Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.239
Peer-review started: November 17, 2021
First decision: December 27, 2021
Revised: January 7, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: April 26, 2022
The estimation of left ventricular ejection fraction (LVEF) by 2D echocardiography (2D-ECHO) is the most used tool to assess LV systolic function (LVSF). Global longitudinal strain (GLS) has recently been suggested as a superior method for several evaluations. This study explored the association and prevalence of LV systolic dysfunction (LVSD) by using these methods in patients with end-stage renal disease (ESRD) and severe hyperparathyroidism (SHPTH); both associated with cardiovascular events (CEs).
To evaluate the myocardial function in patients with ESRD and SHPTH by using the GLS and LVEF measured through conventional 2D-ECHO.
In 62 patients with ESRD and SHPTH, asymptomatic, and without a history of CEs, LVSF was evaluated by 2D-ECHO, obtaining the EF, by the Simpson biplane method, and GLS by speckle tracking.
The total patients with ESRD had a preserved LVEF (> 50%) but abnormal GLS (< 13.55%). Additionally, multivariate analysis showed an independent association of GLS and serum parathyroid hormone (PTH), LV mass index, and hemoglobin. Also, PTH was independently associated with lateral e' wave and tricuspid regurgitation velocity.
In patients with SHPTH linked to ESRD, the use of GLS by 2D-ECHO is a more sensitive tool than LVEF for detecting LVSD.
Core Tip: This study compared global longitudinal strain (GLS) with the often-used left ventricular ejection fraction to estimate ventricular dysfunction in patients with end-stage renal disease. GLS had an advantage to detect dysfunction, but also, it was found that the parathyroid hormone levels were attractive as a complementary tool to predict patient status.
- Citation: Carrasco-Ruiz MF, Ruiz-Rivera A, Soriano-Ursúa MA, Martinez-Hernandez C, Manuel-Apolinar L, Castillo-Hernandez C, Guevara-Balcazar G, Farfán-García ED, Mejia-Ruiz A, Rubio-Gayosso I, Perez-Capistran T. Global longitudinal strain is superior to ejection fraction for detecting myocardial dysfunction in end-stage renal disease with hyperparathyroidism. World J Cardiol 2022; 14(4): 239-249
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/239.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.239
Cardiovascular disease (CVD) is the most common cause of morbidity and mortality in patients with end-stage renal disease (ESRD). Heart failure (HF) is a cardiovascular event (CE) that may occur simultaneously in these patients[1-3]. Chronic elevated pressure, volume overload and nonhemodynamic factors including inappropriate activation of the renin–angiotensin–aldosterone system (RAAS) and alterations in calcium–phosphorus mineral metabolism by an excess of parathyroid hormone[4,5] are involved in left ventricular hypertrophy (LVH)[6,7], then, in the myocardial dysfunction in ESRD[3,8].
LV ejection fraction (LVEF) calculated by 2D echocardiography (2D-ECHO) is the most widely used echocardiographic parameter that provides objective information on LV systolic function (LVSF), and it has been used as a prognostic and treatment indicator for CVD[9-11]. However, in some studies involving patients with ESRD, LVEF fails as a sensitive parameter for detecting subclinical data of LV systolic dysfunction (LVSD). In this sense, research groups have explored new variables and methods to increase the early and efficient diagnosis and prognosis of patients with LVSD[12]. Thus, for example, determination of left atrial volume[13], natriuretic peptides (NPs)[14] and catecholamines metabolites in serum (related to sympathetic overactivity)[15] have been proposed as advantageous tools.
Special attraction is on alternative 2D-ECHO methods, specifically, predialysis and peritoneal dialysis patients with preserved LVEF (> 50%) showed reduced GLS (15%), which was associated with an increased risk of HF hospitalization and increased mortality[3]. Thus, GLS may be a better tool to assess subclinical changes in LVSD in particular cases, for example, where the LVEF is preserved as in those patients subject to volume or pressure overload, or both[3,16]. Additionally, studies have mentioned that the variables used to define a preserved LVEF and a reduced GLS are associated with differences between the characteristics of the population studied (race, stage of ESRD, risk factors, and comorbidities). However, despite this variability, GLS is an important echocardiographic tool that might be used in patients with ESRD to identify those with a high-risk prognosis for developing HF or other CEs.
In the present study, a comparison was made from echocardiographic changes in patients with ESRD and hyperparathyroidism, the degree of LV remodeling, LVSF using 2D-ECHO, EF (by Simpson’s method), and GLS (by Speckle tracking). Additionally, plasma parathyroid hormone (PTH) concentration was considered to increase ability to detect LVSD in patients with ESRD and hyperparathyroidism (a common hormone elevated in ESRD, but poorly explored or linked to CEs in these patients).
This study was done in patients with asymptomatic ESRD with renal replacement therapy (RRT; dialysis and hemodialysis) and with no history of CEs. The degree of LVDD (I, II or III) was assigned by applying the Nagueh SF, 2020 recommendations[17], and the measurement of plasma PTH in relation to the LVSF and LV diastolic function (LVDF) was explored.
This retrospective and observational study included a total of 77 individuals divided into three groups: healthy individuals with no known disease (Control; n = 15), age > 18 years, both genders; patients with ESRD on hemodialysis (ESRD-HD; n = 31) and patients with ESRD on peritoneal dialysis (ESRD-PD; n = 31). The ESRD groups came from the Nephrology service of Centro Médico SXXI, IMSS. This study was carried out from February 2019 to October 2020.
All patients with ESRD maintained their RRT and were supplemented with calcium and calcitriol following clinical practice guidelines[18]. Those individuals with hemodynamic alterations increasing risk on their physical integrity were excluded. A complete medical history was taken. Demographic, clinical and comorbid characteristics were collected. A blood sample was collected in the morning before the RRT, and 4 h later, echocardiography was performed.
Transthoracic echocardiography images were obtained using a commercially available ultrasound system (Ecocardiograph iE33; Philips). Standard 2D Doppler, color, pulsed and continuous wave echocardiographic acquisitions were made. LV dimensions were obtained from images in a long axis parasternal view, and LV mass and body surface area-corrected indexed LV mass were calculated. In addition, the relative wall thickness was obtained as a ratio of (2 × RWTd) / LVIDd, where RWTd is the back-wall thickness, and LVIDd is the internal diameter of the LV at the end of diastole. The LV end-diastolic and systolic volumes were then measured from the apical views (two and four chambers) using the Simpson biplane method[19]. Mitral annulus early diastolic velocity (E') was identified by tissue Doppler imaging in a four-chamber view. Mitral inflow velocities, such as transmitral peak early passive filling velocity (E), late diastolic filling velocity caused by atrial contraction (A), and deceleration time, were determined using Pw-Doppler over the mitral valve in four-chamber views. The degree of diastolic dysfunction of each patient was assessed[17].
GLS was evaluated by obtaining LV grayscale scatter images from all three apical views. From the 2D images, the endocardial border was manually drawn during the end of the systole, and the thickness of the myocardial region of interest was adjusted to include the entire thickness of the myocardial wall. In each echocardiographic view, the myocardium was automatically divided into six segments. Therefore, the overall maximum systolic longitudinal pressure was calculated by averaging the maximum systolic values of all 18 segments, derived from the three apical views (six segments in each apical view) adjusted to the heart rate.
Continuous variables are expressed as the mean ± SD. Continuous variables were compared between groups using ANOVA and then a χ2 test. A multivariate logistic regression analysis was used, which included the clinical and echocardiographic parameters associated with the univariate analysis as independent variables. P < 0.05 was considered statistically significant. The statistical analysis was performed with SPSS version 20.
The characteristics were as follows from the total population: 48 participants were female (62%) and 29 (38%) male. According to the division, in the control group, the age was 34 ± 13 years, body mass index (BMI) 23.4 kg/m2, 67% female and 33% male, without relevant comorbidities. The ESRD-HD group age was 36 ± 2 years, BMI 23.3 kg/m2, 84% female and 16% male, 90% presented with hypertension and 6.45% with type 2 diabetes mellitus (T2DM). In the ESRD-PD group, age was 34 ± 10 years, BMI 22.4 kg/m2, 39% female and 61% male, all presented with hypertension, and 16% with T2DM (Table 1).
| Demographic variables | Control, n = 15 | ESRD-HD, n = 15 | ESRD-PD, n = 15 | Total, n = 15 |
| Age (yr) | 34 ± 13 | 36 ± 2 | 34 ± 10 | 34.6 ± |
| Gender, n (%) | F = 10 (67), M = 5 (33) | F = 26 (84), M = 5 (16) | F = 12 (39), M = 19 (61) | F = 48 (62), M = 29 (38) |
| BMI (kg/m2 ) | 23.4 | 23.3 | 22.4 | 23 ± |
| Hypertension, n (%) | N/A | 28 (90) | 31 (100) | 59 (77) |
| DMT2, n (%) | N/A | 2 (6.45) | 5 (16) | 7 (9.09) |
Patients with ESRD had severe refractory hyperparathyroidism (PTH > 650 pg/mL), but calcium (Ca2+), phosphorus (P), and calcium/phosphorus (Ca2+/P) ratio levels were within normal ranges. There was no significant difference from the control group, suggesting an average plasma calcitriol level. All of our population with ESRD showed LVH. LV mass and LV mass index (LVMI) were substantially increased; LVH concentric type was predominant (ESRD-HD = 80.65% and ESRD-PD = 93.35%) (Table 2).
| Variables | Control | ESRD-HD | ESRD-PD | P value |
| Biochemical | ||||
| PTH (pg/mL) | 50 ± 4.55a,c | 1188 ± 203.9 | 1188 ± 203.9 | < 0.0001 |
| Ca2+ (mg/dL) | 8.55 ± 2.34 | 8.47 ± 0.14 | 8.38 ± 0.09 | NS |
| P (mg/dL) | 4.56 ± 2.28 | 4.75 ± 0.24 | 4.75 ± 0.28 | NS |
| Ca2+/P (mg2/dL2) | 45.32 ± 1.05 | 42.31 ± 2.35 | 39.87 ± 2.5 | NS |
| Albumin (mg/dL) | 4.47 ± 0.10a,c | 4.13 ± 0.12 | 3.94 ± 0.06 | < 0.0284 |
| Hemoglobin (g/dL) | 15.3 ± 0.12a,c | 8.73 ± 0.24 | 8.91 ± 0.22 | < 0.0001 |
| LV remodeling | ||||
| LV mass (g) | 133.8 ± 3a,c | 182.7 ± 12.2 | 186.6 ± 15 | < 0.0001 |
| LVMI (g/m2) | 70.65 ± 2.11a,c | 130.2 ± 6.24 | 127.5 ± 6.55 | < 0.0001 |
| RWT | 0.39 ± 0.016a,c | 0.51 ± 0.01 | 0.51 ± 0.02 | < 0.0001 |
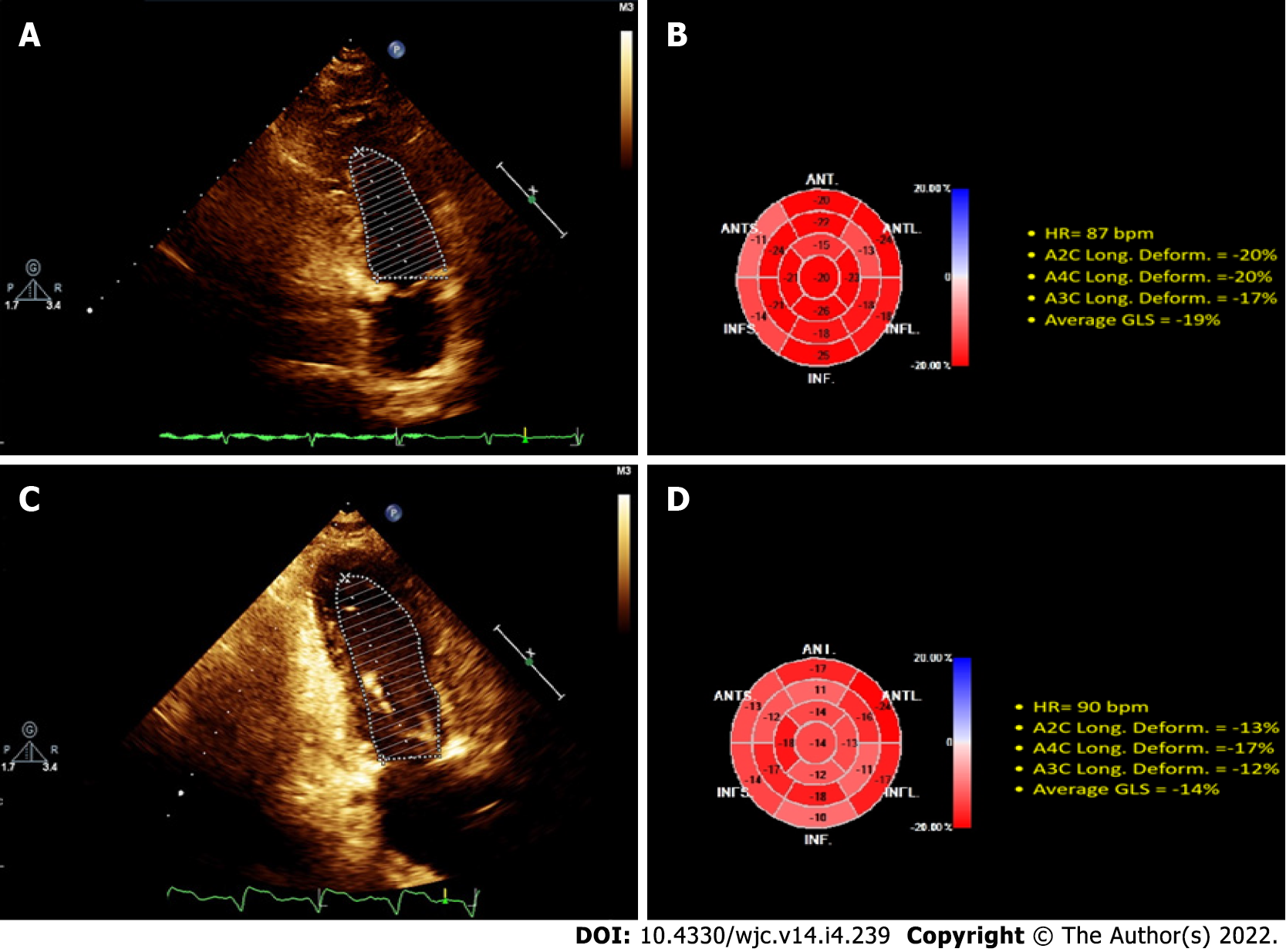
The 2D-ECHO variables of the population studied are shown (illustrative case in Figure 1), demonstrating that by Simpson’s method, LVEF was preserved, while the GLS was substantially reduced compared to the control group (21% ± 0.58% vs 13.93% and 13.18%; P < 0.0001; control vs ESRD-HD and ESRD-PD). The average GLS was 13.55% in all populations with ESRD. This shows that LVEF does not establish systolic dysfunction in patients diagnosed with ESRD, even though they have systolic dysfunction by GLS evaluation (Table 3).
| Variables | Control | ESRD-HD | ESRD-PD | P value |
| LVEF | 60.75 ± 1.30 | 63.5 ± 10.36 | 61.8 ± 11.19 | NS |
| GLS (%) | 21 ± 0.58a,c | 13 ± 0.72 | 12 ± 1.83 | < 0.0001 |
| LAV (mL/m2) | 33.07 ± 0.22a,c | 26.49 ± 1.4 | 25.73 ± 1.57 | < 0.0001 |
| E/A ratio | 1.25 ± 0.03a,c | 1.05 ± 0.06 | 0.91 ± 0.05 | < 0.0001 |
| E/é ratio | 5.38 ± 0.18a,c | 11.62 ± 0.96 | 12.22 ± 1.13 | < 0.0001 |
| E (cm/s) | 60.39 ± 1.71a,c | 80.19 ± 6.26 | 81.29 ± 6.97 | < 0.0001 |
| Lateral e’ (cm/s) | 13.44 ± 0.36a,c | 8.33 ± 0.43 | 7.49 ± 0.16 | < 0.0001 |
| Septal é (cm/s) | 10.85 ± 0.35a,c | 6.18 ± 0.24 | 6.49 ± 0.23 | < 0.0001 |
| TRV (m/s) | 2.21 ± 0.02a,c | 2.94 ± 0.08 | 2.80 ± 0.07 | < 0.0001 |
| LVMI (g /m2) | 70.65 ± 2.11a,c | 130.2 ± 6.24 | 127.5 ± 6.55 | < 0.0001 |
| RWT | 0.39 ± 0.016a,c | 0.51 ± 0.01 | 0.51 ± 0.02 | < 0.0001 |
In the bivariate analysis, an association of GLS with hemoglobin, hypertension, PTH, LVH and LVMI was shown. In comparison, the multivariate analysis showed an independent association of GLS and hemoglobin, PTH and LVMI (Table 4).
| Variables | Univariate | Multivariate using significant variables | Multivariate by step method | ||||||||||||
| B | β | 95%CI for B | P value | B | β | 95%CI for B | P value | B | β | 95%CI for B | P value | ||||
| Lower limit | Upper limit | Lower limit | Upper limit | Lower limit | Upperlimit | ||||||||||
| Association with GLS (%) | |||||||||||||||
| Hemoglobin | -1.09 | -0.61 | -1.42 | -0.77 | 0.00 | -0.58 | -0.32 | -1.12 | -0.05 | 0.03 | -0.62 | -0.35 | -0.99 | -0.26 | 0.00 |
| Hypertension | 5.60 | 0.54 | 3.58 | 7.61 | 0.00 | 1.52 | 0.15 | -1.44 | 4.49 | 0.31 | |||||
| PTH | 0.00 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 0.00 | 0.00 | 0.01 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 |
| LVEF | -0.04 | -0.08 | -0.15 | 0.07 | 0.50 | ||||||||||
| LVH | 6.40 | 0.57 | 4.27 | 8.53 | 0.00 | -1.82 | -0.16 | -7.75 | 4.12 | 0.54 | |||||
| LVMI | 0.06 | 0.51 | 0.04 | 0.08 | 0.00 | 0.03 | 0.28 | 0.01 | 0.05 | 0.01 | 0.03 | 0.29 | 0.01 | 0.05 | 0.00 |
| RWT | 11.36 | 0.25 | 1.27 | 21.46 | 0.03 | -1.21 | -0.03 | -10.14 | 7.73 | 0.79 | |||||
This study analyzed the LVDF by obtaining the pattern of transmitral flow rates and their relationship with the tissue velocities at the mitral ring level. The above is related to the fact that the increase in LV filling pressures is the primary physiological consequence of LVDD. These variables were significantly altered in the ESRD population compared to the controls (Table 3).
The tricuspid regurgitation velocity (TRV) and E/e' ratio associated with the decrease in the E/A ratio and the e' septal lateral waves reflect the pattern of altered relaxation and myocardial distensibility during the diastolic phase. Previously reported recommendations were applied[17], and it was found that among individuals with ESRD-HD, 67.74% (n = 21) had grade I, 22.58% (n = 7) grade II, and 9.67% (n = 3) grade III LVDD. Among individuals with ESRD-PD, 84.37% (n = 27) had grade I and 15.63% (n = 4) had grade II LVDD. The univariate and multivariate analyses (Table 5) showed the associations between the variables studied and PTH. An independent association of serum PTH and lateral e' wave and TRV was shown.
| Variables | Univariate | Multivariate using significant variables | Multivariate by step method | ||||||||||||
| B | β | 95%CI for B | P value | B | β | 95%CI for B | P value | B | β | 95%CI for B | P value | ||||
| Lower limit | Upper limit | Lower limit | Upper limit | Lower limit | Upper limit | ||||||||||
| Association with PTH | |||||||||||||||
| E/é ratio | 54.22 | 0.33 | 18.34 | 90.09 | 0.00 | 28.19 | 0.17 | -11.55 | 67.93 | 0.16 | |||||
| é lateral | -124.,51 | -0.36 | -199.34 | -49.69 | 0.00 | -81.10 | -0.23 | -177.06 | 14.87 | 0.10 | -89.72 | -0.26 | -169.01 | -10.44 | 0.03 |
| é septal | -121.78 | -0.28 | -215.64 | -27.91 | 0.01 | -0.63 | 0.00 | -121.22 | 119.97 | 0.99 | |||||
| TRV | 698.98 | 0.35 | 276.86 | 1121.10 | 0.00 | 360.73 | 0.18 | -141.53 | 862.99 | 0.16 | 500.88 | 0.25 | 53.87 | 947.89 | 0.03 |
Despite the extended use of EF to evaluate myocardial dysfunction, recent proposals promise to improve detection of cardiovascular dysfunction. As an example, 2D-ECHO for measuring left atrial volume is useful to predict diastolic dysfunction in ESRD patients[13]. Measuring plasma biomarkers has been suggested to extend diagnostic advantages. Thus, plasma NP levels are reported to be linked to cardiac remodeling[18], survival[19] and volume expansion-related LV disorders[20] in patients with ESRD[14].
Currently, 2D-ECHO methods remain core in diagnosis and evaluation of patients with CEs. The identification of LV strain changes is crucial for early cardiac damage detection in ESRD[21-24]. This study showed that increased LVMI, low hemoglobin levels but high PTH are independent factors associated with GLS alterations. The overall average GLS was 13.55%, with LVEF > 50% in the patient population, which according to the literature, is related to poor prognosis[25-29]. In this case, it was suggested that in the population with ESRD, the determination of GLS by 2D-ECHO is highly recommended for the assessment of LVSF; being a more powerful tool than LVEF, which can identify systolic myocardial damage[3,18,19] during follow-up of patients[29].
In patients with ESRD, bone mineral disorder is correlated with adverse outcomes from cardiovascular causes[25,30,31]. Recently, the mineral disorders in the early stages of ESRD have been associated with myocardial remodeling, which is a crucial point for LVH development[31-33]. In our study population, the presence of LVH was 100%, with a predominance of the concentric type. In this regard, some studies have directly implicated hyperphosphatemia and hypercalcemia in developing LVH[7,30] by regulating several factors[31,34-36]. However, it must be considered that the kidney is an essential target organ for PTH, and the interaction with its receptor activates multiple signaling pathways[37-39], triggering numerous changes in the kidney and some other systems[40]. As strong evidence of the PTH role in cardioremodeling, studies have shown that patients undergoing parathyroidectomy (PTX) have a substantial reduction in LV mass[41]; in contrast, patients with severe hyperparathyroidism and PTX showed a significant decrease in the risk of death from all causes[42]. Patients in the current study were supplemented with calcium or analogs; plasma values of Ca, P and Ca/P ratio were within normal ranges, while PTH levels were higher than reference values (PTH > 650 pg/mL). Multivariate analysis showed an independent association of GLS and PTH, suggesting a putative role of PTH in LVSF by diverse mechanisms, including those related to remodeling of subendocardial fibers[43].
Additional research is required to elucidate the mechanisms involved in the PTH modulation of the structure and function of the cardiomyocytes, as the pleiotropic effects are attributed to the interaction of PTH with its receptor [33,40].
PTH serum levels showed an independent association with some diastolic function parameters (Table 5): Particularly e' lateral wave and TRV. The alterations in diastolic function primarily involve the mechanisms of myocardial relaxation and compliance. The signaling pathways involving remodeling and cardiac fibrosis in patients with ESRD are complex and multifactorial, involving alterations in the extracellular matrix proteins, type I collagen, elastin, fibroblasts, and myofibroblasts[9,44-46], which reduce myocardial compliance. These effector pathways involve PTH and 25-hydroxy vitamin D3 and several other factors[34-39,46-51]. Elevated levels of catecholamine by sympathetic overactivity are reported to be linked to LV disorders and volume excess in ESRD patients[15].
Finally, our results are in line with recent reports supporting that conventional speckle-tracking echocardiography[52-54] might help identify LVSD in patients with ESRD and preserved LVEF. Early detection of myocardial morphological and functional changes in the routine evaluation of patients with ESRD can tackle the early stages of disease-independent modifiable risk factors that are associated with adverse CEs.
An accurate diagnosis is increasingly important in this type of patient with ESRD with systolic and diastolic myocardial function evaluation, offering integrated, lower cost, and noninvasive information. The use of new diagnostic tools is essential to provide the population with new targeted therapies for defined subsets of patients who may be at risk of developing potential complications. The use of GLS by 2D-ECHO is a more sensitive tool than LVEF to detect of LVSD. For individuals with ESRD and severe hyperparathyroidism refractory to hormone replacement but with normal Ca/P levels, it is essential to recommend the use of GLS as a diagnostic and prognostic tool for systolic myocardial dysfunction; albeit if patients have preserved LVEF.
Echocardiography is the most-used tool for diagnosis of myocardial dysfunction. Among 2D-ecocardiography (2D-ECHO) methods, ejection fraction (EF) is the most used. In patients with myocardial dysfunction and end-stage renal disease (ESRD) parathyroid hormone (PTH) is often altered.
Recent echocardiography methods are being compared with ejection fraction. Global longitudinal strain (GLS) is among the most explored. Additional serum biomarkers could help to increase sensibility of tests.
To compare the EF and global strain methods for detecting myocardial dysfunction. To measure the potential role of some biomarkers as complementary tools in patient evaluation.
Left ventricular systolic function (LVSF) was evaluated in 62 patients with ESRD with altered levels of PTH. LVSF was evaluated by performing 2D-ECHO, obtaining the EF, by the Simpson biplane method, and GLS by speckle tracking.
All patients with ESRD had preserved LVEF (> 50%) but abnormal GLS (< 13.55%). PTH was independently associated with lateral e' wave and tricuspid regurgitation velocity.
In patients with elevated PTH and ESRD, the use of GLS by 2D-ECHO is a more-sensitive tool than LVEF for detecting myocardial dysfunction.
GLS and serum PTH should be widely explored as potential sensitive tools to detect myocardial dysfunction in patients with ESRD. The mechanisms linked to this disrupted condition should be analyzed. Alternative echocardiography methods and biomarkers should be compared to select the most-sensitive and accurate tools.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Malatino L, Italy; Papazafiropoulou A, Greece S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1265] [Cited by in F6Publishing: 1364] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 2. | Shah S, Meganathan K, Christianson AL, Leonard AC, Thakar CV. Pre-dialysis acute care hospitalizations and clinical outcomes in dialysis patients. PLoS One. 2019;14:e0209578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hensen LCR, Goossens K, Delgado V, Abou R, Rotmans JI, Jukema JW, Bax JJ. Prevalence of left ventricular systolic dysfunction in pre-dialysis and dialysis patients with preserved left ventricular ejection fraction. Eur J Heart Fail. 2018;20:560-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1113] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 5. | Tamulėnaitė E, Žvirblytė R, Ereminienė R, Žiginskienė E, Ereminienė E. Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis. Medicina (Kaunas). 2018;54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Kim IY, Kim JH, Kim MJ, Lee DW, Hwang CG, Han M, Rhee H, Song SH, Seong EY, Lee SB. Plasma neutrophil gelatinase-associated lipocalin is independently associated with left ventricular hypertrophy and diastolic dysfunction in patients with chronic kidney disease. PLoS One. 2018;13:e0205848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal Med. 2015;5:254-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Herzog CA. Kidney disease in cardiology. Nephrol Dial Transplant. 2009;24:34-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ponikowski P, Jankowska EA. Pathogenesis and clinical presentation of acute heart failure. Rev Esp Cardiol (Engl Ed). 2015;68:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, Wanner C, Kahn SE, Toto RD, Zinman B, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, Marx N; CARMELINA Investigators. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation. 2019;139:351-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Kümler T, Gislason GH, Køber L, Torp-Pedersen C. Persistence of the prognostic importance of left ventricular systolic function and heart failure after myocardial infarction: 17-year follow-up of the TRACE register. Eur J Heart Fail. 2010;12:805-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Halliday BP, Senior R, Pennell DJ. Assessing left ventricular systolic function: from ejection fraction to strain analysis. Eur Heart J. 2021;42:789-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Tripepi G, Mattace-Raso F, Mallamaci F, Benedetto FA, Witteman J, Malatino L, Zoccali C. Biomarkers of left atrial volume: a longitudinal study in patients with end stage renal disease. Hypertension. 2009;54:818-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Mallamaci F, Zoccali C, Tripepi G, Benedetto FA, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Stancanelli B, Malatino LS; CREED Investigstors. The Cardiovascular Risk Extended Evaluation. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59:1559-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Abd ElHafeez S, Tripepi G, Stancanelli B, Dounousi E, Malatino L, Mallamaci F, Zoccali C. Norepinephrine, left ventricular disorders and volume excess in ESRD. J Nephrol. 2015;28:729-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kamperidis V, Marsan NA, Delgado V, Bax JJ. Left ventricular systolic function assessment in secondary mitral regurgitation: left ventricular ejection fraction vs speckle tracking global longitudinal strain. Eur Heart J. 2016;37:811-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Nagueh SF. Diastology: 2020-A practical guide. Echocardiography. 2020;37:1919-1925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, Bellanuova I, Tripepi R, Seminara G, Parlongo S, Stancanelli B, Bonanno G, Fatuzzo P, Rapisarda F, Belluardo P, Signorelli SS, Heublein DM, Lainchbury JG, Leskinen HK, Bailey KR, Redfield MM, Burnett JC Jr. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc. 2001;76:1111-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, Giacone G, Cottini E, Tripepi G, Malatino LS, Zoccali C. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001;16:1459-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1436] [Cited by in F6Publishing: 2488] [Article Influence: 207.3] [Reference Citation Analysis (0)] |
| 22. | Ewe SH, Haeck ML, Ng AC, Witkowski TG, Auger D, Leong DP, Abate E, Ajmone Marsan N, Holman ER, Schalij MJ, Bax JJ, Delgado V. Detection of subtle left ventricular systolic dysfunction in patients with significant aortic regurgitation and preserved left ventricular ejection fraction: speckle tracking echocardiographic analysis. Eur Heart J Cardiovasc Imaging. 2015;16:992-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | de Brito Galvao JF, Nagode LA, Schenck PA, Chew DJ. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J Vet Emerg Crit Care (San Antonio). 2013;23:134-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Drüeke TB. Hyperparathyroidism in Chronic Kidney Disease. 2021 Oct 18. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] [Cited in This Article: ] |
| 25. | Ke QQ, Xu HB, Bai J, Xiong L, Li MM. Evaluation of global and regional left ventricular myocardial work by echocardiography in patients with chronic kidney disease. Echocardiography. 2020;37:1784-1791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Panoulas VF, Sulemane S, Konstantinou K, Bratsas A, Elliott SJ, Dawson D, Frankel AH, Nihoyannopoulos P. Early detection of subclinical left ventricular myocardial dysfunction in patients with chronic kidney disease. Eur Heart J Cardiovasc Imaging. 2015;16:539-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Tadic M, Cuspidi C. Left ventricular strain and arterial hypertension: Is longitudinal strain ready for primetime? J Clin Hypertens (Greenwich). 2020;22:683-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Park JH, Park JJ, Park JB, Cho GY. Prognostic Value of Biventricular Strain in Risk Stratifying in Patients With Acute Heart Failure. J Am Heart Assoc. 2018;7:e009331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Park JJ, Mebazaa A, Hwang IC, Park JB, Park JH, Cho GY. Phenotyping Heart Failure According to the Longitudinal Ejection Fraction Change: Myocardial Strain, Predictors, and Outcomes. J Am Heart Assoc. 2020;9:e015009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | de Albuquerque Suassuna PG, Sanders-Pinheiro H, de Paula RB. Uremic Cardiomyopathy: A New Piece in the Chronic Kidney Disease-Mineral and Bone Disorder Puzzle. Front Med (Lausanne). 2018;5:206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Kaesler N, Babler A, Floege J, Kramann R. Cardiac Remodeling in Chronic Kidney Disease. Toxins (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 32. | Fujii H, Joki N. Mineral metabolism and cardiovascular disease in CKD. Clin Exp Nephrol. 2017;21:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Iseri K, Dai L, Chen Z, Qureshi AR, Brismar TB, Stenvinkel P, Lindholm B. Bone mineral density and mortality in end-stage renal disease patients. Clin Kidney J. 2020;13:307-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393-4408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1413] [Cited by in F6Publishing: 1437] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 35. | Tanaka S, Fujita S, Kizawa S, Morita H, Ishizaka N. Association between FGF23, α-Klotho, and Cardiac Abnormalities among Patients with Various Chronic Kidney Disease Stages. PLoS One. 2016;11:e0156860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Custódio MR, Koike MK, Neves KR, dos Reis LM, Graciolli FG, Neves CL, Batista DG, Magalhães AO, Hawlitschek P, Oliveira IB, Dominguez WV, Moysés RM, Jorgetti V. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transplant. 2012;27:1437-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Verdelli C, Corbetta S. MECHANISMS IN ENDOCRINOLOGY: Kidney involvement in patients with primary hyperparathyroidism: an update on clinical and molecular aspects. Eur J Endocrinol. 2017;176:R39-R52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell Mol Life Sci. 2011;68:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Vilardaga JP, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends Pharmacol Sci. 2012;33:423-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Fujii H. Association between Parathyroid Hormone and Cardiovascular Disease. Ther Apher Dial. 2018;22:236-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | McMahon DJ, Carrelli A, Palmeri N, Zhang C, DiTullio M, Silverberg SJ, Walker MD. Effect of Parathyroidectomy Upon Left Ventricular Mass in Primary Hyperparathyroidism: A Meta-Analysis. J Clin Endocrinol Metab. 2015;100:4399-4407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Li W, Zhang M, Du S, Yu Y, Liu J, Zhang L, Yao L. Impact of parathyroidectomy on survival among haemodialysis patients: A prospective cohort study. Nephrology (Carlton). 2016;21:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Sanchis L, Andrea R, Falces C, Poyatos S, Vidal B, Sitges M. Differential Clinical Implications of Current Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiogr. 2018;31:1203-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 478] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 45. | Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, Chen DQ, Vaziri ND, Zhao YY. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 46. | van Ballegooijen AJ, Visser M, Kestenbaum B, Siscovick DS, de Boer IH, Gottdiener JS, deFilippi CR, Brouwer IA. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study). Am J Cardiol. 2013;111:418-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Almahmoud MF, Soliman EZ, Bertoni AG, Kestenbaum B, Katz R, Lima JAC, Ouyang P, Miller PE, Michos ED, Herrington DM. Fibroblast Growth Factor-23 and Heart Failure With Reduced Versus Preserved Ejection Fraction: MESA. J Am Heart Assoc. 2018;7:e008334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 705] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 49. | Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 813] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 50. | Falcón D, Galeano-Otero I, Calderón-Sánchez E, Del Toro R, Martín-Bórnez M, Rosado JA, Hmadcha A, Smani T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front Physiol. 2019;10:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 796] [Article Influence: 36.2] [Reference Citation Analysis (2)] |
| 52. | Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351-69; quiz 453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 746] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 53. | Kizilca O, Ozmen D, Demircan T, Yilmaz N, Kir M, Soylu A, Unal N, Kavukcu S. Evaluation of left ventricular systolic functions in two-dimensional speckle-tracking echocardiography in children with chronic renal failure. Cardiol Young. 2021;31:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Wang X, Hong J, Zhang T, Xu D. Changes in left ventricular and atrial mechanics and function after dialysis in patients with end-stage renal disease. Quant Imaging Med Surg. 2021;11:1899-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |