Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.95
Peer-review started: October 24, 2017
First decision: December 1, 2017
Revised: December 7, 2017
Accepted: December 28, 2017
Article in press: December 28, 2017
Published online: January 27, 2018
To assess the usefulness of intra-arterial contrast-enhanced ultrasonography (IAUS) during transarterial chemoembolization (TACE) with drug-eluting beads (DEB) for hepatocellular carcinoma (HCC).
Thirty two patients with 39 HCC underwent DEB-TACE guided with IAUS, and examined by contrast-enhanced ultrasonography (CEUS) or dynamic CT after DEB-TACE were enrolled in this study. CEUS findings before DEB-TACE and IAUS findings were compared. Treatments judged to be complete and incomplete for lesions were appropriate and insufficient, respectively. Findings on CEUS and/or dynamic CT performed 1, 3 and 6 mo after DEB-TACE were evaluated using mRECIST (CR/PR/SD/PD).
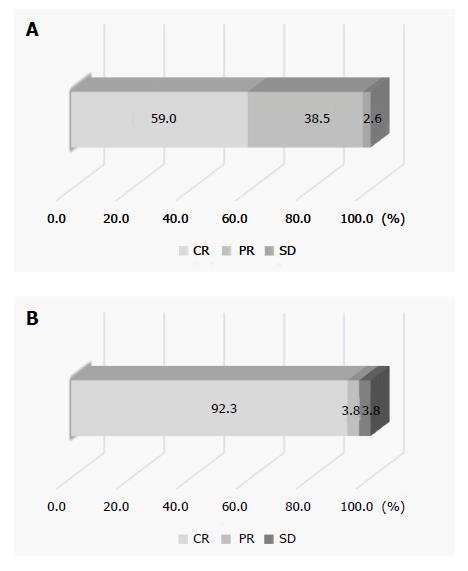
The treatments were complete and incomplete in 26 and 13 lesions, respectively. On imaging evaluation using CEUS and/or dynamic CT one month after treatment, 25 and 1 lesions were judged to be CR and PR, respectively, and at 6 mo after treatment, the results were CR, PR, SD and PD for 24, 1, 0 and 1 of these lesions, respectively, in the 26 completely treated lesions. Of the 13 lesions in which treatment was incomplete, the results on imaging at one month after treatment were CR, PR, SD and PD for 0, 6, 4 and 3 lesions, respectively. The overall CR rate at 6 mo after treatment was 61.5% (24/39).
A combination of DEB-TACE with IAUS can improve the therapeutic effects in patients with HCC.
Core tip: To assess the usefulness of intra-arterial contrast-enhanced ultrasonography (IAUS) during transarterial chemoembolization (TACE) with drug-eluting beads (DEB) for 39 hepatocellular carcinoma (HCC). Complete and incomplete treatments were 26 and 13, respectively. One month after treatment, 25 and 1 lesions were judged to be CR and PR, respectively, and at 6 mo after treatment, the results were CR, PR, SD and PD for 24, 1, 0 and 1 of these lesions, respectively, in the 26 completely treated lesions. The overall CR rate at 6 mo after treatment was 61.5% (24/39). A combination of DEB-TACE with IAUS can improve the therapeutic effects in HCC patients.
- Citation: Shiozawa K, Watanabe M, Ikehara T, Yamamoto S, Matsui T, Saigusa Y, Igarashi Y, Maetani I. Efficacy of intra-arterial contrast-enhanced ultrasonography during transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. World J Hepatol 2018; 10(1): 95-104
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/95.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.95
Transarterial chemoembolization (TACE) improves survival in patients with intermediate-stage (Barcelona Clinic Liver Cancer[1]; BCLC classification B) hepatocellular carcinoma (HCC)[2]. Drug-eluting beads (DEB) are polyvinyl alcohol-based microspheres that can be loaded with anthracycline drugs, such as doxorubicin[3]. DEB-TACE is now used in catheter-based locoregional therapy that takes advantage of the arterial supply to HCC and spares the surrounding hepatic parenchyma, which receives most of its blood supply from the portal vein[4,5]. When injected through a catheter or microcatheter at the tumor site, DEB act as embolic material, causing tumor ischemia, and also release drugs in a sustained and controlled manner[6].
TACE is commonly guided by digital subtraction angiography (DSA). In DEB-TACE, it is particularly important to prevent inflow of DEB into normal hepatic parenchyma and to administer the beads into the tumor artery because of their effect as a permanent embolic material[7]. However, some HCCs are difficult to visualize on DSA for DEB-TACE due to the complex blood supply.
Contrast-enhanced ultrasonography (CEUS) enables tumor visualization without use of ionizing radiation or the risk of nephrotoxicity associated with contrast-enhanced computed tomography (CT) and catheter-based studies[8,9]. In addition, CEUS is useful for evaluation of the hemodynamics of hepatic tumors and surrounding hepatic parenchyma in real time. We have evaluated the efficacy of HCC treatment with sorafenib (Nexavar; Bayer Healthcare, Leverkusen, Germany) and CyberKnife® (Accuray Incorporated, Sunnyvale, CA, United States) by CEUS using Sonazoid® (Daiichi Sankyo, Tokyo, Japan)[10,11].
Transcatheter (intra-arterial) CEUS (IAUS) has recently been used in DEB-TACE for HCC and metastatic hepatic tumors, and its safety and efficacy in identifying the feeding artery have been evaluated[12-16]. However, the therapeutic effect using IAUS as support for DEB-TACE in HCC has not been examined. In this study, we evaluated the usefulness of IAUS using Sonazoid® in DEB-TACE for HCC.
Of patients who received TACE for HCC at our hospital between June 2015 and September 2016, 32 patients (39 lesions) with lesions visualizable by ultrasonography (US) who gave consent to DEB-TACE and IAUS and could be examined by CEUS or dynamic CT at 1, 3 and 6 mo after DEB-TACE and every 3-4 mo thereafter were included in the study. The patients were 28 males and 4 females; the median age was 73 years old; the underlying liver disease was hepatitis B in 4 patients, hepatitis C in 12, alcoholic liver disease in 14, and non-alcoholic steatohepatitis (NASH) in 2; and the median tumor diameter was 21 mm (Table 1). All patients were diagnosed with HCC using gray-scale US, dynamic CT and Gd-EOB-DTPA-enhanced magnetic resonance imaging, based on the new guidelines of the American Association for the Study of Liver Diseases[17]. Serum α-fetoprotein (AFP), AFP-L3 fraction, and des-γ-carboxyprothrombin (DCP) levels were referred to for diagnosis, as needed.
| Characteristics | All (n = 32) |
| Age (yr) (median) | 72 (range 44-89) |
| Gender: Male/female | 28/4 |
| Etiology | |
| Alcohol/HBV/HCV/NASH | 14/4/12/2 |
| Child-Pugh classification A/B | 23/9 |
| Previous treatment (y/n) | 18/14 |
| Tumor number | 39 |
| Tumor size (mm) (median) | 21 (range 8-50) |
| Tumor location | |
| Peripheral/central | 26/13 |
| DCBead (100-300 μm, mL) (median) | 0.6 (range 0.2-1.5) |
| Vascular lake (y/n) | 7/32 (22%) |
We chose all patients based on the Evidence-based Clinical Practice Guidelines for HCC developed by the Japan Society of Hepatology[18]. All 32 patients had hepatic cirrhosis with Child-Pugh classification A or B and an Eastern Cooperative Oncology Group performance status < 2[19]. The all lesions were single HCC ≤ 50 mm in diameter or up to 3 HCCs ≤ 30 mm in diameter. Tumors were selected that were difficult to treat with radiofrequency ablation, such as those with the presence of ascites, close to vessels, or on the liver surface in patients who did not want surgical resection. Exclusion criteria were non-visualizable lesions on US in the supine position, advanced-stage HCC in the BCLC classification, serum total bilirubin > 3 mg/dL, history of heart or renal impairment, iodine allergy, and egg allergy.
The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and local laws and regulations. The study was performed after approval by the Ethics Committee of Toho University Medical Center Omori Hospital. All patients provided written informed consent for DEB-TACE and IAUS.
Gray-scale US and CEUS using Sonazoid® were performed within one month before DEB-TACE in all patients. All CEUS was performed by two sonographers with 25 and 18 years of experience, respectively, using an Aplio XG (Toshiba Medical Systems, Tokyo, Japan) with a convex probe (PVT-375BT, 3.75-MHz center frequency). The mechanical index (MI) for the acoustic output was set to 0.2 and the dynamic range was set to 60-65 dB. In patients in whom lesions were detected by gray-scale US, the single focus point was set at the lower margin of the lesion. An intravenous bolus injection of Sonazoid® (0.5 mL) was administered via a left cubital venous line, followed by flushing with 10 mL of normal saline. The dynamics of enhancement of the lesion were observed in the vascular phase (arterial phase, 0-40 s; portal venous phase, 40-120 s; late phase, > 120 s), and in the postvascular phase 10 min after injection. Subsequently, the feeding blood vessel was identified by re-injection of Sonazoid®[20] in as many lesions as possible.
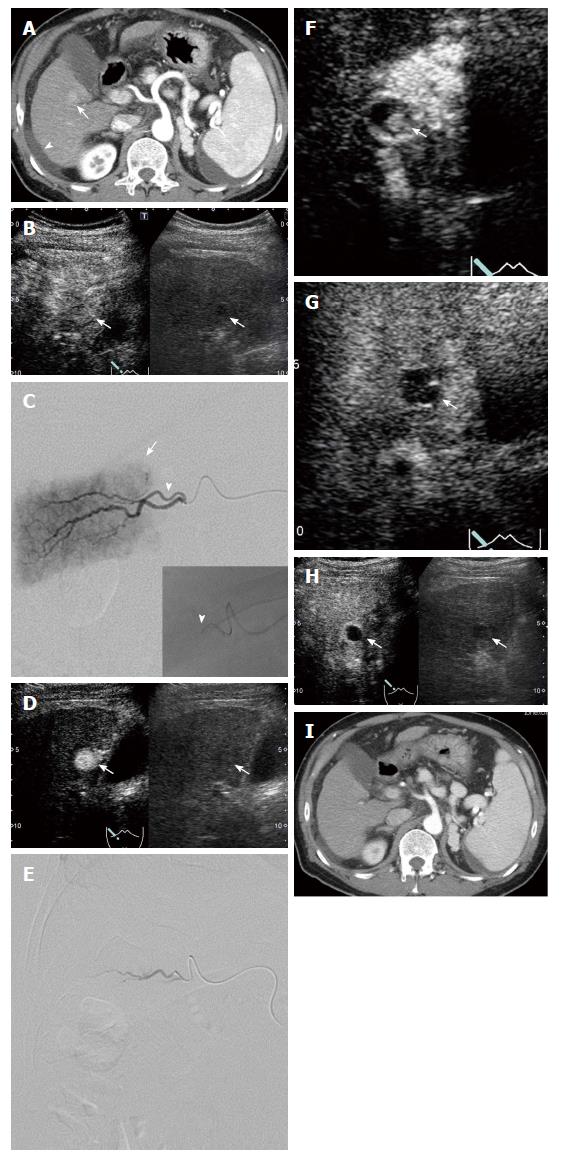
Dynamic CT was performed within one month before DEB-TACE in all patients, a 3D-CT angiogram was prepared, and abdominal angiography was performed with reference to the CT findings. DEB-TACE was performed by an expert interventional radiologist and hepatologist with 31 years of experience and a hepatologist with 19 years of experience. The right groin and upper abdomen were cleansed with iodine and the patient was draped under sterile cloths with exposure of the right groin and upper abdomen. Through a right femoral artery access and after placement of a 3 Fr shepherd hook catheter (Terumo, Tokyo, Japan), the celiac trunk was examined through the arterial and venous phases to define the hepatic artery anatomy and to assess portal vein patency. Then, the hepatic artery was selectively catheterized (segment/subsegment) to study the arterial supply of the target lesions. A coaxial microcatheter [Haruka® (JMS Co., Ltd. Tokyo, Japan) or Wonder III® (UTM Co., Ltd. Aichi, Japan)] was advanced in every feeding artery to allow embolization of the lesion with drug-eluting microspheres [DC beads® (Eisai Co., Ltd. Tokyo, Japan), 100-300 μm in diameter, 1 vial loaded with 50 mg of epirubicin® (Nippon Kayaku Co., Ltd. Tokyo, Japan)] until contrast medium disappeared from the blood vessel within 5-6 heart beats. At this time, DSA and IAUS were performed and tumor enhancement was evaluated. If residual tumor enhancement was observed in any images, DEB-TACE was repeated and disappearance of as much tumor enhancement as possible was confirmed using IAUS, after which the treatment was considered complete.
The lesion location was identified with gray-scale US, immediately prior to an echo-contrast study. IAUS was performed by administration of Sonazoid® through the microcatheter and imaging of the area of the target lesion with a dedicated, contrast-specific technique. IAUS was performed by the same expert interventional radiologist and hepatologist with 31 years of experience using an Aplio 400 (Toshiba Medical Systems, Tokyo, Japan) with a convex probe (PVT-375BT, 3.75-MHz center frequency). The MI for the acoustic output was 0.2-0.3 and the dynamic range was 60-65 dB. A single focal point was set at the deep site of the lesion. Sonazoid® (0.5 mL diluted with 19.5 mL of distilled water) was used as the contrast medium in IAUS. The diluted Sonazoid® was introduced into the feeding artery by intermittent injection of 0.3-0.5 mL through a microcatheter placed in the artery and flushing with saline at the same flow rate.
The following items were performed in DEB-TACE using IAUS: (1) Before DEB-TACE, the feeding arteries were identified and tumor enhancement was confirmed by IAUS. When a hypoenhanced area was partially observed in the tumor on IAUS, another feeding artery was identified when possible; (2) during DEB-TACE, DSA and IAUS were performed when contrast medium disappeared from the blood vessel within 5-6 heartbeats on fluoroscopy, and the presence or absence of tumor enhancement was evaluated. If tumor enhancement disappeared on DSA, but was detected by IAUS, DEB-TACE was repeated until the tumor enhancement disappeared on IAUS. Treatment was considered complete after disappearance of as much of the contrast image as possible; and (3) video images of all IAUS were stored on the hard disk of the scanner and transferred to a high-performance personal computer.
The items below were investigated after completion of all procedures: (1) In all lesions, CEUS findings before treatment and stored IAUS video images were compared, and disappearance of the tumor enhancement and untreated regions were evaluated. Treatments to be complete and incomplete for lesions were appropriate and insufficient, respectively; and (2) findings on CEUS and/or dynamic CT performed 1, 3, 6 and 12 mo after DEB-TACE and every 3 mo thereafter were evaluated using modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria [Complete response (CR), Partial response (PR), Stable disease (SD), Progressive disease (PD)][21]. Normally, mRECIST is used to evaluate treatment using dynamic CT, but we applied this method to CEUS findings. In cases judged as SD or PD on imaging after treatment, TACE (DEB-TACE or conventional TACE) was repeated within one month.
Following each procedure, patients were hospitalized for about 6 d and reevaluated with physical examinations and blood tests on days 1, 5 and 30. Safety was monitored by recording postprocedure clinical complications and liver and renal function. The severity of complications was retrospectively graded according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0 (v4.03; June 14, 2010)[22]. An adverse event was considered to be treatment-related if it occurred within 30 d after DEB-TACE.
The median duration of observation in all patients was 363 d. Since an enhanced area was noted on IAUS when the contrast medium was retained in the feeding vessel on fluoroscopy in all patients, i.e., when contrast medium disappeared from the blood vessel within 5-6 heart beats, additional DEB-TACE was applied. A comparison of CEUS findings before treatment and stored IAUS video images showed that the treatment was complete and incomplete in 26 and 13 lesions, respectively (Figure 1). Of 32 lesions judged to have received complete treatment at the end of treatment, 6 were judged to have received incomplete treatment based on evaluation of stored video images; therefore, combining these two evaluations, complete treatment was achieved in 26 lesions (Figure 2). On imaging evaluation using CEUS and/or dynamic CT one month after treatment, 25 and 1 lesions were judged to be CR and PR, respectively, in the 26 completely treated lesions. At 6 mo after treatment, the results were CR, PR, SD and PD for 24, 1, 0 and 1 of these lesions, respectively, reflecting changes to PR and PD in one lesion each of the 25 lesions judged as CR one month after treatment, and a change to CR in the lesion judged as PR at one month. Of the 24 CR lesions at 6 mo after treatment, imaging evaluation was possible in 21 lesions at 12 mo, and 18 and 3 were judged to be CR and PD, respectively. Of the 13 lesions in which treatment was incomplete (Figure 3), the results on imaging at one month after treatment were CR, PR, SD and PD for 0, 6, 4 and 3 lesions, respectively. No lesion achieved CR at 6 mo after treatment, and additional TACE was applied to all 13 lesions. The overall CR rate at 6 mo after treatment was 61.5% (24/39) (Figure 4).
There was no DEB-TACE-related mortality and no major adverse events were recorded. Within 7 d after DEB-TACE, 3/39 lesions (7.7%) showed a mild transient increase (an increase of more than double compared to before treatment) in liver enzymes, and 19/39 (48.7%) had fever (≥ 37.5 °C) and 3/39 (7.7%) had abdominal pain, as symptoms of post-embolization syndrome that were managed with conservative therapies. The median hospitalization after each course of treatment was 6 d. All patients were asymptomatic upon discharge from hospital. There were no adverse events that were directly due to IAUS (Table 2).
IAUS has previously been shown to be safe and effective in facilitation of TACE to treat malignant liver tumors such as HCC[12-16,23]. Use of IAUS during TACE allows accurate identification of feeding blood vessels, which is difficult with DSA alone, and the frequency of DSA during treatment can be decreased, which reduces the risk of embolization of non-target regions[14]. In addition, IAUS can identify tumors that are not stained through an artery and are fed by a branch of the portal vein, which may provide information comparable to that from CT angiography (CTA)[16].
In DEB-TACE, DEB must be administered selectively because of its effect as a permanent embolic agent, and thus identification of feeding arteries is very important[7]. In addition, DEB-TACE can be simply evaluated during treatment because of the high visibility on CEUS due to the property of the microspheres, unlike conventional TACE using Lipiodol® (Laboratoire Guerbet, Aulnay-Sous-Bois, France). Thus, we evaluated the therapeutic effect of DEB-TACE in 39 lesions in 32 patients with HCC, using IAUS with Sonazoid® as treatment support. The reported CR rate of HCC, including advanced HCC, to DEB-TACE is 12-27%[24-27], but CR on imaging at 6 mo after treatment in our study was achieved for 24 of 26 completely treated lesions (92.3%), and the overall CR rate at 6 mo was 61.5% (24/39).
When two blood vessels are present in an anteroposterior arrangement at the same level, the positional relationship is difficult to identify using two-dimensional imaging with DSA. Moreover, small tumors and tumors with poor blood flow are often not visualized by DSA, but can be visualized by IAUS[12,14,23]. IAUS is also sensitive to hemodynamics and was useful for identification of lesions in our study. Therefore, use of IAUS may have enabled effective treatment.
In particular, in all cases in which residual tumor enhancement was judged to have disappeared on DSA, IAUS showed a residual enhancement in the tumor, and DEB-TACE was repeated until this image disappeared on IAUS. Disappearance of contrast medium within 5-6 heartbeats in fluoroscopy is generally used to indicate completion of DEB-TACE, but this criterion may be insufficient. Continuing treatment until disappearance of the contrast enhancement in the tumor on IAUS is important, and this may have been one reason for the high CR rate in this study.
Manini et al[28] showed the efficacy of DEB-TACE for treatment of BCLC A stage HCC patients prior to liver transplantation based on the Milan criteria[29]. The median diameter of the target tumors was 24 mm, similar to that in our study, and achieving CR with initial DEB-TACE was found to be an important prognostic factor. Generally, TACE is used for BCLC B stage (intermediate stage) HCC, and DEB-TACE is frequently indicated for very large HCC and multiple HCCs, as an option to improve the outcome[2]. Our results suggest that reliable DEB-TACE may also increase the CR rate for small tumors, and thus may also improve the therapeutic effect for these tumors.
However, although the effect of intratumoral drug release in DEB-TACE has been reported[6], only one lesion changed from PR at one month after treatment to CR at 6 mo on imaging evaluation, suggesting the importance of ensuring CR just after treatment, i.e., treatment should be completed until enhancement in the tumor disappears. Two lesions changed from CR at one month after treatment to PR and PD at 6 mo after treatment, respectively, in 25 lesions judged as CR one month after treatment. For these lesions, peritumoral invasion could not be ruled out before treatment.
DEB-TACE was repeated until the contrast enhancement in the tumor disappeared on IAUS, but there were no cases with severe postembolization syndrome, such as biloma and liver abscess, and no other adverse events that extended hospitalization. Intravenous administration is generally used for contrast agent for US, but there was no adverse event directly due to IAUS, which suggests that CEUS can be safely used with transarterial administration of the contrast agent Sonazoid®.
Information similar to that provided by IAUS may be acquired using CTA and cone-beam CT. Oblique acquisition may also be performed to define the positional relationship of the tumor on DSA, but the procedure is complicated and time-consuming, and increases the exposure dose and contrast medium volume, all of which are disadvantageous[15,30]. Also, renal function is decreased in many patients with hepatic cirrhosis, and worsens with iodine contrast medium. In contrast, IAUS may reduce the exposure dose and iodine contrast medium volume, which is likely to shorten the treatment time. Furthermore, Sonazoid® can be repeatedly administered[31], which is advantageous when making a judgment using a first IAUS procedure is difficult.
The limitations of this study include the small number of cases and short observation period. Similarly to normal gray-scale US, IAUS may depend on the experience and skill of operating physicians. Moreover, IAUS is difficult to apply for lesions that cannot be visualized in the supine position, for cases with multiple lesions, in obese patients, and for deep lesions. The treatment was incomplete in 13 lesions, even though IAUS was frequently performed during DEB-TACE to identify feeding arteries. Since this study was a pilot study performed early after introduction of IAUS, the operator had limited experience, and the operator of DEB-TACE and rater of IAUS was the same physician, which made the evaluation complex. Thus, the presence of small regions fed by micro-blood vessels in the tumor and other tumor blood vessels may not have been recognized on IAUS, and thus completion of DEB-TACE may not have been judged appropriately (Figure 3). We are planning to perform a long-term study of the effect of IAUS on the therapeutic efficacy of DEB-TACE in a larger number of patients with HCC. Further, in the future, we want to run this future study at multiple centers, and compare conventional TACE and DEB-TACE using IAUS to make more solid conclusions with prospective design. In conclusion, a combination of DEB-TACE with IAUS can improve the therapeutic effects in patients with HCC.
In transarterial chemoembolization with drug-eluting beads (DEB) (DEB-TACE) for hepatocellular carcinoma (HCC), it is particularly important to prevent inflow of DEB into normal hepatic parenchyma and to administer the beads into the tumor artery because of their effect as a permanent embolic material. However, some HCCs are difficult to visualize on digital subtraction angiography for TACE due to the complex blood supply. On the other hand, contrast-enhanced ultrasonography (CEUS) is useful for evaluation of the hemodynamics of hepatic tumors and surrounding hepatic parenchyma in real time. Transcatheter (intra-arterial) CEUS (IAUS) has recently been used in DEB-TACE for HCC, and its safety and efficacy in identifying the feeding artery have been evaluated.
Generally, the complete response (CR) rate of DEB-TACE for small HCC is reported to be low. It is thought that DEB-TACE is mainly performed for giant and multiple HCCs in many facilities. The authors wanted to know the true therapeutic effect of DEB-TACE for small HCCs less than 50 mm by considering whether feeding artery can be selected reliably and whether the timing of completion of treatment is appropriate.
IAUS has recently been used in DEB-TACE for HCCs and metastatic hepatic tumors, and its safety and efficacy in identifying the feeding artery have been evaluated. However, the therapeutic effect using IAUS as support for DEB-TACE in HCC has not been examined. In this study, the authors evaluated the usefulness of IAUS using Sonazoid® in DEB-TACE for HCC.
The authors evaluate the identification of feeding arteries and the appropriate timing of completion of DEB-TACE for HCC by IAUS using Sonazoid®.
DEB-TACE with IAUS can improve the therapeutic effects in patients with HCC. This study includes the small number of cases and short observation period. A same study with much larger number of patients and much longer observation period are awaited.
IAUS is very useful to obtain CR in HCC treatment with DEB-TACE. IAUS is very useful to obtain CR in HCC treatment with DEB-TACE. In all cases in which residual tumor enhancement was judged to have disappeared on Digital subtraction angiography (DSA), IAUS showed a residual enhancement in the tumor. Disappearance of contrast medium within 5-6 heartbeats in fluoroscopy is generally used to indicate completion of DEB-TACE, but this criterion may be insufficient. The appropriate treatment using IAUS is possible to obtain CR in DEB-TACE for relatively small HCC. There is a possibility of obtaining CR by appropriate treatment in DEB-TACE for HCC. The appropriate treatment using IAUS is possible to obtain CR in DEB-TACE for HCC. The authors treated HCCs with IAUS using Sonazoid®. In DSA, disappearance of contrast medium within 5-6 heartbeats in fluoroscopy is generally used to indicate completion of DEB-TACE, but this criterion may be insufficient. The appropriate treatment using IAUS is possible to obtain CR in DEB-TACE for HCC. The therapeutic effect of DEB-TACE for HCC may improve.
In DSA, disappearance of contrast medium within 5-6 heartbeats in fluoroscopy is generally used to indicate completion of DEB-TACE, but this criterion may be insufficient. IAUS is very useful for obtaining CR in DEB- TACE for HCC. The authors are planning to perform a long-term study of the effect of IAUS on the therapeutic efficacy of DEB-TACE in a larger number of patients with HCC. The authors prospectively compare therapeutic efficacy of DEB-TACE with/without IAUS.
The authors wish to thank Takahide Kudo, Kenichi Maruyama and the staff of Department of Clinical Functional Physiology, Toho University Medical Center, Omori Hospital.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kohla MAS, Preda CM, Sirin G, Thomopoulos KC S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [PubMed] [Cited in This Article: ] |
| 2. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, Lloyd AW. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691-1699. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Cazejust J, Bessoud B, Colignon N, Garcia-Alba C, Planché O, Menu Y. Hepatocellular carcinoma vascularization: from the most common to the lesser known arteries. Diagn Interv Imaging. 2014;95:27-36. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563-2567. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980-985. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Kakeda S, Korogi Y, Ohnari N, Moriya J, Oda N, Nishino K, Miyamoto W. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J Vasc Interv Radiol. 2007;18:1508-1516. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: a pilot study. World J Gastroenterol. 2012;18:5753-5758. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Shiozawa K, Watanabe M, Ikehara T, Kobayashi K, Ochi Y, Suzuki Y, Fuchinoue K, Yoneda M, Kenmochi T, Okubo Y. Evaluation of contrast-enhanced ultrasonography for hepatocellular carcinoma prior to and following stereotactic body radiation therapy using the CyberKnife® system: A preliminary report. Oncol Lett. 2016;11:208-212. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Schacherer D, Girlich C, Zorger N, Wiest R, Schoelmerich J, Feuerbach S, Jung EM. Sono-hepatic-arteriography (Sono-HA) in the assessment of hepatocellular carcinoma in patients undergoing transcatheter arterial chemoembolization (TACE). Ultraschall Med. 2010;31:270-275. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Uller W, Wiggermann P, Gössmann H, Klebl F, Salzberger B, Stroszczynski C, Jung EM. Evaluation of the microcirculation of hepatocellular carcinomas using contrast-enhanced ultrasound with intraarterial and intravenous contrast application during transarterial chemoembolization with drug-eluting beads (DEB-TACE): preliminary data. Clin Hemorheol Microcirc. 2011;49:55-66. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Moschouris H, Malagari K, Kalokairinou M, Stamatiou K, Marinis A, Papadaki MG. Contrast-enhanced ultrasonography with intraarterial administration of SonoVue for guidance of transarterial chemoembolization: an initial experience. Med Ultrason. 2011;13:296-301. [PubMed] [Cited in This Article: ] |
| 15. | Burgmans MC, van Erkel AR, Too CW, Coenraad M, Lo RH, Tan BS. Pilot study evaluating catheter-directed contrast-enhanced ultrasound compared to catheter-directed computed tomography arteriography as adjuncts to digital subtraction angiography to guide transarterial chemoembolization. Clin Radiol. 2014;69:1056-1061. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Lekht I, Nayyar M, Luu B, Guichet PL, Ho J, Ter-Oganesyan R, Katz M, Gulati M. Intra-arterial contrast-enhanced ultrasound (IA CEUS) for localization of hepatocellular carcinoma (HCC) supply during transarterial chemoembolization (TACE): a case series. Abdom Radiol (NY). 2017;42:1400-1407. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45:123-127. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] [Cited in This Article: ] |
| 20. | Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:40-45. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Zhong X, Lim EA, Hershman DL, Moinpour CM, Unger J, Lee SM. Identifying Severe Adverse Event Clusters Using the National Cancer Institute’s Common Terminology Criteria for Adverse Events. J Oncol Pract. 2016;12:e270-e280, 245-246. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Zorger N, Jung EM, Schreyer AG, Heiss P, Mueller-Wille R, Wiest R, Feuerbach S, Rennert J. Ultrasound-arterioportography (US-AP): A new technical approach to perform detection of liver lesions. Clin Hemorheol Microcirc. 2010;46:117-126. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100-1108. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Manini MA, Sangiovanni A, Martinetti L, Viganò D, La Mura V, Aghemo A, Iavarone M, Crespi S, Nicolini A, Colombo M. Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transpl. 2015;21:1259-1269. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Gunsar F. Liver Transplantation for Hepatocellular Carcinoma Beyond the Milan Criteria. Exp Clin Transplant. 2017;15:59-64. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Kakeda S, Korogi Y, Hatakeyama Y, Ohnari N, Oda N, Nishino K, Miyamoto W. The usefulness of three-dimensional angiography with a flat panel detector of direct conversion type in a transcatheter arterial chemoembolization procedure for hepatocellular carcinoma: initial experience. Cardiovasc Intervent Radiol. 2008;31:281-288. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol. 2007;42:643-651. [PubMed] [DOI] [Cited in This Article: ] |