Introduction
Multiple sclerosis (MS) is a chronic, demyelinating disease that affects humans by progressively degenerating neurons in the central nervous system (CNS) – axons and myelin – with consequent loss of brain volume.
The website www.pacjent.gov.pl estimates that there are over 46 thousand people in Poland currently suffering from MS and about 2.8 million worldwide.
The main symptoms of MS include the following: motor disability and balance problems, visual disturbances, chronic fatigue, depression, cognitive disorders, sensory loss, and bladder dysfunction.
All the symptoms listed above have a strong negative impact on quality of life, work ability, and social participation [1]. It is therefore crucial to diagnose the disease and begin treatment as early as possible.
Diagnosis of MS is based on proof of disease dissemination in time (DIT) and dissemination in space (DIS) while excluding other disorders that can mimic MS in laboratory tests and clinical manifestation [1].
Beginning with the first Mc Donald criteria published in 2001, and through further revisions, we can observe the care and attention paid to clarify the guidelines and to facilitate earlier and proper diagnosis. There is also a growing role of imaging and laboratory tests in making the MS diagnosis.
The aim of this review is to analyse the McDonald criteria from 2017 and compare them with the criteria from 2010, to present the newest recommendations of the Polish Medical Society of Radiology and the Polish Society of Neurology, MAGNIMS-CMSC-NAIMS, and to demonstrate new fields for research.
The role of MRI in the diagnosis of multiple sclerosis
Magnetic resonance imaging (MRI) criteria for MS were first included in the diagnostic work-up for MS in 2001 [1]. Since then, the criteria have evolved several times. In general they are based on the presence of focal lesions in the white matter of CNS – hyperintense on T2-weighted and T2-weighted fluid-attenuated inversion recovery (FLAIR) [1].
The lesions must be typical for MS by the morpho-logy, diameter, and localization (Table 1).
Table 1
MRI characteristic lesion features typical for multiple sclerosis (Polman et al., 2011; Filippi et al., 2019)
Regarding the diameter of lesions, the threshold of 3 mm in the long axis is reasonable in our opinion. In an analysis comparing 2 cohorts in a study including a total of 232 patients suffering from relapsing-remitting multiple sclerosis (RRMS) and a control group, the authors confirmed that the lesions with a diameter of about 3 mm best discriminated the control group from MS patients [2].
Lesions localized in periventricular white matter are not specific only for MS, but they may also be observed in older patients, especially those with vascular risk factors such as migraine.
It is well known that in MS patients, demyelinated lesions are found also in deep grey matter in the brain – mostly in the hypothalamus and caudate. They are connected to atrophy in this structure and cognitive disorders and disability.
White matter lesions tend to be formed by inflammatory changes, whereas deep grey matter lesions present an intermediate grade of inflammation [3].Growing awareness of the role of deep grey matter involvement in MS pathology, in our opinion, may lead to incorporation of these lesions in future MR guidelines.
It is important to recognize the enhancement pattern in post gadolinium sequences – typical for MS is open-ring or closed-ring enhancement. Large, multiple closed-ring enhancement indicates other entities like malignancy, acute disseminated encephalomyelitis (ADEM), or infection; if punctuated, we should consider vasculitis or Susac syndrome [4] (Figure 1).
Radiologically isolated syndrome
The term “radiologically isolated syndrome” (RIS) was introduced in 2009 to describe lesions in CNS that are strongly suggestive of demyelinating lesions but without any clinical symptoms of MS or other medical condition [5].
Clinically isolated syndrome
Clinically isolated syndrome (CIS) is defined as a first presentation of neurological disturbance highly suggestive for MS but in a patient not known to have MS and not meeting the criterion of DIT.
The symptoms are usually monofocal and depend on the anatomical location of the lesions (e.g. optic neuritis or an isolated brainstem or spinal cord syndrome), and they are typically of rapid onset and last for more than 24 hours with no fever or signs of infection [6].
CIS may progress in clinically definite MS (CDMS) in the future. For the patient and the neurologists, it is crucial to predict the probability of the course of CIS. The risk of developing SM is higher in younger patients, is not related with sex, and is described for 42-82% (depending on the duration of follow-up in the study). The risk is similar in different CIS subtypes [7].
It is important to emphasize that about one-third of patients with CIS will not develop further neurological dysfunction, which was proven in a study with 30 years of observation [7,8] (Table 2).
Table 2
Comparison between McDonald 2010 and 2017 criteria for DIS and DIT recognition
Table 3
Comparison between McDonald 2010 and 2017 criteria – summary
The role of CSF analysis in the diagnosis of multiple sclerosis
Cerebrospinal fluid (CSF) analysis is used at the time of diagnosis. Routine CSF examination includes measuring the oligoclonal band (OCB) status, IgG index, albumin ratio, and cell counts [9]. That allows the exclusion of alternative pathologies (e.g. inflammatory process or migraine).
Immunoglobulin G in CNS is produced specifically by small amounts of B-lymphocytes, only in the case of neuroinflammation; normally IgG is not produced intra-thecally in any significant amount, which is why its pre-sence in CSF and absence in serum is indicative for inflammatory activity within the CNS [10].
The presence of at least 2 specific oligoclonal immunoglobulin G bands in CSF is confirmed by agarose isoelectric focusing (IEF) combined with immunoblotting [11].
The IgG index is the ratio of the quotients for IgG and albumin (IgGcsf/IgGs)/(Albcsf/Albs). It is a quantitative analysis of the relationship between CSF IgG and serum IgG, divided by the same relationship for albumin [10].
OCB in CSF is detected in about 95% of CDMS patients and in 68-83% of CIS patients [12]; however, there is a long list of other pathologies that are CSF OGB positive, like systemic lupus erythematous, neuroborreliosis, aseptic meningitis, and others [13], and the neuromyelitis optica (NMO) and its spectrum disorders are the most frequent reason for misdiagnosis. Metanalysis [14] shows that the specificity of OGB in MS is 94%, and it falls to 64% when regarding other inflammatory processes of CNS.
That is why, if there are any features suggesting other disorders, further tests, like serological testing for AQP4, should be provided [15].
It is well known that the presence of OGB is associated with worse prognosis – conversion from RIS to CIS and to RRMS, greater brain atrophy [16]. It is connected with chronic inflammatory activity within the CNS and is the second clearest diagnostic marker after MR in MS diagnosis [11].
Oligoclonal bands
CSF oligoclonal bands appeared for the first time in MS criteria in the modification of the Schumacher criteria in the early 1970s and were later enforced by the Poser criteria published in 1983. Subsequently, positive CSF criterion appeared in McDonald 2001 [17].
In Mc Donald 2010 the value of oligoclonal bands was very limited. DIT could have been recognized by the pre-sence of a new T2 and/or gadolinium-enhancing lesion(s) on follow-up MRI or simultaneous presence of asymptomatic gadolinium-enhancing and non-enhancing lesions at any time. Positive CSF analysis was the main criterion used in making the diagnosis of the subtypes of MS – primary progressive multiple sclerosis (PPMS) [18].
This generated a diagnostic dilemma concerning patients with first CIS typical for demyelination, who fulfil only DIS at baseline [18].
McDonald 2017 criteria include the presence oligoclonal bands in CSF in the absence of atypical findings of CSF as a fulfilment of DIT, which practically means that in patients with CIS fulfilling the DIS criteria, the presence of CSF oligoclonal bands is sufficient for a diagnosis of MS even with no other evidence (clinical or radiological) of DIT [7].
Arrambide et al. [11], in a large cohort study, proved that CSF-specific oligoclonal bands are the second clearest marker for MS after MRI. In another study, the authors observed that the presence of CSF oligoclonal bands (as well as age of onset and number of T1 lesions) significantly predicts the risk of developing MS in patients with CIS [19].
Diagnostic criteria of multiple sclerosis
Criteria for diagnosing MS were described by Charcot for the first time in 1868 as a triad of nysta-gmus, intention tremor, and scanning speech [17]. From that time the diagnostic criteria for MS have been constantly and intensively evolving.
In the Poser classification from 1983, clinical symptoms and CSF analysis (IgG and oligoclonal bands) were sufficient for making the diagnosis [20].
However, the introduction of MR in 1981, its rapid development, and growing importance led to the publication of new diagnostic criteria in 2001. For the first time, MR criteria were incorporated and the terms of dissemination of lesions in space (DIS) and in time (DIT) appeared [21].
In subsequent years, the McDonald criteria have been revised 3 times: McDonald 2005, McDonald 2010, and McDonald 2017.
Independently the MAGNIMS Study Group (Magnetic Resonance Imaging in MS), focusing on the utility of MRI in clinical practice, has gathered regularly twice a year from 2002 until now, to summarise and clarify the MRI criteria. The recommendations and guidelines published by this group were also taken into consideration when establishing the McDonald criteria.
Differences between McDonald 2017 and McDonald 2010 criteria
Symptomatic and asymptomatic lesions are counted in the determination of DIS and DIT
According to the 2010 McDonald criteria, DIS is defined as the presence of at least one asymptomatic lesion typical for MS in at least 2 of the 4 locations characteristic of MS: juxtacortical, periventricular, infratentorial, and spinal cord [18]. Symptomatic lesions in patients with infratentorial or spinal cord IS were not included, to avoid double counting. The practical meaning of this rule was that patients with CIS and only one lesion in the brainstem or spinal cord were treated as patients with CIS without any brain lesions [22]. Two important studies performed by Tintore et al. and Brownlee et al. in 2016 revealed that including lesions in the symptomatic region in the DIS criterion increases the sensitivity of MRI criteria with no reduction of specificity [23].
In the MAGNIMS recommendations no distinctions were made between symptomatic and asymptomatic lesions for DIT and DIS.
Based on these studies and recommendations, the panel in McDonald 2017 criteria includes in the determination of DIS and DIT both symptomatic and asymptomatic lesions.
Of interest, in the MAGNIMS 2016 recommendations it was proposed to include the optic nerve as one of the typical MS localizations, and to increase the number of periventricular lesions necessary to confirm the involvement of this area from 1 to 3 [1].
However, the McDonald 2017 criteria do not include optic neuritis as a site to fulfil the DIS criteria and maintain the requirement of one periventricular lesion as sufficient for DIS determination, but for some patients caution is suggested (older individuals, with migraine or vascular risk factors) and searching for a higher number of periventricular lesions.
Cortical and juxtacortical lesions can be used in fulfilling MRI criteria for dissemination in space
The McDonald 2010 criteria for DIS included only juxtacortical lesions.
The first study demonstrating the occurrence and extension of cortical demyelination was by Bø et al. [24]. They described 4 types of cortical lesions (type 1 – extending both white and grey matter; type 2 – localized only in the cortex, without extend to the surface of brain or to the subcortical white matter; type 3 – the most common, subpial; type 4 – lesion in the full width of the cerebral cortex but not reaching the subcortical white matter).
Pirko et al. 2007 stated that cortical lesions, despite their different, less inflammatory character compared to white matter lesions (WML), are typical for MS.
Despite the development of magnetic resonance techniques (double inversion recovery, phase-sensitive inversion recovery) in the MAGNIMS guidelines, it was concluded that in most clinical scanners the highly advanced MRI techniques cannot be implemented. That is why the expert consensus was that these lesion should be treated as a single term: cortical/juxtacortical lesions [1].
Recommendations of the Polish Medical Society of Radiology and the Polish Society of Neurology
In 2020 the Polish Medical Society of Radiology and Neurology published recommendations for the MR protocol concerning imaging in patients with MS. They contain practical information for radiologists, which should be included in the exam description, emphasize the growing need for volumetric brain analysis to measure brain atrophy [25].
Also, the experts recommend the performance of brain MR in MS patients every 12 month during the first years of disease; performance of spine MR depends on clinical indications (Table 4).
Table 4
Head MRI protocol – recommendations of the Polish Medical Society of Radiology and the Polish Society of Neurology
Table 5
Spinal cord MRI protocol
MAGNIMS-CMSC-NAIMS recommendations
In 2021 new recommendations were made by the international group of North American Imaging in Multiple Sclerosis (NAIMS) and Consortium of Multiple Sclerosis Centers (CMSC). The specialists intended to unify European and North American guidelines mainly for usage of MRI in diagnosis and disease monitoring. The group took into consideration the clinical needs and proposed standardization of brain and spinal cord MR protocols, also precisely pointing to the scheme of control MR scans.
In these recommendations, the protocols were modified: mainly shortened and simplified (for example, the group recommend that spinal cord MRI not be performed routinely in control examination), and they emphasized the value of 3-dimensional FLAIR sequence.
Also, the recommendations include precise indications for gadolinium contrast usage. Mainly they are needed at the point of diagnosis, and they are extended to specific groups of patients: children, pregnant and post-partum women [26].
For pregnant women, gadolinium-based contrast is contraindicated, which is why, for the assessment of disease activity, it is recommended for the detection of T2 lesion enlargement/appearance of a new lesion.
During lactation gadolinium-based contrasts may be used only if it is highly necessary; afterwards, macrocyclic gadolinium-based contrast breastfeeding may be con-tinued.
Clinical implications of the McDonald criteria
Filippi et al., in a recent (2022) large cohort study, compared the specificity and sensitivity of the McDonald criteria from 2010 and 2017 in predicting MS after CIS.
After retrospectively analysing a group of 785 patients, the authors showed that the 2017 McDonald criteria have greater sensitivity (83% vs. 66%), lower specificity (39% vs. 60%), and similar accuracy overall compared with the 2010 McDonald criteria in predicting CDMS development independently of the type of clinical onset.
The study proved also that the 2017 Mc Donald criteria allow for earlier diagnosis of SM – the median time to diagnosis was about 3.2 months, without OCB 11.4 months, compared with the 2010 criteria at 13 months, and 58.5 months when waiting for a second clinical attack [7].
Another retrospective cohort study (250 patients) [27] revealed that the application of the 2017 revised McDonald criteria significantly shortened the time to diagnosis compared with the McDonald 2010 criteria. The authors concluded that the greatest impact on early diagnosis was from the OCB identification, with a median reduction in diagnostic time of 7.2 months.
This is crucial in the context of 2 multicentre studies that have shown that patients with CIS treated accurately have a delayed conversion to CDMS [28].
However, there is also a diagnostic dilemma: on one hand, early diagnosis helps to avoid the further development of the disease and in fact protects patients from disability, but on the other hand, the lower specificity of the McDonald 2017 criteria carry the implication of false positive diagnosis and the danger of unnecessary treatment, which brings the risk of possible side effects (Table 3).
Future directions
In a recent article by Filipp et al. 3 MR imaging markers of MS for further clinical research were indicated: the central vein sign, leptomeningeal enhancement, subpial demyelination and chronic active lesions [29].
The central vein sign (CVS) can be detected on susceptibility-weighted image sequences on MRI, where it appears as linear hyperintensity (corresponding with vein) within the plaque [30].
The big disadvantage of this sign is that its detectability depends on the scanner field strength. In a meta-analysis, the authors showed that 1.5T MRI scanners had a lower detectability of CVS in MS lesions (58%) than both 3T and 7T (respectively, 74 % and 82%).
Also, the usage of advanced SWI sequences , like 3D-epi, allows for higher effectiveness [31]. In our opinion, all the above make this imaging marker available mainly for a big scientific centres.
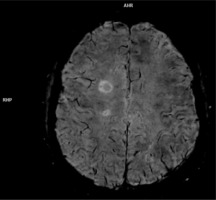
Chronic active lesions means MS white matter lesions that do not show post-contrast enhancement, but in SWI sequence there is a hypointense rim, which means a smouldering, demyelinating process and in fact indicates axonal degeneration and, most importantly, remye-lination failure [32] (Figure 2).
Figure 2
SWI sequence obtained on 3T scanner,showing central vein sign and hypointnense focus (arrow) in the periphery of chronic active lesion
Leptomeningeal enhancement. It is well known that in MS patients there is an accumulation of immune cells in different parts of brain. The presence of B-cell “follicle-like” structures was depicted along the meninges, which was connected with its diffuse inflammation, microglial activation, and grey matter cortical demyelination [33]. However, this inflammation does not cause T1 postcontrast enhancement but is best detected on delayed 3D flair post-contrast sequence. Because this feature is not specific only for MS (it can be present in other neuroinflammatory disorders, neoplastic and infectious disease) it is a subject for further investigation [29].
It is worth mentioning that the immunological processes in the pathophysiology of MS are nowadays a “hot topic” of new research, trying to find a new biomarkers of disease activity. For example, in our recent study we observed that the choroid plexus volume correlates with the activity of the inflammatory process in the CNS in MS patients. We proposed that it could become a valuable radiological biomarker of MS activity [34].
As well as leptomeningeal enhancement, also cortical demyelination is not specific for MS, but is still the subject of further investigation, especially regarding improvement of the quality of imaging. So far, it is best depicted on 7T scanners, which is why it cannot be routinely used in daily practice [29].
We can observe very intense and incessant research progress in the field of multiple sclerosis diagnosis, treatment, and monitoring of therapy.
This is the reason for continuous changes in the recom-mendations, which seem to be better and better adjusted to clinical needs.
It is mandatory to be aware that the goal is to achieve the proper diagnosis by taking into consideration all available and recommended examinations, not forgetting about the holistic judgement of multiple-sclerosis-related experts.