Published online May 8, 2015. doi: 10.4254/wjh.v7.i7.968
Peer-review started: August 31, 2014
First decision: November 14, 2014
Revised: January 21, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: May 8, 2015
Oxidative stress is becoming recognized as a key factor in the progression of chronic liver disease (CLD) and hepatocarcinogenesis. The metabolically important liver is a major reservoir of mitochondria that serve as sources of reactive oxygen species, which are apparently responsible for the initiation of necroinflammation. As a result, CLD could be a major inducer of oxidative stress. Chronic hepatitis C is a powerful generator of oxidative stress, causing a high rate of hepatocarcinogenesis among patients with cirrhosis. Non-alcoholic steatohepatitis is also associated with oxidative stress although its hepatocarcinogenic potential is lower than that of chronic hepatitis C. Analyses of serum markers and histological findings have shown that hepatocellular carcinoma correlates with oxidative stress and experimental data indicate that oxidative stress increases the likelihood of developing hepatocarcinogenesis. However, the results of antioxidant therapy have not been favorable. Physiological oxidative stress is a necessary biological response, and thus adequate control of oxidative stress and a balance between oxidative and anti-oxidative responses is important. Several agents including metformin and L-carnitine can reportedly control mechanistic oxidative stress. This study reviews the importance of oxidative stress in hepatocarcinogenesis and of control strategies for the optimal survival of patients with CLD and hepatocellular carcinoma.
Core tip: Oxidative stress is a key biological response that correlates with the progression of chronic liver disease. However, oxidative stress is an essential survival mechanism and thus to erase it is an unsuitable approach to disease control. As hepatocarcinogenesis is closely associated with increased oxidative stress via viral proteins or chronic inflammation and lipids, controlling oxidative stress should be effective against progressive liver disease. Agents that can control oxidative stress might represent a more effective approach than reactive oxygen species-scavenging agents.
- Citation: Takaki A, Yamamoto K. Control of oxidative stress in hepatocellular carcinoma: Helpful or harmful? World J Hepatol 2015; 7(7): 968-979
- URL: https://www.wjgnet.com/1948-5182/full/v7/i7/968.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i7.968
The risk and pathogenesis of hepatocellular carcinoma (HCC) has been investigated in detail because about 80% of this type of cancer is due to chronic infection with hepatitis B (HBV) and C (HCV) viruses[1]. Hepatocellular carcinoma differentiates from low- and high-grade dysplastic nodules and sequentially advances into well-, moderately- and poorly differentiated HCC. Such multistep carcinogenesis associated with chronic inflammation suggests that numerous complex pathogeneses are involved in hepatocarcinogenesis. Whole exome sequencing has revealed that HCC contains many oncogene and tumor suppressor gene mutations[2]. The most common pathways can be p53-, Wnt- and RB1-dependent[3]. Poor outcomes of HCC include p53 signaling-related genes such as the protein kinase TTK[4]. Activation of the Wnt-catenin pathway is frequently anomalous in HCC and high expression levels correlate with poor outcomes[5]. Mutations in RB1 are associated with cancer-specific and recurrence-free survival after resection[6]. Such changes could be induced via HBV, HCV and lipid-induced cellular stress and chronic inflammation.
The pathogenic mechanisms of HBV- and HCV-related chronic liver disease (CLD) and hepatocarcinogenesis include viral protein-related immune function interference, tumor initiation or suppression interference and CLD-related environmental changes[1]. Tumor-related gene methylation is induced via HBV and HCV infection without inflammation and it is increased stepwise under conditions of chronic hepatitis, dysplastic nodules and HCC[7,8]. Oxidative stress is included in this process via the direct effects of viral proteins or secondarily to chronic inflammation[1].
Recent advances in HBV- and HCV-targeted anti-viral therapy have enabled control of HBV and HCV[9,10]. So far, the HBV load can only be decreased by nucleos(t)ide analogues that cannot eradicate the virus[11]. Pegylated interferon (IFN) combined with ribavirin and HCV NS3 protease inhibitor, can eradicate HCV in nearly 80% of therapy recipients. However, patients with liver cirrhosis have difficulties enduring the many side-effects of these drugs such as anemia, leukocytopenia, and skin rash[12]. New treatment modalities with IFN-free oral direct-acting antiviral agents combined with other types of therapy do not generate many of the side effects that are associated with IFN, but they are not indicated for decompensated liver cirrhosis because metabolism is decreased in patients with defective liver function[13]. Therefore, the direct hepatocarcinogenic activities of HBV and type C liver cirrhosis remain a major threat in endemic areas.
The incidence of non-alcoholic steatohepatitis (NASH) has recently increased and it progresses to HCC. Non-alcoholic steatohepatitis is an advanced stage of non-alcoholic fatty liver disease (NAFLD) that is involved in metabolic syndrome, a condition that becoming increasingly prevalent[14]. Both NASH and related HCC require urgent investigation because obesity is widespread in first-world countries. Oxidative stress is a key factor in NASH progression and NASH-related hepatocarcinogenesis[15,16]. The standard treatment for NASH is supplementation with the representative antioxidant, vitamin E[17].
The administration of antioxidant therapies for diseases involving oxidative stress is controversial because reactive oxygen species (ROS) are essential for maintaining defense by anti-pathogenic microorganisms, or by anti-carcinogenic mechanisms. Although many antioxidants are already on the market, their proven antioxidant activities in vitro have not been confirmed in vivo[18]. Many studies of cerebrovascular diseases and mortality have associated vitamin E with unfavorable outcomes[14]. Therefore, the notion of controlling oxidative stress in this manner requires re-evaluation. This article reviews current understanding of oxidative stress in viral hepatitis- and NASH-related HCC and the controversy surrounding antioxidant therapy for these diseases.
The mechanisms of hepatocarcinogenesis include several common functions such as oncogene activation, oxidative stress and tumor suppressor function attenuation, but the upstream functions differ. HBV related HCC can be found in non-cirrhotic carriers, whereas HCV-related HCC is found mainly in patients with cirrhosis[19]. The incidence of NASH related HCC is increasing and more clinical evidence is needed.
Oxidative stress could be induced via chronic hepatic inflammation regardless of etiology. Acute liver injury and hepatic inflammation induce ROS via neutrophils and Kupffer cell activation. As these cells invade liver parenchyma, hepatocytes could also be affected by the induced ROS. Although the free diffusion of superoxide is blocked by the plasma membrane, superoxide dismutase in the membrane can convert superoxide anions to H2O2 after internalization into hepatocytes[20]. The ROS in mitochondria are by-products of the beta oxidation pathway for fatty acid metabolism and they are generated via electron leakage from mitochondrial electron transport resulting in the activation of oncogenic pathways[21].
Enveloped HBV is a DNA virus containing a relaxed circular DNA genome enclosed by envelope protein[22,23]. After envelopment and the release of mature virions, HBV converts into a covalently closed circular DNA that persists in the nucleus of infected cells as minichromosomes that are difficult to eradicate[24]. After initial infection, HBV persists in the liver for life, even if a patient achieves a clinical cure with seroclearance of HBV envelope antigen (HBsAg) and the emergence of anti-HBs antibody[25]. Chronic inflammation and liver fibrosis caused by chronic HBV infection contributes to the development of HCC[26]. However, in addition to these host factors, HBV itself plays a direct role in the development of HCC[27,28]. A significant proportion of HBV-related HCC arises in otherwise normal livers[19,29], and animal models transfected with this virus genome develop HCC, which confirms the oncogenic potential of HBV in the liver[27,30]. Gene expression profiles of HBV-related HCC indicate that several genes related to signal transduction, transcription and metastasis play direct hepatocarcinogenic roles[31]. Despite a considerable amount of research, the molecular basis of HBV-related hepatocarcinogenesis remains unclear[32,33].
Chronic inflammation is a common feature of chronic hepatitis B and C that results in induction of oxidative stress. The HBV evades immune surveillance resulting in altered viral-specific and non-specific immune responses[34]. Kupffer cells or macrophages exert both immunostimulatory and immunoregulatory activities upon exposure to HBV. The addition of HBV particles and HBsAg induces production of the proinflammatory cytokines interleukin (IL)-1β, IL-6, CXCL-8 and tumor necrosis factor (TNF)-α by human CD68+ macrophage-enriched cells via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation[34]. However, another study did not find cytokine production with immunoregulatory cytokine transforming growth factor-β production[35]. The immune system activates Kupffer cells to eradicate HBV, while HBV evades the Kupffer cell-related pathway to reduce the inflammatory pathway and render the environment favorable for survival. Such persistent atypical cytokine production and the resulting ROS affect hepatocarcinogenesis.
The HBV genome encodes several gene products such as DNA polymerase (Pol), capsid protein (core), envelope proteins L, M and S, and the multifunctional protein HBx. Among these products, the oncogenic potential of HBx protein has been analyzed in detail. Transactivating HBx protein stimulates viral gene expression and replication, and also protects virally-infected cells against immune-mediated destruction[36]. High and low levels of HBx protein are expressed in the cytoplasm and nucleus, respectively, of hepatocytes infected with HBV[37]. HBx protein regulates some oncogenes and affects several apoptosis-related signaling pathways[36]. Through binding to transcription factors such as CREB, RPB5, TFIIB, XAP-1, C/EBPalpha and XAP3, HBx can upregulate oncogenes such as Rab18 or Yes-associated protein[38,39]. HBx induces apoptosis by upregulating FasL protein through activating MLK3/MKK7/JNKs signaling and interacting with Bcl-2/CED-9 signaling[40,41].
Concentrated cytoplasmic HBx co-localizes with mitochondria that are sources of ROS[42]. The C-terminal region of HBx produced by HBx truncation is required for ROS production[37] and it is found in 46% of HCC, but not in non-tumor tissues[43]. The C-terminal truncated HBx found in HCC suggests that ROS are involved and that it is significantly associated with increased venous invasion and metastasis. The stable expression of C-terminal truncated HBx in vitro results in increased C-Jun kinase transcriptional activity and the enhanced invasiveness of hepatoma cell lines.
Integration of the HBV gene into the house genome is an important mechanism that is responsible for HCC development. The frequency of integration is reportedly higher in tumors than in adjacent liver tissues (86.4% vs 30.7%). Several cancer-related genes such as TERT, MLL4, and CCNE1 are integrated by HBV, especially in tumors[44] and among these, HBx is most frequently integrated into the human genome.
Several studies have analyzed mutations within the HBV genome that might be associated with HCC. Genotypic diversity is related to differences in clinical and virological characteristics. For example, patients infected with genotype C have more severe chronic liver disease, including cirrhosis and HCC[29,45]. Deletions and point mutations, which are more subtle genetic variations than genotypes, have been identified, such as codon 38 in the X gene, the core promoter region, G1613A and C1653T, basal core promoter region A1762T/G1764A mutations and deletions of pre-S and X protein[46-49]. The analytical findings of HCC-related HBV mutations emphasized the importance of HBx and suggested the involvement of oxidative stress. The envelope protein pre-S is also involved in HCC development. The pre-S region might affect the pathway of hepatocarcinogenesis via oxidative stress.
The HCC-associated HBV variant pre-S has been identified in the pre-S2 start codon and/or in deletions of the 5’-terminal of the pre-S2 region and pre-S1 mutation with deletions of the 3’-terminal of pre-S1[50,51]. These pre-S region mutant products induce the accumulation of mutated L protein in the endoplasmic reticulum (ER) of hepatocytes. The ER plays a major role in the synthesis, folding and trafficking of secretory and membrane proteins that correlate with the cellular response known as ER stress. A considerable amount of experimental data have confirmed the potential pro-oncogenic role of pre-S mutated gene products via accumulation in the ER with enhanced ER stress and ROS[51]. The pre-S mutated proteins accumulating in the ER can trigger c-Raf-1/Erk2 signaling, which results in AP-1 and NF-κB activation, enhanced proliferative activity of hepatocytes and an increased incidence of liver tumors in transgenics[52]. Pre-S2 mutated proteins also have non-ER related functions such as interacting with Jun activation domain-binding protein 1, which results in cyclin-dependent kinase inhibitor p27 degradation and cell cycle progression[53].
Oxidative stress must be involved to some degree in HBV-related hepatocarcinogenesis, possibly via HBx and pre-S-related functions. Relatively weak oxidative stress has been defined in HBV-related hepatocarcinogenesis in vitro. HBV with HBx protein expressed in mitochondria, binds to voltage-dependent anion channels 3 and alters the mitochondrial transmembrane potential resulting in ROS generation and the activation of several transcription factors[54]. Analyses of serum from patients have shown that oxidative stress-related markers are significantly increased in HCV-, but not in HBV-related HCC[55]. One reason for this might be that HBV has other prevailing features such as the induction of mutations in oncogenic and tumor suppressor genes by viral proteins. Anti-oxidant therapy is seemingly logical for HBV-related HCC as HBx and pre-S proteins have promising effects on oxidative stress. However the inferior outcomes of oxidative stress in human serum and in vitro indicate that expectations should not be too high.
Since HCV, like HBV, is not a cytopathic virus, immune reactions play a central role in the development of chronic hepatitis[56,57]. However, the clinical course and direct viral and hepatitis-related effects on hepatocarcinogenesis differ. The expression of several genes associated with detoxification and the immune response suggest that indirect and immune or detoxifying response-related hepatocarcinogenic roles are involved in HCV-related HCC[31].
Immune systems are disrupted by HCV proteins. Antigen-presenting cells such as Kupffer cells, macrophages, or dendritic cells exhibit both immunostimulatory and immunoregulatory activities upon exposure to HCV[58]. The HCV core and Kupffer cells affected by NS3 secrete pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and the immunosuppressive cytokine, IL-10, in vitro[59]. The release of pro-inflammatory cytokines might explain the induction and persistent inflammation of patients with chronic hepatitis C, while immunosuppressive cytokine release explains the difficulty of eradicating HCV-infected hepatocytes. The direct effects of HCV on the inflammatory signal in Kupffer cells upregulates the immunoregulatory molecule PD-L1[60]. In addition to interference with Kupffer cell-related anti-viral activities, HCV induces a sufficient amount of inflammatory cytokines to result in chronic inflammation. Kupffer cells accumulate around inflammatory foci and express cytotoxic molecules such as granzyme B, perforin and ROS that induce inflammation and fibrosis[61]. Sustained inflammation results in hepatocyte apoptosis and repeated regeneration cycles followed by spontaneous DNA mutation and damage resulting in HCC[1].
HCV antigens, especially core protein, play major roles in chronic hepatitis C pathogenesis and hepatocarcinogenesis[62]. Because microarray analysis has not suggested a direct hepatocarcinogenic pathway in HCV-related HCC, the direct effects of viral proteins are seemingly less powerful than HBV. However, HCV core protein seems to play direct roles in TNFR, PKR and Stat3 pathways that are associated with cell proliferation, apoptosis, transformation and immortalization[63,64]. Transgenic mice expressing HCV core protein exhibit hepatocarcinogenesis via fatty metamorphosis and increased oxidative stress[65,66]. The glutathione pool is oxidized and NADPH content is decreased via the direct interaction of HCV core and mitochondria from the livers of transgenic mice expressing HCV proteins[67]. Patients with HCV-related HCC have more oxidative stress in the liver and higher levels of serum oxidative stress markers such as 8-hydroxy-2’-deoxyguanosine or reactive oxygen metabolites than those with HBV-related HCC[55,68,69]. A weak, direct carcinogenic effect of HCV core protein might coordinate with oxidative stress, chronic inflammation and damage to apoptosis regeneration cycle DNA to result in hepatocarcinogenesis at a more advanced age than patients with HBV-related infection[70]. Oxidative stress is associated with aging that also drives hepatocarcinogenesis in patients with HCV-related HCC[71].
Serum markers and hepatocyte deposition associated with iron especially in lysosomes are frequently elevated in patients with chronic hepatitis C infection[72]. Reticuloendothelial systems including Kupffer cells are also targets of iron deposition that might affect chronic inflammation[73]. Excess divalent iron atoms are highly toxic, as they induce the Fenton reaction and produce highly toxic ROS, hydroxyl radicals. Because some investigators have reported that phlebotomy and a low-iron diet lowers the risk of HCC developing in patients with chronic hepatitis C infection, iron toxicity is thought to be involved in hepatocarcinogenesis[74,75]. Oxidative stress induced by HCV reduces hepcidin transcription followed by ferroportin expression in enterocytes and increases duodenal iron uptake[72,76]. An excess of dietary iron fed to HCV transgenic mice induces excess hepatocarcinogenesis[77]. Iron and resultant oxidative stress closely correlate with the progress of chronic hepatitis C and related HCC development that should be incorporated as treatment options. As described above, the lipid-related, direct pro-oxidant functions of HCV proteins, especially the core and iron accumulating functions, indicate the importance of the relationship between oxidative stress and hepatocarcinogenesis in patient with chronic hepatitis C.
The pathophysiology of NASH has been considered to comprise a “two hit” theory[78]. The first hit is hepatocyte steatosis, which is characterized by the accumulation of triglyceride in hepatocytes. The second hit consists of various types of cellular stresses, such as apoptosis, oxidative stress, ER stress, and intestinal circumstances. The recent genome-wide association study discovered that the patatin-like phospholipase 3 (PNPLA3) gene correlates with NASH progression[79]. This genetic mechanism might be involved in the first hit and fatty deposition could be the second hit; nonetheless, this two-hit theory is too simple to explain all aspects of NASH[15,80]. Other studies have found that inflammation induces fatty deposition in hepatocytes. Such findings have recently led to a “multiple hit” theory to explain the fact that inflammation promotes steatosis or that genetic factors such as PNPLA3 correlate with disease progression[80]. Lipid droplets were originally thought to function simply as cellular energy-storage structures. However, they now considered to be complicated organelles that are involved in many processes such as metabolic, inflammatory and immunological responses. Lipid toxicity induces multiple hits such as oxidative stress, ER stress, and immune reactions[14]. These cellular stresses are also involved in carcinogenesis.
Obesity-related symptoms such as hypertriglyceridemia and hypertension are established risk factors for NAFLD[81]. Central obesity presenting as visceral fat accumulation is associated with various pathologies such as cerebrovascular diseases, type 2 diabetes, NASH and gastrointestinal cancers. Visceral obesity induces several cytokines including the inflammatory cytokine IL-17[82], which induces neutrophil chemokine expression via IL-17 receptor A which is extensively expressed in the liver. Controlling the IL-17-related pathway effectively prevents NASH progression in mouse models[83]. Elevated pre-therapy serum IL-17 levels in patients with HCC correlate with risk of early recurrence after curative hepatectomy[84]. Co-cultured HCC cell lines and T cells producing IL-17 in vitro augment the proliferation of HCC cells, suggesting the importance of IL-17 for HCC pathogenesis. Neutrophil infiltration might be involved in NAFLD progression in human NASH. Therefore, visceral adipocytes that accumulate in patients with central obesity are related to such cytokines and should be involved in the pathogenesis of NAFLD.
Visceral fat accumulation correlates with increased adipokine levels, significant risks for HCC and recurrence after curative treatment[85,86]. Adiponectin is a “good” adipokine that modulates many metabolic processes including glucose regulation and fatty acid oxidation. Adiponectin is the most abundant adipokine mainly secreted from mature, white adipose tissue, and levels of expression and secretion increase during adipocyte differentiation. Adiponectin levels inversely correlate with BMI, visceral obesity contents and insulin resistance. Serum adiponectin is significantly higher in females than in males, in whom serum androgens become more evident during puberty[87]. Adiponectin has anti-inflammatory, anti-diabetic and anti-lipid storage effects. Furthermore, weight-loss induces adiponectin synthesis, whereas proinflammatory adipokines such as TNF-α and IL-6 suppress adiponectin[88]. Adiponectin increases the expression of CXCL-8 in primary hepatocytes that functions in cell survival and in anti-apoptotic activities to guard cells; however, this also induces uncontrolled cell survival resulting in malignant transformation[89]. High levels of serum adiponectin are associated with reduced risk for several cancers such that prostate, breast, colorectal and pancreatic cancers[90,91]. However, higher levels of low- and medium-molecular weight adiponectin are also associated with a higher risk of HCC through a relationship with the inflammatory response[92]. Increased levels of adiponectin might be induced via a compensatory mechanism to dampen inflammation. However, several studies have also found that adiponectin has direct proinflammatory activities. More evidence is needed to confirm this.
The relationship between hepatic iron deposition and disease progression in NASH remains controversial[93]. Iron accumulation in hepatocytes correlates with more severe damage[94]. The iron metabolic pathway is implicated in the insulin resistance and hepatic cholesterol synthesis pathways. Hepatic lipid-induced ER stress results in an unfolded protein response and hepatic iron accumulation[95]. Several reports describe the risk of iron overload in NASH[96].
Oxidative stress closely correlates with HCV- and NASH-related hepatocarcinogenesis, but relatively weakly with HBV-related HCC. Thus, anti-oxidant therapy would seem reasonable for controlling HCV- and NASH-related hepatocarcinogenesis. Liver inflammation is definitely associated because hepatocarcinogenesis arises mostly in patients with chronic hepatitis. The major inflammatory cytokine TNF-α alters mitochondrial integrity by mimicking a mild uncoupling effect in liver cells, as indicated by a reduction in membrane potential and ATP depletion[97]. The TNF-α induced ROS activation of NF-κB and downstream target genes such as CXCL1, IκBα and A20 results in enhanced migration activity of hepatoma cell lines. Reduced hepatic inflammation via nucleos(t)ide analogues in hepatitis B and interferon in hepatitis C correlates with reduced oxidative stress. However, preventing or controlling HCC using antioxidants is a matter of debate.
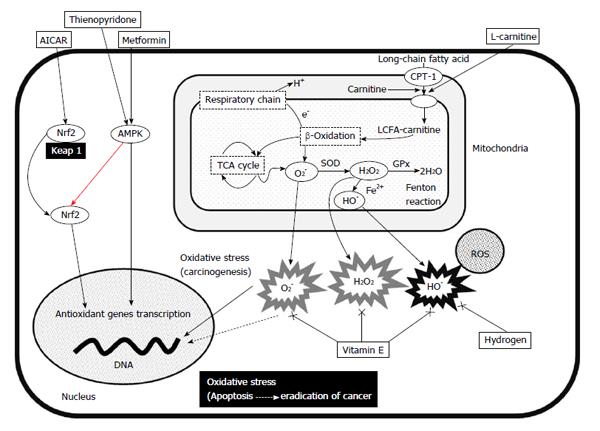
Oxidative stress is increased through the generation of ROS and defects in redox defense mechanisms with glutathione (GSH), catalase or superoxide dismutase (SOD)[98]. Mitochondria comprise the most important and abundant source of intracellular ROS. Mitochondrial dysfunction therefore plays a central role in the pathological mechanisms of chronic hepatic inflammation and subsequent hepatocarcinogenesis (Figure 1). An imbalance in the mitochondrial respiratory chain is the main source of ROS, O2-, H2O2 and hydroxyl radicals (•OH). The transport of high-energy electrons through the mitochondrial electron transport chain (ETC; complexes I, III and IV) is an important step for ATP production. This energy-producing pathway also produces ROS. High-energy electrons in ETC complexes I-III react with O2 and produce superoxide (O2-) which accounts for up to 4%-5% of the consumed O2. The amount of resulting O2- increases in damaged mitochondria. The tricarboxylic acid cycle and the β-oxidation of fatty acids generate reduced (NADH and FADH2) cofactors from oxidized (NAD+ and FAD) cofactors. The NADH uses two electrons from the oxidation pathway in the inner membrane to supply the rest of the ETC for the reduction of O2 to water. As the substrates and cofactors of complex I have the lowest reduction potential in the mitochondrial respiratory chain, side reactions could occur such as nonspecific electron transfer to species in solution. Under physiological conditions, reactive and incompletely reduced forms of oxygen such as superoxide (O2-) are detoxified into water by anti-oxidant defenses and repair enzymes to maintain a low steady state of toxic oxidants[14].
The mitochondrial capacity to control the oxidative balance would be destroyed under conditions of continuous oxidative stress. Excess O2- could be produced within damaged mitochondria through electron leakage and SOD would convert the resulting excess O2- into H2O2. Glutathione plays an important role in the balance between pro- and anti-oxidants correlating with the detoxification of H2O2 and other ROS. Glutathione-dependent enzymes as glutathione peroxidase, glutathione reductase (GR), and glutathione S-transferase (GST) play important roles in the stimulation of glutathione function. Glutathione peroxidase catalyzes H2O2 to non-toxic H2O, GR regenerates the pool of reduced glutathione and GST catalyzes glutathione reactions. Despite such anti-oxidant mechanisms, the Fenton and/or Haber-Weiss reactions generate the highly reactive toxic ROS, •OH from H2O2 and oxidative stress produced by Fenton reactions is mediated by iron.
Iron loading of the liver causes further hepatic oxidative stress that is a main cause of HCC[99]. Iron overload can induce hepatocarcinogenesis in animals and is associated with the high incidence of HCC among African and Taiwanese individuals[100,101]. Iron depletion in HCV- and NASH-related hepatocarcinogenesis should play a more important role in the prevention of HCC.
Several antioxidant agents and foods are widely available and the effects of several of them in vitro and in vivo are under investigation. The optimal choice of agents to control chronic hepatitis or related hepatocarcinogenesis without suppressing the physiological roles of oxidative stress is difficult to determine.
Phlebotomy is a method of reducing iron overload that has been effective against chronic hepatitis C and NASH in selected patients[102,103]. Iron depletion improves not only iron overload but also insulin resistance, suggesting that iron toxicity is involved in several metabolic pathways[104]. Iron depletion for NASH remains a matter of debate but it could nevertheless function as an antioxidant treatment strategy.
Candidate antioxidant treatments for HCC include antioxidant genes, inducible transcriptional factors such as AMP-activated protein kinase (AMPK) or nuclear factor erythroid 2-related factor (Nrf2) activators, ROS scavengers and agents that support mitochondrial uptake. The following are representative drugs that are associated with reducing oxidative stress.
Metformin: Metformin is an anti-diabetic drug, but it also has an anti-oxidative function. Metformin increases intracellular levels of AMP after activating AMPK, which is a highly conserved heterodimeric serine-threonine kinase that serves as an energy sensor in eukaryotic cells and bridges metabolism to carcinogenesis[105]. It is activated by an increase in the cellular AMP/ATP ratio under hypoglycemia, hypoxia, ischemia and heat shock[106]. The activation of AMPK suppresses cell proliferation in non-malignant and malignant cells via regulation of the cell cycle, apoptosis, autophagy and the inhibition of fatty acid synthesis[107]. Phospho (p)-AMPK is down-regulated in HCC tissues from patients and low levels of p-AMPK expression correlates with a poor prognosis, indicating the importance of AMPK signaling in HCC[108]. Adding metformin to hepatoma cell lines results in AMPK activation as well as dose- and time-dependent growth inhibition. Metformin also induces cell-cycle block, apoptosis, STAT3-induced IL-6 production[109] and the antioxidant enzyme heme oxygenase-1 (HO-1) in human endothelial cells via the Nrf2 signaling pathway[110]. The recently-discovered direct AMPK activator thienopyridone also activates AMPK through distinct mechanisms with metformin[111]. This might be a future approach to activate AMPK.
A meta-analysis of anti-diabetic drugs found that metformin, sulfonylurea and insulin induce a 50% reduction, and 62% and 161% increases, respectively in HCC incidence[112]. However, randomized controlled trials have not shown significant effects. Metformin reduced the occurrence of HCC and liver-related death, and increased survival rates in patients with diabetes and HCC who underwent radiofrequency ablation without any severe side effects[113]. The standard treatment for NASH according to the guidelines of the American Association for the Study of Liver Disease (AASLD) is vitamin E. This was derived from the findings of a clinical trial that has shown improvements in the clinical profile and histological findings of NASH activity within two years[114]. Metformin improves histological activity in the livers of mouse models of non-diabetic NASH, but not in human NASH[115]. Thus, metformin is preferential for treating NASH-related HCC in mouse models[116], but clinical trials are needed to confirm the effects of metformin on HCC management.
Nrf2-acting agents: Under basal conditions, Nrf2 binds to Kelch-like ECH associating protein 1 (Keap 1) which exists in the cytoplasm in an inactive form[117]. The AMPK activator 5-aminoimidazole-4-carboxamide-1-b-D-ribofuranoside (AICAR) induces an increase in Nrf2 protein and antioxidant enzyme expression in endothelial cells, whereas AICAR activates Nrf2 in hepatoma cell lines resulting in antioxidant enzyme expression[118]. A combination of metformin and AICAR might activate AMPK and Nrf2 to control HCC. A more detailed analysis in a basic approach with clinical trials might be needed to determine the value of this new type of drug.
Vitamin E: Controversy surrounds the value of ROS-scavenging agents because ROS have essential functions for life. Scavengers of ROS consistently exert effective chemical activities in vitro, but not often in vivo[18]. Scavenging ROS is considered effective in preventing the development of cancer and cancer stemness, yet ROS also contribute to the prevention of cancer[119]. Stem cell-like cancer cells that express the CD44 variant have an antioxidative phenotype. The CD44 variant protects cancer stem-like cells from oxidative stress and prevents their apoptosis[120]. Oxidative stress upon normal cells might induce transition to a cancer cell phenotype that is highly resistant to further oxidative stress. Several clinical trials are presently investigating the induction of oxidative stress under these conditions as a cancer treatment[121]. The AASLD recommends treating NASH with vitamin E at a dose of 800 IU/d, which is higher than that usually administered to treat NASH[17]. This recommendation is based on a two-year randomized study of NASH that demonstrated improved alanine transaminase and histological activity[114]. However, that trial did not find an improvement in liver fibrosis[122]. Further investigations of longer duration are required to determine the effects of vitamin E on hepatocarcinogenesis.
Peroxisome proliferator-activator-γagonist (pioglitazone): The transcription factor peroxisome proliferator-activator (PPAR)γ regulates lipid metabolism and its activation suppresses hepatic lipoperoxide and reduces the production of the hepatic pro-inflammatory cytokines, TNF-α and IL-6[123]. The effects of PPARγ on a model of diethylnitrosamine (DEN)-induced HCC via cyclin-dependent kinase inhibitor p27 expression are favorable[124]. Pioglitazone serve as an anti-oxidant to treat NASH. A large clinical study has shown fair results of using pioglitazone to treat NASH with respect to serum alanine aminotransferase levels[114]. Although an improvement in histological activity was not proven, this drug will be assessed for patients with NASH-related diabetes. A long observational assessment of hepatocarcinogenesis will be needed later.
Hydrogen: Molecular hydrogen (H2) has powerful and selective antioxidant effects with unique features[125]. Hydrogen scavenges toxic hydroxyl radicals, but not O2-, H2O2 or nitric oxide in cultured cells. This selective reduction of ROS has been explained by the strong oxidative activity of hydroxyl radicals reacting with mild anti-oxidative function of hydrogen. The easy distribution of hydrogen is one characteristic of the effects. Most hydrophilic compounds are retained at membranes and cannot pass into the cytoplasm, whereas hydrophobic compounds such as vitamin E need specific carriers or receptors to penetrate biomembranes. However, H2 can penetrate biomembranes, diffuse into the cytosol and easily reach the nucleus where it can protect nuclear DNA and prevent mitochondrial damage. Hydrogen is effective against DEN-induced HCC and HCC associated with type 1 diabetes and NASH[126,127]. Data from patients with NASH are not yet available and thus clinical trials are required to further evaluate the effects of molecular hydrogen on HCC.
L-carnitine: L-carnitine is an essential nutrient that converts fat into energy in mitochondria and ameliorates liver damage. It acts as a fatty acid carrier across the mitochondrial membrane and it also exists as free or acyl forms in plasma[128]. L-carnitine plays an important role in lipid metabolism as it is an essential cofactor for the β-oxidation of fatty acids through facilitating the transport of long-chain fatty acids and its ability to activate carnitine palmitoyltransferase, the key enzyme in fatty acid oxidation[129]. L-carnitine has recently been proposed as treatment for various diseases, including liver damage. Several studies have shown that L-carnitine can ameliorate or prevent liver damage with various etiologies. Animal studies have shown that dietary supplementation with L-carnitine prevents chemically induced hepatitis and subsequent HCC, as well as NASH-related HCC[130,131]. L-carnitine supplementation greatly improves plasma glucose levels in patients with NASH, lipid profiles and histological manifestations[132]. The results of these clinical trials were fair, and more clinical trials of larger populations are required to further evaluate the effects of L-carnitine on hepatocarcinogenesis.
Because oxidative stress is an essential survival mechanism, erasing it is not a feasible approach to disease control. Rather, controlling oxidative stress should be effective because hepatocarcinogenesis is closely associated with increased oxidative stress via viral proteins or chronic inflammation and lipids. Agents that can control oxidative stress such as the AMPK activator metformin or the mitochondrial support agent L-carnitine probably comprise a more effective approach than ROS scavengers such as vitamin E.
P- Reviewer: Czaja MJ, Ding MX, He ST S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 599] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 2. | Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 962] [Cited by in F6Publishing: 1059] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 3. | Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, Matsuda M, Fujii H, Scoazec JY, Ohgaki H. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Miao R, Luo H, Zhou H, Li G, Bu D, Yang X, Zhao X, Zhang H, Liu S, Zhong Y. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol. 2014;61:840-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450-456. [PubMed] [Cited in This Article: ] |
| 6. | Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM, Sung CO, Baek D, Haq F, Ansari AA, Lee SY. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology. 2014;60:1972-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 7. | Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Okamoto Y, Shinjo K, Shimizu Y, Sano T, Yamao K, Gao W, Fujii M, Osada H, Sekido Y, Murakami S. Hepatitis virus infection affects DNA methylation in mice with humanized livers. Gastroenterology. 2014;146:562-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitou S, Arase Y. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669-1679.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Everson GT, Sims KD, Rodriguez-Torres M, Hézode C, Lawitz E, Bourlière M, Loustaud-Ratti V, Rustgi V, Schwartz H, Tatum H. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146:420-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Takaki A, Kawai D, Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2014;15:7352-7379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 16. | Tanaka S, Miyanishi K, Kobune M, Kawano Y, Hoki T, Kubo T, Hayashi T, Sato T, Sato Y, Takimoto R. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J Gastroenterol. 2013;48:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2413] [Cited by in F6Publishing: 2449] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 18. | Bast A, Haenen GR. Ten misconceptions about antioxidants. Trends Pharmacol Sci. 2013;34:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [PubMed] [Cited in This Article: ] |
| 20. | Fisher AB. Redox signaling across cell membranes. Antioxid Redox Signal. 2009;11:1349-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Higgs MR, Chouteau P, Lerat H. ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. J Gen Virol. 2014;95:991-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Seitz S, Urban S, Antoni C, Böttcher B. Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions. EMBO J. 2007;26:4160-4167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 25. | Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108. [PubMed] [Cited in This Article: ] |
| 26. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [PubMed] [Cited in This Article: ] |
| 27. | Wang Y, Cui F, Lv Y, Li C, Xu X, Deng C, Wang D, Sun Y, Hu G, Lang Z. HBsAg and HBx knocked into the p21 locus causes hepatocellular carcinoma in mice. Hepatology. 2004;39:318-324. [PubMed] [Cited in This Article: ] |
| 28. | Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39:1683-1693. [PubMed] [Cited in This Article: ] |
| 29. | Kao JH. Hepatitis B viral genotypes: clinical relevance and molecular characteristics. J Gastroenterol Hepatol. 2002;17:643-650. [PubMed] [Cited in This Article: ] |
| 30. | Zheng Y, Chen WL, Louie SG, Yen TS, Ou JH. Hepatitis B virus promotes hepatocarcinogenesis in transgenic mice. Hepatology. 2007;45:16-21. [PubMed] [Cited in This Article: ] |
| 31. | Iizuka N, Oka M, Yamada-Okabe H, Mori N, Tamesa T, Okada T, Takemoto N, Tangoku A, Hamada K, Nakayama H. Comparison of gene expression profiles between hepatitis B virus- and hepatitis C virus-infected hepatocellular carcinoma by oligonucleotide microarray data on the basis of a supervised learning method. Cancer Res. 2002;62:3939-3944. [PubMed] [Cited in This Article: ] |
| 32. | Koike K, Tsutsumi T, Fujie H, Shintani Y, Kyoji M. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62 Suppl 1:29-37. [PubMed] [Cited in This Article: ] |
| 33. | Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56-S61. [PubMed] [Cited in This Article: ] |
| 34. | Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Li H, Zheng HW, Chen H, Xing ZZ, You H, Cong M, Jia JD. Hepatitis B virus particles preferably induce Kupffer cells to produce TGF-β1 over pro-inflammatory cytokines. Dig Liver Dis. 2012;44:328-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Xu C, Zhou W, Wang Y, Qiao L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014;345:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Jung SY, Kim YJ. C-terminal region of HBx is crucial for mitochondrial DNA damage. Cancer Lett. 2013;331:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | You X, Liu F, Zhang T, Li Y, Ye L, Zhang X. Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis. 2013;34:1644-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, Shan C, Kong G, Wang Y, Yang X. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Tang RX, Kong FY, Fan BF, Liu XM, You HJ, Zhang P, Zheng KY. HBx activates FasL and mediates HepG2 cell apoptosis through MLK3-MKK7-JNKs signal module. World J Gastroenterol. 2012;18:1485-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Geng X, Harry BL, Zhou Q, Skeen-Gaar RR, Ge X, Lee ES, Mitani S, Xue D. Hepatitis B virus X protein targets the Bcl-2 protein CED-9 to induce intracellular Ca2+ increase and cell death in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2012;109:18465-18470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Clippinger AJ, Bouchard MJ. Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J Virol. 2008;82:6798-6811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Sze KM, Chu GK, Lee JM, Ng IO. C-terminal truncated hepatitis B virus x protein is associated with metastasis and enhances invasiveness by C-Jun/matrix metalloproteinase protein 10 activation in hepatocellular carcinoma. Hepatology. 2013;57:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 619] [Cited by in F6Publishing: 660] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 45. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. [PubMed] [Cited in This Article: ] |
| 46. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554-559. [PubMed] [Cited in This Article: ] |
| 47. | Tsubota A, Arase Y, Ren F, Tanaka H, Ikeda K, Kumada H. Genotype may correlate with liver carcinogenesis and tumor characteristics in cirrhotic patients infected with hepatitis B virus subtype adw. J Med Virol. 2001;65:257-265. [PubMed] [Cited in This Article: ] |
| 48. | Muroyama R, Kato N, Yoshida H, Otsuka M, Moriyama M, Wang Y, Shao RX, Dharel N, Tanaka Y, Ohta M. Nucleotide change of codon 38 in the X gene of hepatitis B virus genotype C is associated with an increased risk of hepatocellular carcinoma. J Hepatol. 2006;45:805-812. [PubMed] [Cited in This Article: ] |
| 49. | Tatsukawa M, Takaki A, Shiraha H, Koike K, Iwasaki Y, Kobashi H, Fujioka S, Sakaguchi K, Yamamoto K. Hepatitis B virus core promoter mutations G1613A and C1653T are significantly associated with hepatocellular carcinoma in genotype C HBV-infected patients. BMC Cancer. 2011;11:458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006;97:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 51. | Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. J Hepatol. 2014;61:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 52. | Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH. The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002;21:525-535. [PubMed] [Cited in This Article: ] |
| 53. | Hsieh YH, Su IJ, Wang HC, Tsai JH, Huang YJ, Chang WW, Lai MD, Lei HY, Huang W. Hepatitis B virus pre-S2 mutant surface antigen induces degradation of cyclin-dependent kinase inhibitor p27Kip1 through c-Jun activation domain-binding protein 1. Mol Cancer Res. 2007;5:1063-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Nishimura M, Takaki A, Tamaki N, Maruyama T, Onishi H, Kobayashi S, Nouso K, Yasunaka T, Koike K, Hagihara H. Serum oxidative-anti-oxidative stress balance is dysregulated in patients with hepatitis C virus-related hepatocellular carcinoma. Hepatol Res. 2013;43:1078-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152-160. [PubMed] [Cited in This Article: ] |
| 57. | Dustin LB, Cashman SB, Laidlaw SM. Immune control and failure in HCV infection--tipping the balance. J Leukoc Biol. 2014;96:535-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 59. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 60. | Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, Beaugrand M, Sasportes M. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver. 1998;18:391-397. [PubMed] [Cited in This Article: ] |
| 62. | Kato N, Yoshida H, Ono-Nita SK, Kato J, Goto T, Otsuka M, Lan K, Matsushima K, Shiratori Y, Omata M. Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology. 2000;32:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196:641-653. [PubMed] [Cited in This Article: ] |
| 64. | Shen S, Niso-Santano M, Adjemian S, Takehara T, Malik SA, Minoux H, Souquere S, Mariño G, Lachkar S, Senovilla L. Cytoplasmic STAT3 represses autophagy by inhibiting PKR activity. Mol Cell. 2012;48:667-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 65. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 893] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 66. | Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365-4370. [PubMed] [Cited in This Article: ] |
| 67. | Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481-37488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 68. | Fujita N, Sugimoto R, Ma N, Tanaka H, Iwasa M, Kobayashi Y, Kawanishi S, Watanabe S, Kaito M, Takei Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15:498-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Tanaka H, Fujita N, Sugimoto R, Urawa N, Horiike S, Kobayashi Y, Iwasa M, Ma N, Kawanishi S, Watanabe S. Hepatic oxidative DNA damage is associated with increased risk for hepatocellular carcinoma in chronic hepatitis C. Br J Cancer. 2008;98:580-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Ng J, Wu J. Hepatitis B- and hepatitis C-related hepatocellular carcinomas in the United States: similarities and differences. Hepat Mon. 2012;12:e7635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Hino K, Nishina S, Hara Y. Iron metabolic disorder in chronic hepatitis C: mechanisms and relevance to hepatocarcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 4:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449-456. [PubMed] [Cited in This Article: ] |
| 74. | Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T. Normalization of elevated hepatic 8-hydroxy-2’-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697-8702. [PubMed] [Cited in This Article: ] |
| 75. | Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 77. | Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567-576. [PubMed] [Cited in This Article: ] |
| 79. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2233] [Cited by in F6Publishing: 2311] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 80. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1543] [Cited by in F6Publishing: 1629] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 81. | Bhala N, Jouness RI, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Curr Pharm Des. 2013;19:5169-5176. [PubMed] [Cited in This Article: ] |
| 82. | Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366-74.e1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 83. | Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 84. | Wu J, Du J, Liu L, Li Q, Rong W, Wang L, Wang Y, Zang M, Wu Z, Zhang Y. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Zhao J, Lawless MW. Stop feeding cancer: pro-inflammatory role of visceral adiposity in liver cancer. Cytokine. 2013;64:626-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, Masuzaki R, Goto T, Hamamura K, Kanai F. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58:839-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 87. | Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, Bondioni S, Beck-Peccoz P, Spada A. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005;90:2397-2402. [PubMed] [Cited in This Article: ] |
| 88. | Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 89. | Wanninger J, Neumeier M, Weigert J, Bauer S, Weiss TS, Schäffler A, Krempl C, Bleyl C, Aslanidis C, Schölmerich J. Adiponectin-stimulated CXCL8 release in primary human hepatocytes is regulated by ERK1/ERK2, p38 MAPK, NF-kappaB, and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G611-G618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Izadi V, Farabad E, Azadbakht L. Serum adiponectin level and different kinds of cancer: a review of recent evidence. ISRN Oncol. 2012;2012:982769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Aleksandrova K, Boeing H, Nöthlings U, Jenab M, Fedirko V, Kaaks R, Lukanova A, Trichopoulou A, Trichopoulos D, Boffetta P. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60:858-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 93. | Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 94. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [PubMed] [Cited in This Article: ] |
| 95. | Sharp PA. New insights into the role of iron in the development of nonalcoholic fatty liver disease. Hepatology. 2010;52:408-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Sorrentino P, D’Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 97. | Kastl L, Sauer SW, Ruppert T, Beissbarth T, Becker MS, Süss D, Krammer PH, Gülow K. TNF-α mediates mitochondrial uncoupling and enhances ROS-dependent cell migration via NF-κB activation in liver cells. FEBS Lett. 2014;588:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 98. | Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 99. | O’Brien J, Powell LW. Non-alcoholic fatty liver disease: is iron relevant? Hepatol Int. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Asare GA, Paterson AC, Kew MC, Khan S, Mossanda KS. Iron-free neoplastic nodules and hepatocellular carcinoma without cirrhosis in Wistar rats fed a diet high in iron. J Pathol. 2006;208:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Moyo VM, Makunike R, Gangaidzo IT, Gordeuk VR, McLaren CE, Khumalo H, Saungweme T, Rouault T, Kiire CF. African iron overload and hepatocellular carcinoma (HA-7-0-080). Eur J Haematol. 1998;60:28-34. [PubMed] [Cited in This Article: ] |
| 102. | Hino K, Hara Y, Nishina S. Mitochondrial reactive oxygen species as a mystery voice in hepatitis C. Hepatol Res. 2014;44:123-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Sumida Y, Yoshikawa T, Okanoue T. Role of hepatic iron in non-alcoholic steatohepatitis. Hepatol Res. 2009;39:213-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 105. | Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1111] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 106. | Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1847] [Cited by in F6Publishing: 2091] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 107. | Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 108. | Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, Qiu B, Li Y, Guo L, Wu M. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372-5380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 109. | Cai X, Hu X, Cai B, Wang Q, Li Y, Tan X, Hu H, Chen X, Huang J, Cheng J. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol Rep. 2013;30:2449-2457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84-H93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 111. | Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 112. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881-891; quiz 892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 113. | Harris K, Smith L. Safety and efficacy of metformin in patients with type 2 diabetes mellitus and chronic hepatitis C. Ann Pharmacother. 2013;47:1348-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in F6Publishing: 2196] [Article Influence: 156.9] [Reference Citation Analysis (1)] |
| 115. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 116. | Tajima K, Nakamura A, Shirakawa J, Togashi Y, Orime K, Sato K, Inoue H, Kaji M, Sakamoto E, Ito Y. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab. 2013;305:E987-E998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 117. | Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887-2900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 118. | Sid B, Glorieux C, Valenzuela M, Rommelaere G, Najimi M, Dejeans N, Renard P, Verrax J, Calderon PB. AICAR induces Nrf2 activation by an AMPK-independent mechanism in hepatocarcinoma cells. Biochem Pharmacol. 2014;91:168-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3:120144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 120. | Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 121. | Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3513] [Cited by in F6Publishing: 3878] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 122. | Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 123. | Wu CW, Chu ES, Lam CN, Cheng AS, Lee CW, Wong VW, Sung JJ, Yu J. PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther. 2010;17:790-798. [PubMed] [Cited in This Article: ] |
| 124. | Borbath I, Leclercq I, Moulin P, Sempoux C, Horsmans Y. The PPARgamma agonist pioglitazone inhibits early neoplastic occurrence in the rat liver. Eur J Cancer. 2007;43:1755-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1482] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 126. | Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q, Chang Y, Liu Q, Sun X, Wu M. The protective role of hydrogen-rich saline in experimental liver injury in mice. J Hepatol. 2011;54:471-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 127. | Kawai D, Takaki A, Nakatsuka A, Wada J, Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 128. | Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 129. | Kerner J, Bieber L. Isolation of a malonyl-CoA-sensitive CPT/beta-oxidation enzyme complex from heart mitochondria. Biochemistry. 1990;29:4326-4334. [PubMed] [Cited in This Article: ] |
| 130. | Chang B, Nishikawa M, Nishiguchi S, Inoue M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int J Cancer. 2005;113:719-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T, Koike K, Shimomura Y, Seki H, Matsushita H, Miyake Y, Ikeda F. L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS One. 2014;9:e100627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G, Galvano F. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis--a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |