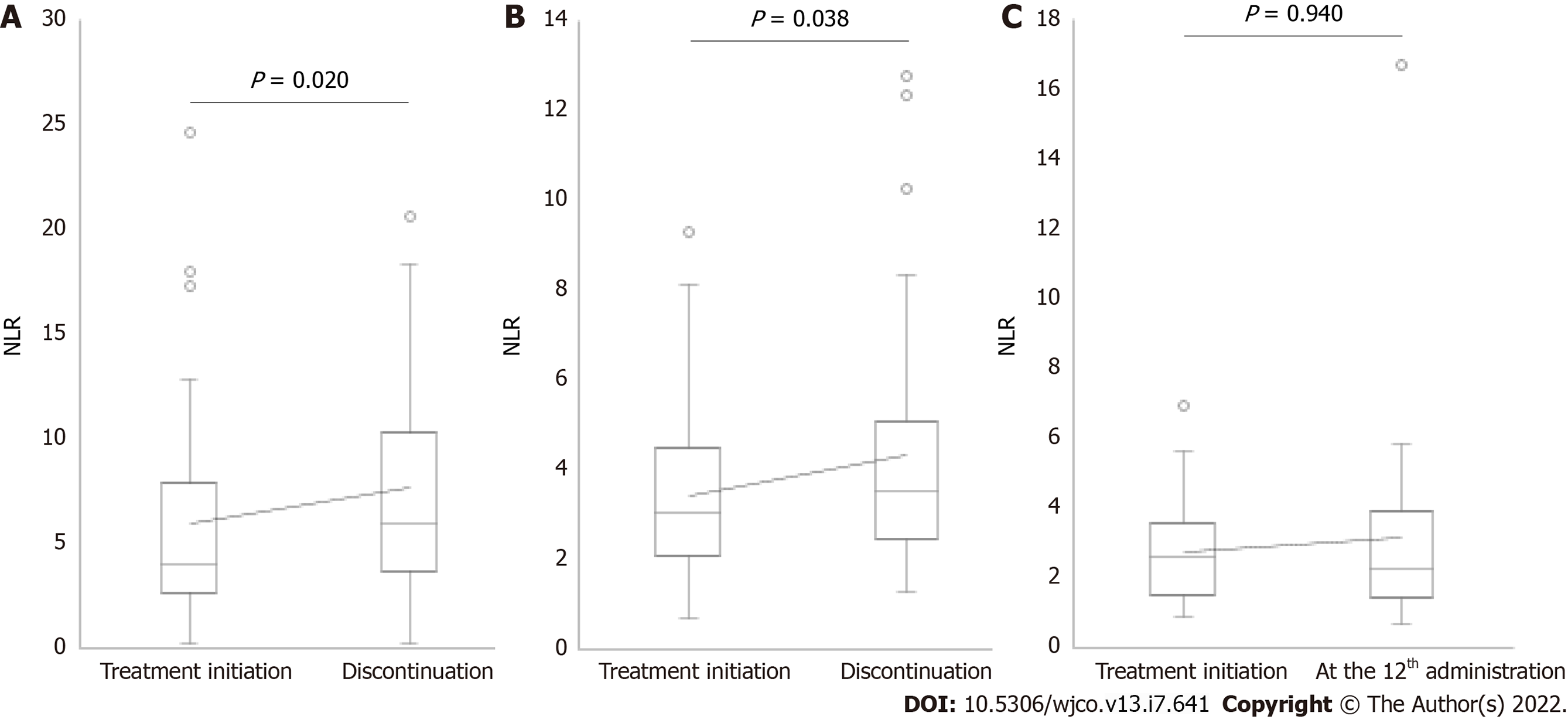
The median values of NLR at treatment initiation in patients who received nivolumab administration < 6, 6-11, and ≥ 12 times were 4.01, 3.03, and 2.64, respectively (Figure 1). A significant increase in NLR was observed at discontinuation in 40 patients who discontinued treatment after administering nivolumab < 6 times (median NLR, 4.01 vs 5.92, P = 0.020; Figure 1A). The reasons for the discontinuation of nivolumab in these patients were progression of disease in 34 patients and development of severe irAEs in six patients (pneumonitis: two patients, rashes: one patient, myocarditis: one patient, hypophysitis: one patient, and eosinophilia: one patient). A significant increase in NLR was observed at discontinuation in 32 patients who discontinued treatment after administering nivolumab 6-11 times (median NLR, 3.03 vs 3.50, P = 0.038; Figure 1B). The reasons for the discontinuation of nivolumab in these patients were progression of disease in 26 patients and severe irAEs in six patients (pneumonitis: three patients, rashes: two patients, and colitis: one patient). Finally, no significant differences in NLR were observed between the treatment initiation and the 12th administration in 32 patients who received nivolumab ≥ 12 times (median NLR, 2.64 vs 2.32, P = 0.940; Figure 1C).
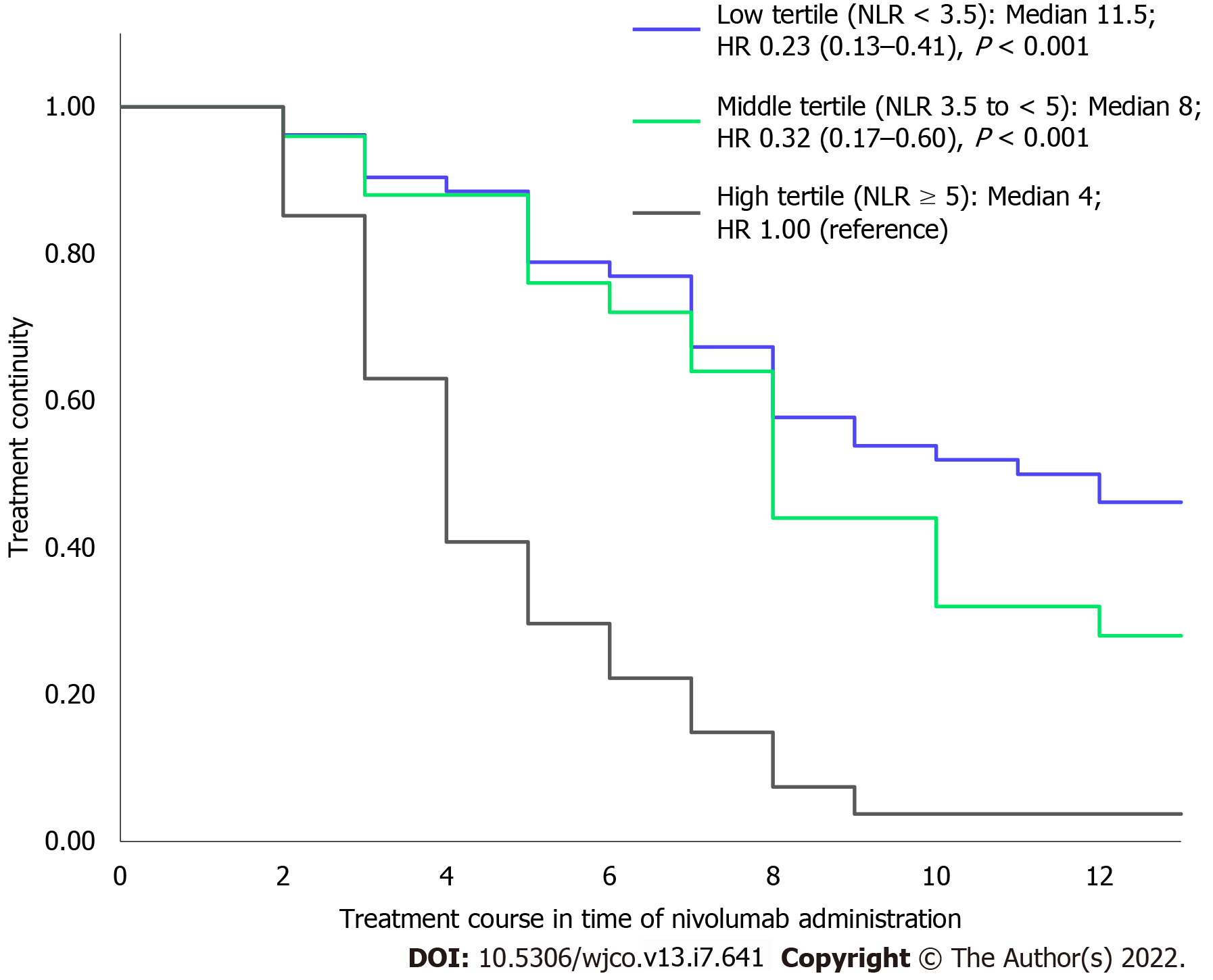
When the population was categorized into three groups based on the tertiles of their mean NLR during the observation period as NLR < 3.5, 3.5 to < 5, and ≥ 5, we observed a significant difference in treatment continuity between the three groups, as shown in Figure 2. The median number of times that nivolumab was administered in each group with mean NLR < 3.5, 3.5 to < 5, and ≥ 5 was 11.5, 8, and 4, respectively. The groups with mean NLR < 3.5 and 3.5 to < 5 had significantly longer treatment continuity than the group with NLR ≥ 5 (hazard ratio [HR] for low tertile compared with high tertile: 0.23; 95% confidence interval [CI]: 0.13-0.41, P < 0.001; HR for middle tertile compared with high tertile: 0.32; 95%CI: 0.17-0.60; P < 0.001).
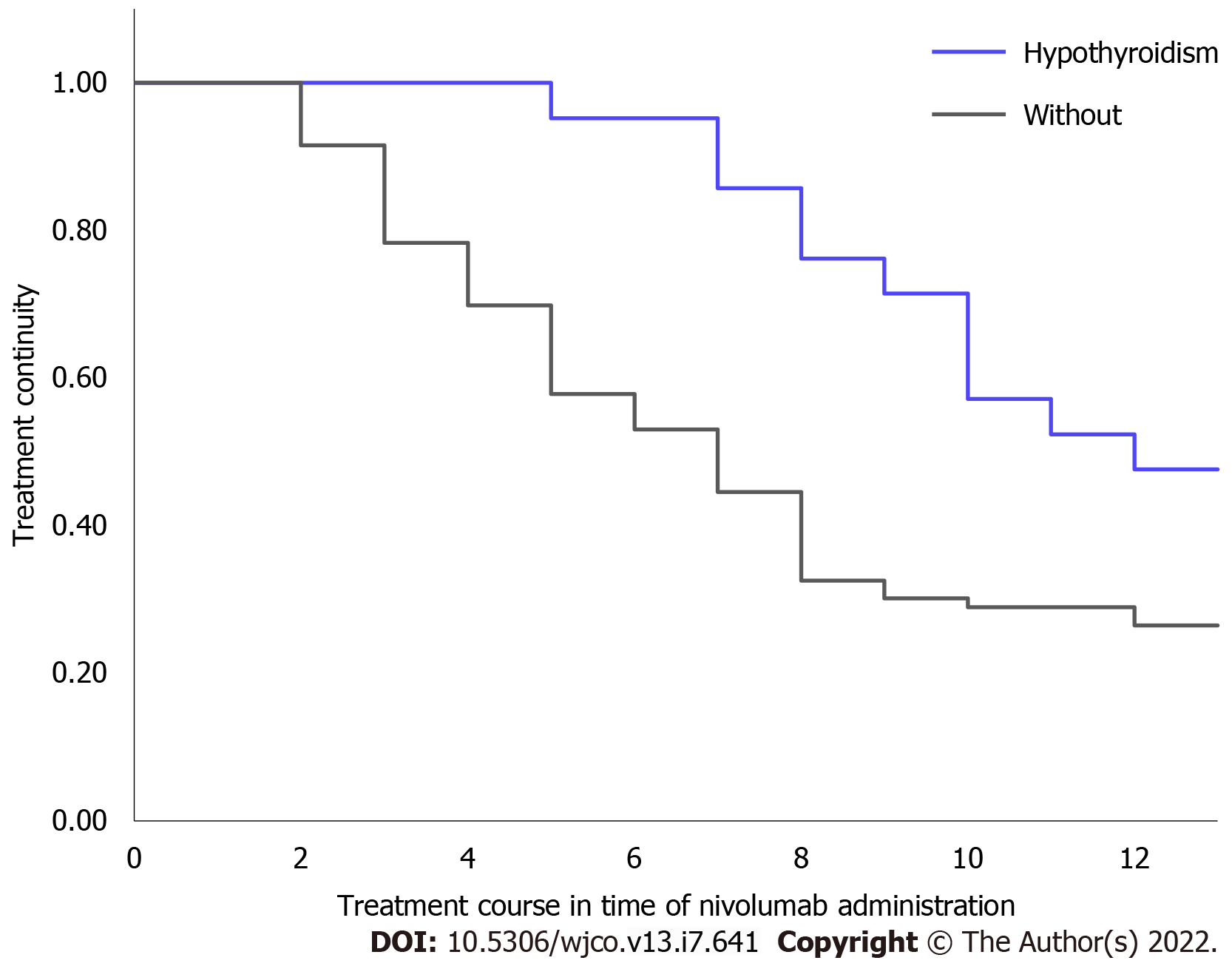
Treatment continuity was significantly longer in patients who developed hypothyroidism than in patients without hypothyroidism (median not reached vs 7 times administration, P = 0.010; Figure 3).
No patients discontinued nivolumab due to hypothyroidism. In patients who developed hypothyroidism, the reasons for discontinuing nivolumab during the observation period were progression of disease in nine patients and severe irAEs in two patients (pneumonitis: one patient and rashes: one patient). In patients without hypothyroidism, the reasons for discontinuing nivolumab during the observation period were progression of disease in 51 patients and severe irAEs in ten patients (pneumonitis: four patients, rashes: two patients, myocarditis: one patient, colitis: one patient, eosinophilia: one patient, and hypophysitis: One patient).
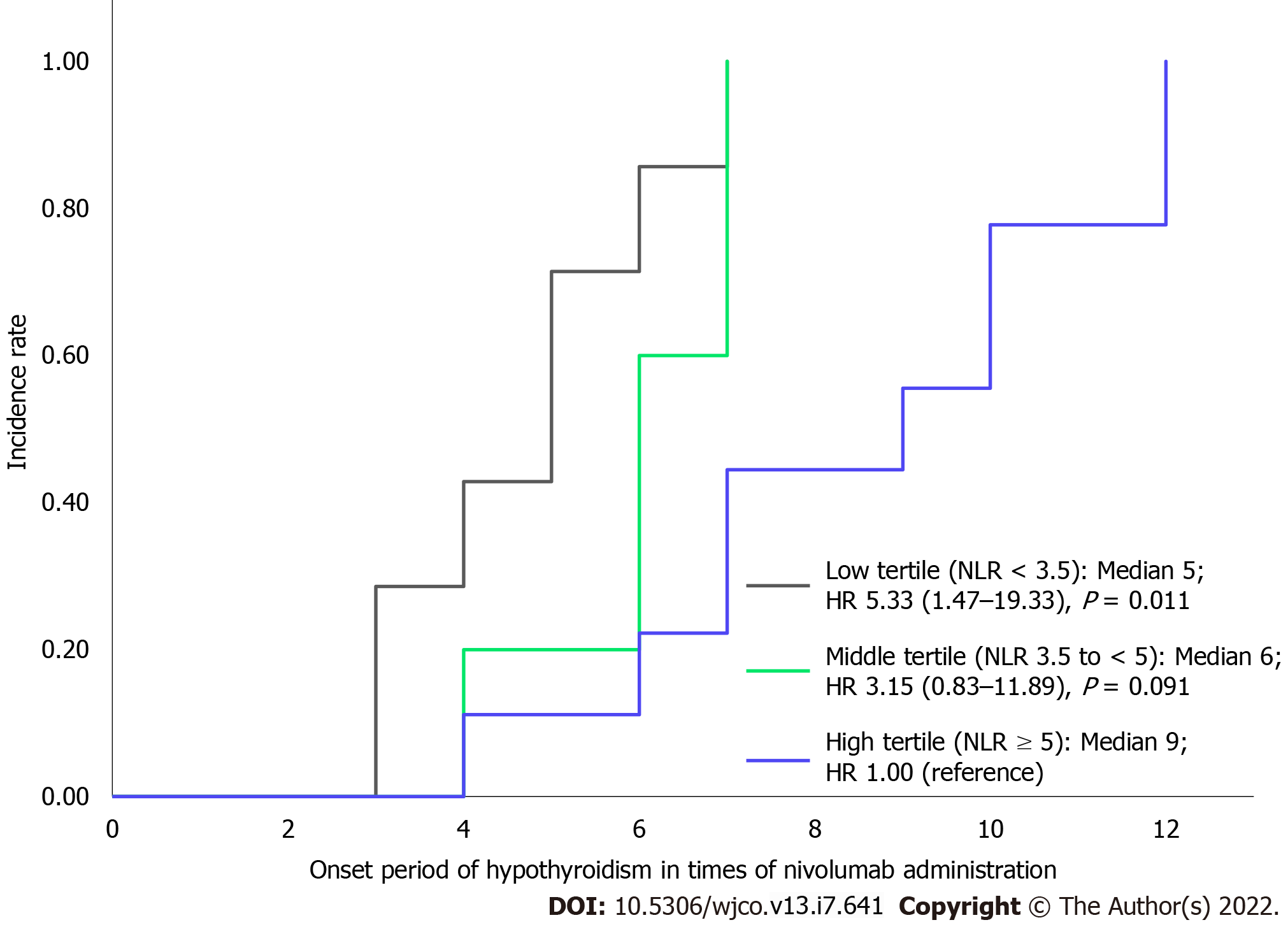
When the population was categorized into three groups based on the tertiles of their maximum NLR from treatment initiation to development of hypothyroidism, we observed a significant difference in the onset period, as shown in Figure 4. The median onset periods of each group with maximum NLRs of < 3.5, 3.5 to < 5, and ≥ 5 were at 5th, 6th, and 9th administrations, respectively. The groups with maximum NLR < 3.5 had a significantly earlier onset of hypothyroidism than the group with NLR ≥ 5, whereas there was no significant difference in the onset periods of the groups with maximum NLRs of 3.5 to < 5 and ≥ 5 (HR for low tertile compared with highest tertile: 5.33; 95%CI: 1.47-19.33, P = 0.011; HR for middle tertile compared with highest tertile: 3.15; 95%CI: 0.83-11.89, P = 0.091).
This study evaluated treatment outcomes as the number of times of nivolumab administration. The median values of NLR at treatment initiation in patients who administered nivolumab < 6, 6-11, and ≥ 12 times were 4.01, 3.03, and 2.64, respectively. Previous studies have found that low NLR at treatment initiation is associated with favorable therapeutic outcomes[16-20]; the results of this study are similar to those previously reported. Because the cancer treatment response to nivolumab is assessed up to the 6th administration[2-10], patients who discontinue after administering nivolumab < 6 times are considered to show a lack of therapeutic effect, whereas those who discontinue after administering nivolumab 6-11 and ≥ 12 times are considered to show a therapeutic effect. Therefore, patients with high NLR at treatment initiation may not show a therapeutic effect until the 6th administration, increasing the possibility of discontinuation.
A previous study reported that low NLR at the 4th administration of nivolumab was associated with prolongation in overall survival and that responding patients showed a decline in their longitudinal NLR over time[22,23]. We found that patients with mean NLR < 3.5 and 3.5 to < 5 had significantly longer treatment continuity than those with mean NLR ≥ 5. Thus, we suggest that low NLR (mean NLR < 5) can be useful for predicting treatment continuity. Interestingly, a significant increase in NLR was observed at treatment discontinuation (Figure 1A-C). PD-1 expressed on activated T cells binds to PD-L1 expressed on cancer cells to transmit an inhibitory signal to T cells; however, nivolumab promotes the reactivation of the immune response by suppressing this inhibitory signal[1,24]. Thus, low NLR levels indicates that the antitumor effect of nivolumab sustains the lymphocyte-dominant immune state, whereas an increase in NLR indicates that the weakened immune activation affects the discontinuation of nivolumab.
Patients who developed irAEs have shown favorable treatment response to nivolumab[12,13]. Furthermore, it has recently been reported that patients who developed hypothyroidism, one of the irAEs, during treatment also showed a favorable therapeutic response[14,15]. Our study showed that patients with hypothyroidism have a longer treatment continuity than those without hypothyroidism, supporting the results of the previous studies.
Although it has been mentioned above that monitoring NLR fluctuations during treatment is useful for predicting the therapeutic effect, whether NLR fluctuations can be used to predict the onset period of hypothyroidism is an interesting topic. However, Matsukane et al[25] showed that there was no significant change in NLR from the period of treatment initiation to development of hypothyroidism in patients who developed hypothyroidism after administering nivolumab. Thus, NLR fluctuations during treatment cannot predict the development of hypothyroidism. However, the present study revealed that patients who developed hypothyroidism showed significantly lower NLR at treatment initiation and patients with NLR < 5 showed a significantly higher incidence of hypothyroidism than those with NLR ≥ 5. We further investigated whether the persistence of low NLR affected the difference in the onset period of hypothyroidism. In particular, we investigated whether patients with NLR < 3.5 and NLR 3.5 to < 5 at treatment initiation had an earlier onset period than those with NLR ≥ 5. This study showed that patients with maximum NLR of < 3.5 until the development of hypothyroidism had a significantly earlier onset of hypothyroidism than those with NLR ≥ 5. Thus, persistently low NLR may be a risk factor for the early development of hypothyroidism. Monitoring the maximum NLR using a cutoff value of < 3.5 as a reference is clinically helpful in predicting the early onset of hypothyroidism.
This study has several limitations. First, this was a retrospective study conducted at a single institution, and the cancer types of patients were not specified. Additionally, there was a bias in cancer types of the patient population. Second, due to the limited sample size of this study population, follow-up with larger populations is needed for verification. Third, the follow-up period was limited to the 12th dose of nivolumab. In fact, in some patients, hypothyroidism develops after 12 doses; hence, the incidence of hypothyroidism should be evaluated throughout the treatment period. Fourth, we analyzed the treatment continuity of nivolumab rather than its therapeutic response as a criterion of therapeutic effect. Further studies are needed on NLR fluctuations via treatment response.
The involvement of antithyroid peroxidase antibody or antithyroglobulin antibody has been shown as a factor related to the development of hypothyroidism[26]. However, these laboratory data are not measured regularly in daily clinical practice. Alternatively, as the neutrophil and lymphocyte counts are regularly measured, the possibility of using NLR as a predictive factor was considered to be useful for the evaluation of the treatment continuity of nivolumab and associated adverse effects.
Low NLR at treatment initiation increased the incidence of treatment-induced hypothyroidism. Low NLR levels were also associated with the treatment continuity of nivolumab. Thus, the persistence of low NLR may be a risk factor for the early development of hypothyroidism.
The activation of immunocompetent cells by nivolumab exerts an antitumor effect. However, excessive immune responses developed in autologous organs along with the breakdown of self-tolerance causes immune-related adverse events (irAEs), such as hypothyroidism.
Low neutrophil-to-lymphocyte ratio (NLR) values have been shown to be associated with a favorable therapeutic response to nivolumab. The possibility that NLR is associated with immune response implies that NLR can be not only a predictive factor for good response to nivolumab but also a predictive factor for the development of hypothyroidism.
To evaluate whether continuous monitoring of NLRs during nivolumab treatment is useful for predicting the incidence and onset period of hypothyroidism.
NLR of patients who received nivolumab treatment was measured before each administration. NLR at treatment initiation was compared between patients with and without hypothyroidism during the treatment period. Patients who developed hypothyroidism were categorized into three groups as those with NLR < 3.5, NLR 3.5 to < 5, and NLR ≥ 5 according to their maximum NLR from treatment initiation to hypothyroidism development, and the onset periods of hypothyroidism were compared.
Patients with hypothyroidism showed significantly lower NLR at treatment initiation, and the incidence of hypothyroidism was higher among those with NLR < 5. Patients with persistently low NLR (< 3.5) developed hypothyroidism earlier than those with NLR 3.5 to < 5 and NLR ≥ 5.
Low NLR at treatment initiation increases the incidence of treatment-induced hypothyroidism. Moreover, its persistence may be a risk factor for the early onset of hypothyroidism.
The follow-up period in this study was limited to the 12th dose of nivolumab. The incidence of hypothyroidism should be evaluated throughout the treatment period.
We would like to thank all the patients and investigators who participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YH, China; He XH, China; Li D, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, Singh S, Wong S, Garner N, Leblanc H, Bunch RT, Blanset D, Selby MJ, Korman AJ. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 2. | Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, Juergens RA, Laurie SA, Nathan FE, Shen Y, Harbison CT, Hellmann MD. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980-2987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 387] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 3. | Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhäeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crinò L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924-3933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 613] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 4. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab vs Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4375] [Cited by in F6Publishing: 4258] [Article Influence: 473.1] [Reference Citation Analysis (0)] |
| 5. | Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab vs chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1853] [Cited by in F6Publishing: 1978] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 6. | Robert C, Long GV, Brady B, Dutriaux C, Di Giacomo AM, Mortier L, Rutkowski P, Hassel JC, McNeil CM, Kalinka EA, Lebbé C, Charles J, Hernberg MM, Savage KJ, Chiarion-Sileni V, Mihalcioiu C, Mauch C, Arance A, Cognetti F, Ny L, Schmidt H, Schadendorf D, Gogas H, Zoco J, Re S, Ascierto PA, Atkinson V. Five-Year Outcomes With Nivolumab in Patients With Wild-Type BRAF Advanced Melanoma. J Clin Oncol. 2020;38:3937-3946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3044] [Cited by in F6Publishing: 3205] [Article Influence: 400.6] [Reference Citation Analysis (0)] |
| 8. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1496] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 9. | Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB, Collins G, Savage KJ, Trneny M, Kato K, Farsaci B, Parker SM, Rodig S, Roemer MG, Ligon AH, Engert A. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 664] [Cited by in F6Publishing: 696] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 10. | Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, Oulkhouir Y, Bautista Y, Cornelissen R, Greillier L, Grossi F, Kowalski D, Rodríguez-Cid J, Aanur P, Oukessou A, Baudelet C, Zalcman G. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 553] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 11. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2308] [Cited by in F6Publishing: 2578] [Article Influence: 429.7] [Reference Citation Analysis (0)] |
| 12. | Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4:374-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 13. | Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, Imai Y, Ishida K, Fukuoka J, Tomii K. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol. 2017;12:1798-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 14. | Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 15. | Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, Otsuka A, Yamasaki T, Saito R, Kitamura M, Kitawaki T, Hishizawa M, Kawaguchi-Sakita N, Fujii T, Taura D, Sone M, Inagaki N. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14:e0216954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 17. | Ueda T, Chikuie N, Takumida M, Furuie H, Kono T, Taruya T, Hamamoto T, Hattori M, Ishino T, Takeno S. Baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in recurrent or metastatic head and neck cancer patients treated with nivolumab. Acta Otolaryngol. 2020;140:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 19. | Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, Yasui H. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. 2018;9:34520-34527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, Warsch J, Elias R, Chae YK, Kim DW, Warsch S, Ishkanian A, Ikpeazu C, Mudad R, Lopes G, Jahanzeb M. Developing a Predictive Model for Clinical Outcomes of Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Clin Lung Cancer. 2018;19:280-288.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Nakazawa Y, Gannichida A, Kageyama A, Utsumi H, Kuwano K, Kawakubo T. Involvement of neutrophil-lymphocyte ratio in nivolumab therapy-induced hyperthyroidism. Jpn J Pharm Health Care Sci. 2020;46:481-488. [DOI] [Cited in This Article: ] |
| 22. | Dusselier M, Deluche E, Delacourt N, Ballouhey J, Egenod T, Melloni B, Vergnenègre C, Veillon R, Vergnenègre A. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS One. 2019;14:e0219060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Ameratunga M, Chénard-Poirier M, Moreno Candilejo I, Pedregal M, Lui A, Dolling D, Aversa C, Ingles Garces A, Ang JE, Banerji U, Kaye S, Gan H, Doger B, Moreno V, de Bono J, Lopez J. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer. 2018;89:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T, Masuda S, Okamoto I, Nakagawa T, Ito T, Eto M, Mori M, Nakanishi Y, Egashira N. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep. 2021;11:1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, Takagi H, Hagiwara D, Ito Y, Morishita Y, Goto M, Suga H, Banno R, Yokota K, Hase T, Morise M, Hashimoto N, Ando M, Kiyoi H, Gotoh M, Ando Y, Akiyama M, Hasegawa Y, Arima H. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc. 2018;2:241-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |