Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1196
Peer-review started: February 6, 2021
First decision: May 8, 2021
Revised: June 1, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: September 15, 2021
Neoadjuvant chemoradiotherapy (Neo-CRT) is the current standard strategy for treating locally advanced rectal cancer. However, it delays the administration of optimal chemotherapy and increases toxicity.
To compare the feasibility and efficacy of neoadjuvant chemotherapy (Neo-CT) and Neo-CRT for patients with locally advanced rectal cancer.
The Cochrane, EMBASE, and PubMed databases were searched for relevant articles using MESH terms and free words. The hazard ratio of overall survival and the risk ratio (RR) for the pathological complete response, the sphincter preservation rate, and treatment-related adverse events were analyzed.
A total of 19 studies of 60870 patients were included in the meta-analysis. There was no significant difference in overall survival [hazard ratio = 1.09, 95% confidence interval (CI) = 0.93–1.24; P = 0.19] or the pathological complete response (RR = 0.79, 95%CI = 0.61–1.03; P = 0.086) between the Neo-CT and Neo-CRT groups. As compared to the Neo-CRT group, the incidences of anastomotic fistula (RR = 0.49, 95%CI = 0.35–0.68; P = 0.000) and temporary colostomy (RR = 0.69, 95%CI = 0.58–0.83; P = 0.000) were significantly lower in the Neo-CT group, with a simultaneous increase in the sphincter preservation rate (RR = 1.07, 95%CI = 1.01–1.13; P = 0.029). However, there was no significant difference in the tumor downstaging rate, overall complications, and urinary complications.
Neo-CT administration can lower the incidences of anastomotic fistula and temporary colostomy and increase the sphincter preservation rate as to compared to Neo-CRT and could provide an alternative to chemoradiotherapy for locally advanced rectal cancer.
Core Tip: Neoadjuvant chemoradiotherapy acts as standard treatment in locally advanced rectal cancer (LARC). However, it delays the administration of optimal chemotherapy and increases toxicity. We designed the meta-analysis to compare the feasibility and efficacy of neoadjuvant chemotherapy vs neoadjuvant chemoradiotherapy for the treatment of LARC. The present study showed that neoadjuvant chemotherapy was effective for the treatment of LARC, especially to lower the incidences of anastomotic fistula and temporary colostomy and increase the sphincter preservation rate as compared to neoadjuvant chemoradiotherapy. This could have the potential to provide an alternative to chemoradiotherapy for LARC.
- Citation: Wu P, Xu HM, Zhu Z. Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer: A meta-analysis. World J Gastrointest Oncol 2021; 13(9): 1196-1209
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/1196.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.1196
Preoperative administration of fluorouracil-based chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and postoperative systemic therapy has been the standard strategy for treatment of locally advanced rectal cancer (LARC) since a 2004 randomized controlled study reported a lower local recurrence rate as compared to postoperative chemoradiation[1].
In subsequent clinical trials, neoadjuvant CRT (Neo-CRT) achieved significantly lower recurrence rates, greater accuracy of degrading staging, and higher R0 resection rates. However, with increasing application, the deficiencies of such regimens have started to arouse attention, including the long treatment cycle, including 25 d of concurrent CRT, 6–8 wk of recovery followed by TME, and 6 mo of perioperative treatment as well as the relatively high incidence (9%–20%) of anastomotic fistula formation in lower rectal cancer (RC)[2]. In addition, a second surgery is required after 3 mo to address the stoma. Long-term toxicity and the incidence of side effects, such as rectovaginal fistula, vaginal wall perforation, intestinal obstruction, perineal incision healing delay, peritoneal fibrosis, urethral irritation, control dysfunction, and sexual dysfunction, increase over time. Further, most clinical trials found that Neo-CRT did not improve overall survival (OS) due to higher distant metastatic rates. Finally, the presence of peritoneal fibrosis may increase the difficulty of surgery.
Therefore, the clinical utility of Neo-CRT regimens should be re-evaluated to make the best of their advantages, while avoiding their various disadvantages. Also, it is unclear whether it safe to selectively omit chemoradiation for RC patients with clinical evidence of a response to neoadjuvant chemotherapy (Neo-CT). For these reasons, many clinical trials have been carried out to explore the optimization of neoadjuvant therapy without radiation[3-6]. The FOWARC study (mFOLFOX6 with or without radiation in neoadjuvant treatment of LARC) claimed that perioperative mFOLFOX6 alone followed by TME, led to a similar downstaging rate as CRT but with less toxicity, fewer postoperative complications, and a lower pathological complete response (pCR) rate than CRT. Preoperative FOLFOX/bevacizumab is reportedly a safe and effective treatment option for patients with LARC, as this regimen achieved a better clinical response, pCR rate, and local recurrence rate than consistent use of CRT.
Although Neo-CT alone has shown promising results, many studies failed to compare Neo-CT with Neo-CRT, and the data tended to be limited or were collected from relatively small studies. In the present study, a meta-analysis of related clinical studies was performed to evaluate the pCR rate, sphincter preservation rate, incidences of anastomotic fistula and temporary colostomy, tumor downstaging rate, overall complications, urinary complications, and OS of RC patients receiving Neo-CT with or without radiotherapy. Ultimately, the aim of this meta-analysis was to provide a summary and reference for future research and treatment.
The meta-analysis was conducted in accordance with the preferred Reporting Items for Systematic Review and Meta-analysis guidelines. The Cochrane, EMBASE, and PubMed databases were systematically searched for relevant articles published from 1964 to 2020 using the following search terms “rectal neoplasm,” “chemotherapy,” and “radiotherapy.” Wu P and Zhu Z conducted the searches independently.
The diagnoses of all patients were pathologically confirmed as primary RC with or without metastasis. For all patients, if Neo-CT was deemed unnecessary, then neoadjuvant radiotherapy was selected. All studies included related data of endpoints, such as OS, pCR, sphincter preservation rate, or tumor downstaging rate. If related data could not be extracted, the study was excluded from analysis. The quality of all studies was deemed as medium or high. The study design (e.g., randomized control trial or cohort study) was not considered in the selection process. The sample size of all studies was greater than 20 patients.
The quality of the randomized control trials, including randomization, concealment of allocation, double-blinding, withdrawals, and dropouts, was evaluated using the Modified Jadad Score. Meanwhile, the Newcastle Ottawa Scale was applied to assess the quality of the cohort studies based on three items: Selectivity, comparability, and outcome. The primary endpoint was OS, the secondary endpoints were the pCR rate, sphincter preservation rate, incidences of anastomotic fistula and temporary colostomy, tumor downstaging rate, overall complications, and urinary complications.
The hazard ratio (HR) was used to estimate OS, while the risk ratio (RR) was applied to evaluate pCR, sphincter preservation rate, incidences of anastomotic fistula and temporary colostomy, tumor downstaging rate, overall complications, and urinary complications. The HRs were extracted directly from the studies or from a Kaplan–Meier curve generated with the Engauge Digitizer (http://digitizer.sourceforge.net/). Moreover, a credibility interval was calculated based on the HR and probability (P) value, as described by Tierney et al[7], and an I statistic (I2) > 50% and P < 0.05 were considered to indicate significant heterogeneity. In this case, a random effects model was used for analysis. Sensitivity analysis was conducted to explore the source of heterogeneity in all studies. Subgroups were created to lower heterogeneity and identify factors influencing the final results. Furthermore, possible publication bias was assessed using the Egger’s and Begg’s tests, where P < 0.1 was regarded as confirmation of significant publication bias.
Images were processed with the Engauge Digitizer and Adobe Photoshop CC 2018 (Adobe Inc., San Jose, CA, United States). Furthermore, all data analyses were conducted using STATA 14.0 software (Stata LLC, College Station, TX, United States). A P value < 0.05 was considered statistically significant.
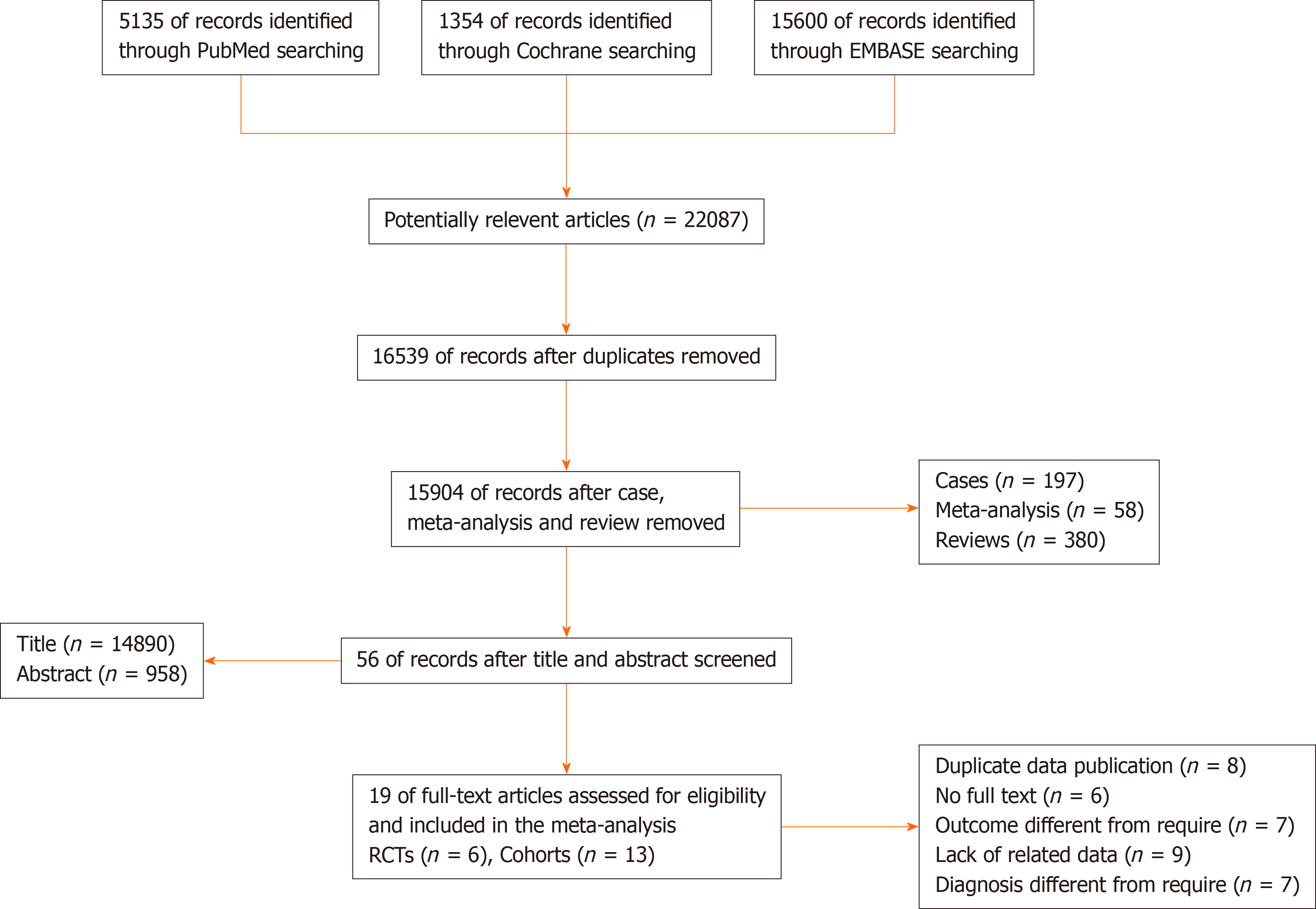
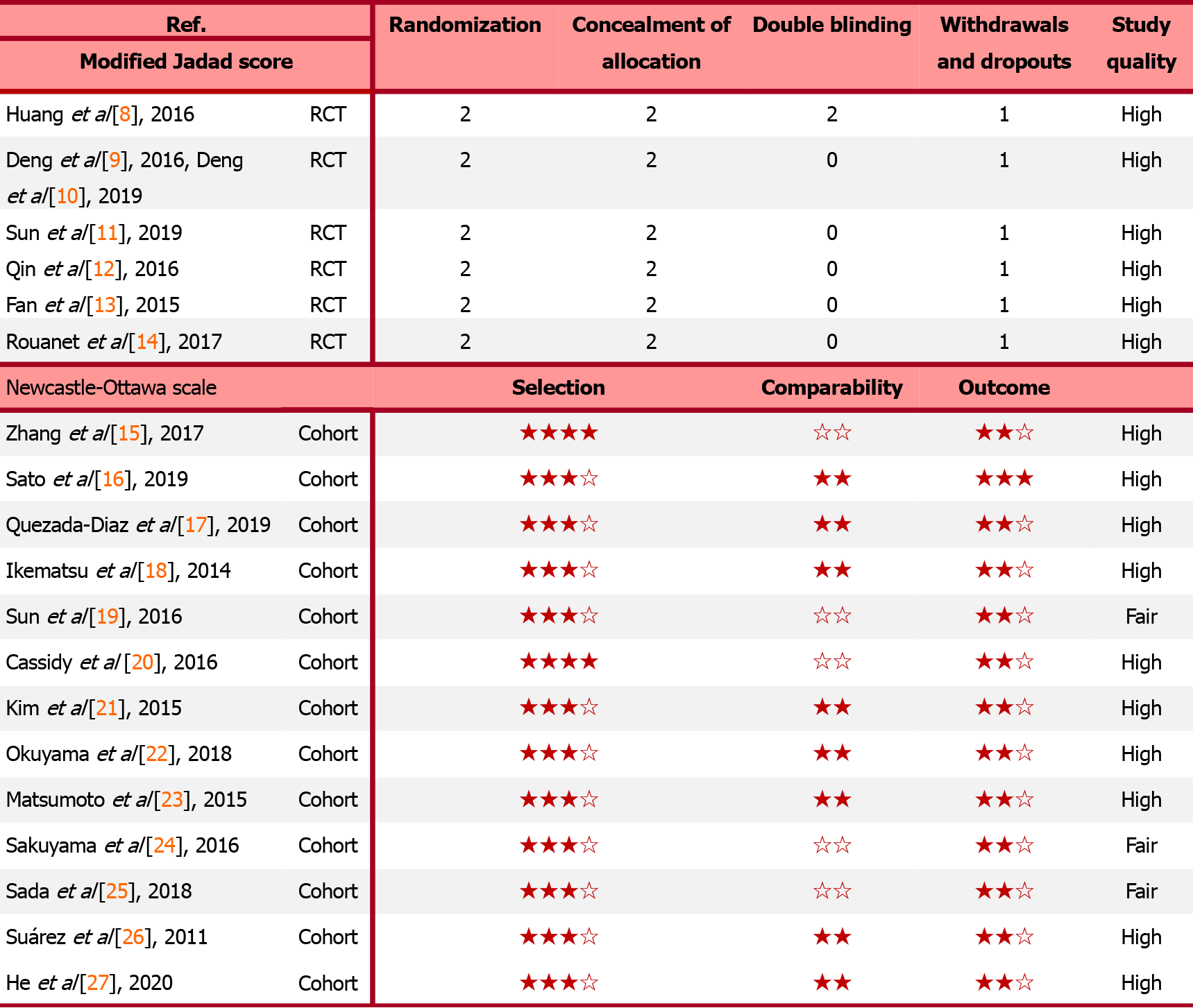
A total of 22087 potentially relevant articles were retrieved from the PubMed, Cochrane, and EMBASE databases. After exclusion of duplicate articles, case reports, meta-analyses, and reviews, the titles, abstracts, and full texts of the articles were screened. Finally, 19 articles (6 randomized control trials[8-14] and 13 cohort studies[15-27], Figure 1) with 60870 patients met the eligibility criteria and were included in the meta-analysis. In total, 2908 and 57962 patients had been treated with Neo-CT alone and Neo-CRT, respectively. The characteristics of the included studies (i.e., first author, year of publication, sample size, tumor stages, surgery regimens, chemotherapy regimens, and radiotherapy regimens) are shown in Table 1. The corresponding study was represented using the last name of the first author plus the year of publication. The quality of all studies was deemed medium or high (Figure 2).
| Ref. | Race | Sample size | Tumor stage | Surgery | Chemotherapy | Radiotherapy (Gy) | ||
| nCT | nCRT | nCT | nCRT | |||||
| Huang et al[8], 2016 | Asian | 102 | II–III | AR, LAR, etc. | AR, LAR, etc. | mFOLFOX6 | mFOLFOX6 | 50 |
| Deng et al[9], 2016, Deng et al[10], 2019 | Asian | 475 | II–III | TME | TME | mFOLFOX6 | mFOLFOX6/FOLFOX6 | 46.0–50.4 |
| Sun et al[11], 2019 | Asian | 220 | II–III | TME | TME | mFOLFOX6 | 5-FU; mFOLFOX6 | 46.0–50.4 |
| Fan et al[13], 2015 | Asian | 184 | II–III | TME | TME | XELOX | XELOX | 46 |
| Qin et al[12], 2016 | Asian | 318 | II–III | NA | NA | 5-FU, FOLFOX | 5-FU, FOLFOX | 50 |
| Zhang et al[15], 2017 | Asian | 142 | LARC | NA | NA | mFOLFOXIRI | mFOLFOXIRI | NA |
| Sato et al[16], 2019 | Asian | 26 | LARC | TME; LLND | TME; LLND | S1 + oxaliplatin | 5FU-based | 4045 |
| Diaz et al[17], 2019 | NA | 46 | II/III | TME | TME | mFOLFOX6; CAPOX; FLOX | Fluorouracil/oral capecitabine | 50.0 or 50.4 |
| Ikematsu et al[18], 2014 | NA | 77 | III - IV | ISR | ISR | mFOLFOX6 | 5-fluorouracil | 45 |
| Sun et al[19], 2016 | Asian | 22094 | II–III | NA | NA | NA | NA | NA |
| Cassidy et al[20], 2016 | NA | 21707 | III | NA | NA | NA | NA | NA |
| Kim et al[21], 2015 | Asian | 86 | IV | TME | TME | FOLFOX4; FOLFIRI; 5-FU/LV, + capecitabine | 5-FU/LV or capecitabine | 45.0-50.4 |
| Matsumoto et al[23], 2015 | Asian | 124 | II–III | LAR, APR/ASR, Hartmann, ISR | LAR, APR/ASR, Hartmann, ISR | FOLFOX; IRIS; FOLFIRI | FOLFOX; IRIS; FOLFIRI | NA |
| Sakuyama et al[24], 2016 | Asian | 88 | Ⅰ-Ⅳ | ISR, etc. | ISR, etc. | FOLFOX | FOLFOX | 45 |
| Sada et al[25], 2018 | Asian | 11839 | II–III | NA | NA | NA | NA | 40-55 |
| Suárez et al[26], 2011 | NA | 25 | Ⅳ | AR, APR, Hartmann | AR, APR, Hartmann | 5-FU + oxaliplatin-based | fluoropyrimidine-based | 50.40 |
| Rouanet et al[14], 2017 | NA | 29 | LARC | TME, etc. | TME, etc. | FOLFIRINOX | FOLFIRINOX | 50 |
| Okuyama et al[22], 2018 | Asian | 55 | II–III | LAR, APR | LAR, APR | oxaliplatin-based | 5-FU-based | 45 |
| He et al[27], 2020 | Asian | 3233 | II–III | TME | TME | FOLFOX; CAPOX | FOLFOX; CAPOX | 50 |
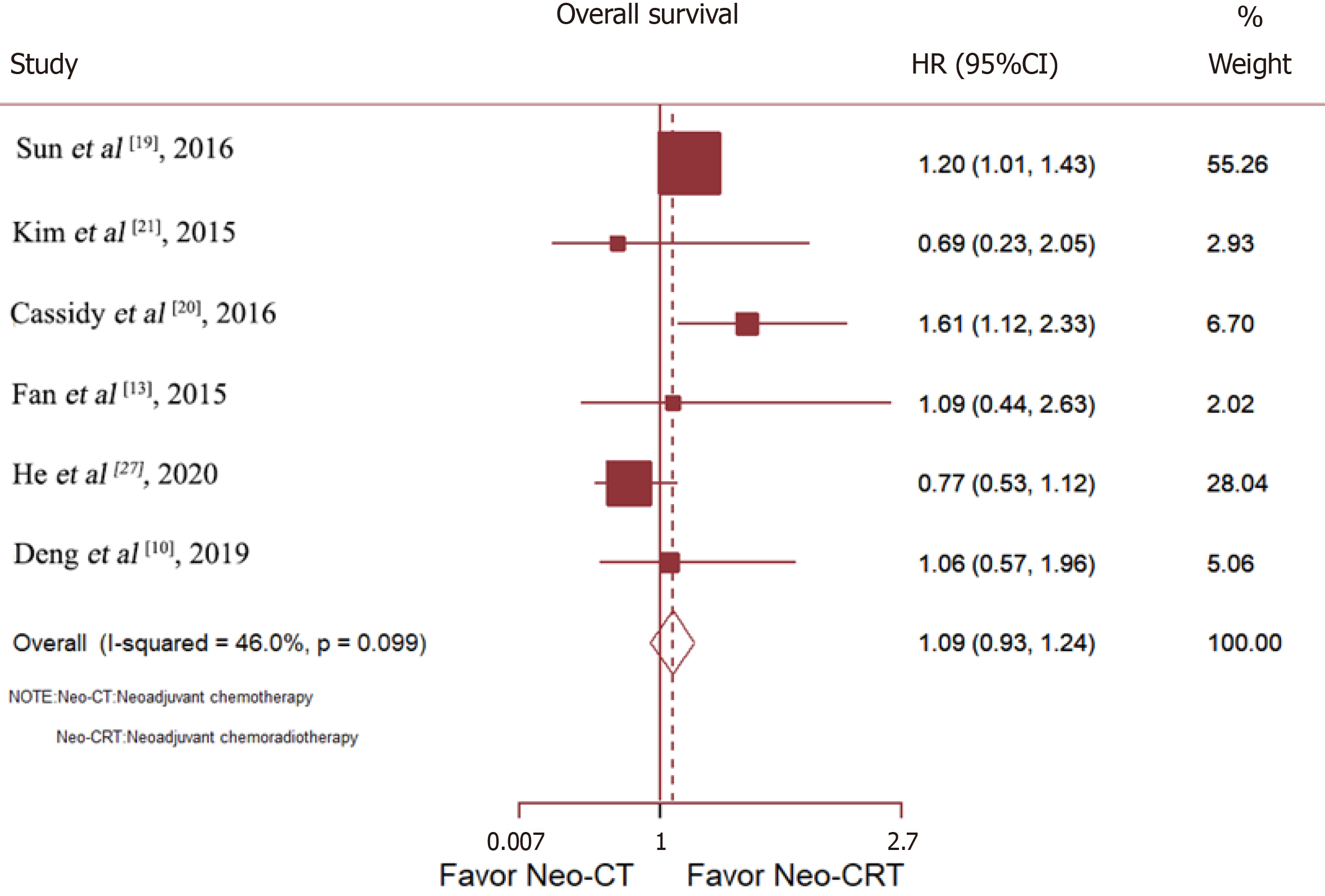
Six studies compared the OS of patients with RC treated with Neo-CT vs Neo-CRT. Sun et al[19] and Cassidy et al[20] reported that Neo-CRT improved OS, while Deng et al[10], He et al[27], Fan et al[13], and Kim et al[21] reported no benefit. After merging all studies, Neo-CRT did not improve OS [HR = 1.09, 95% confidence interval (CI) = 0.93–1.24; p = 0.19] (Figure 3).
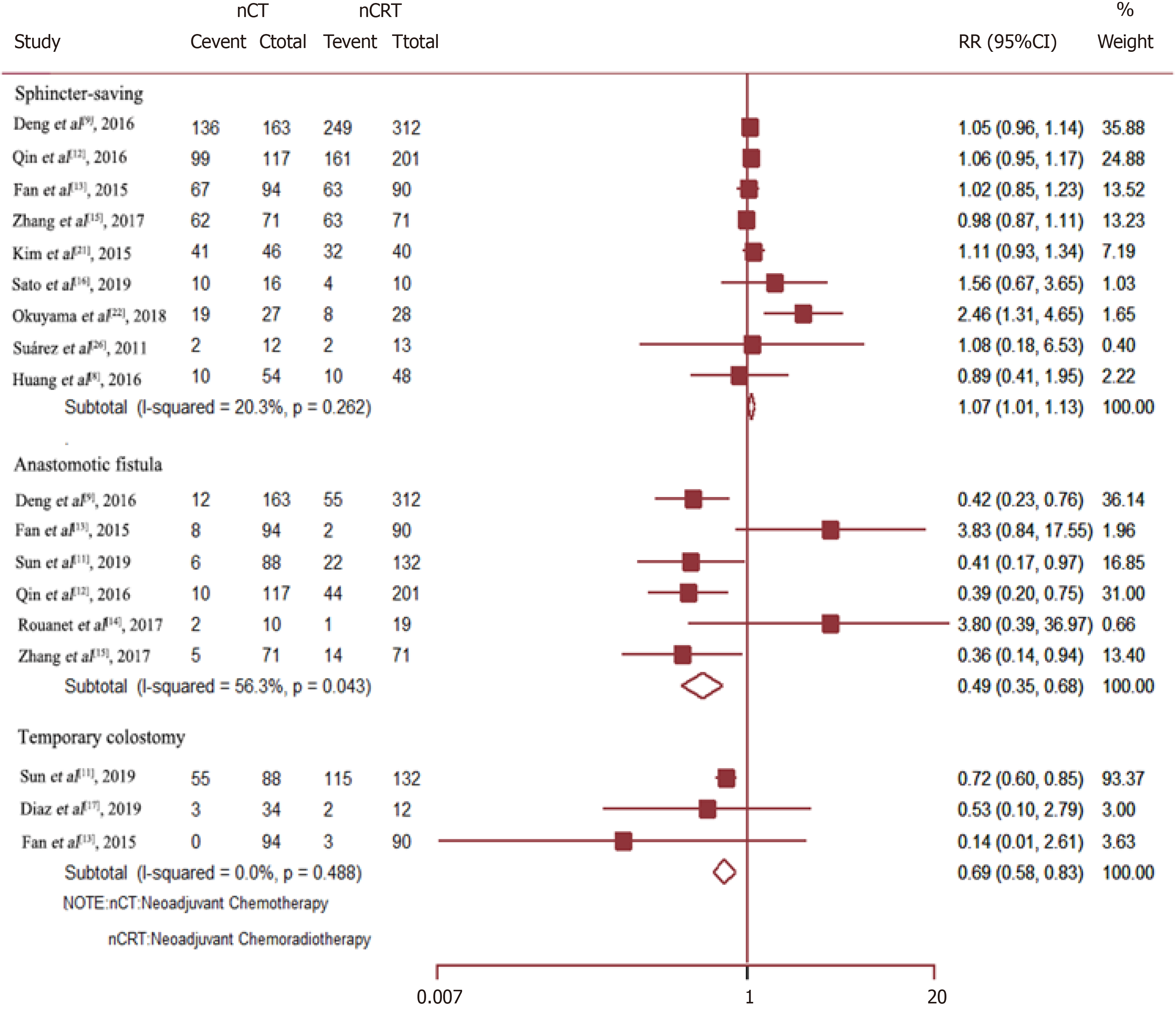
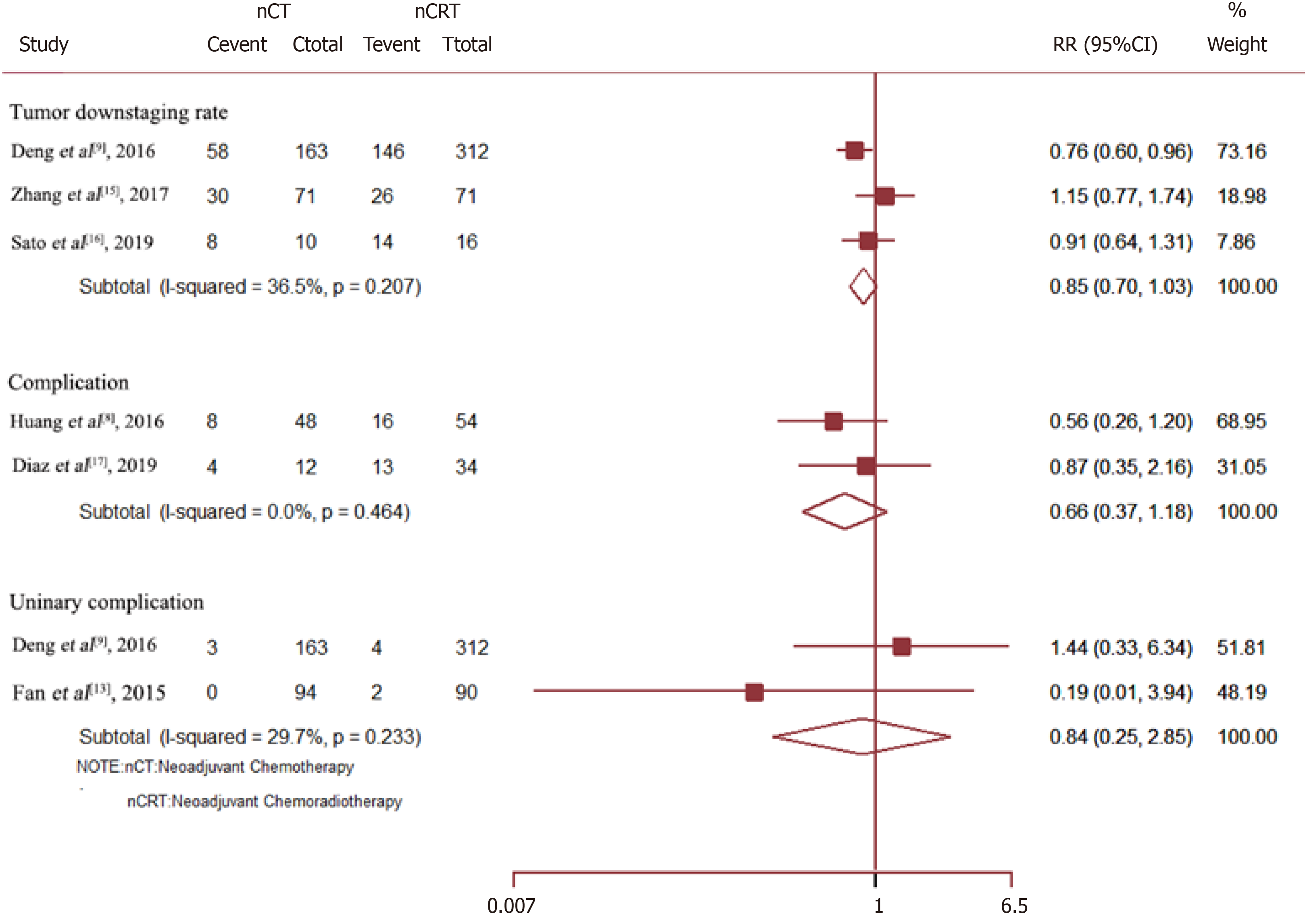
Data on the rate of sphincter preservation, incidences of temporary colostomy, and anastomotic fistula were available from nine, three, and six trials, respectively. As compared to Neo-CRT, Neo-CT without radiotherapy was associated with a significant decrease in the incidences of anastomotic fistula (RR = 0.49, 95%CI = 0.35–0.68; P = 0.000) and temporary colostomy (RR = 0.69, 95%CI = 0.58–0.83; P = 0.000). Moreover, Neo-CT without radiotherapy resulted in a higher sphincter preservation rate (RR =1.07, 95%CI = 1.01–1.13; P = 0.029) (Figure 4).
Neo-CRT had no significant effect on the tumor downstaging rate (RR = 0.85, 95%CI = 0.70–1.03; P = 0.088), overall complications (RR = 0.66, 95%CI = 0.37–1.18; P = 0.158), or urinary complications (RR = 0.84, 95%CI = 0.25–2.85; P = 0.775) (Figure 5).
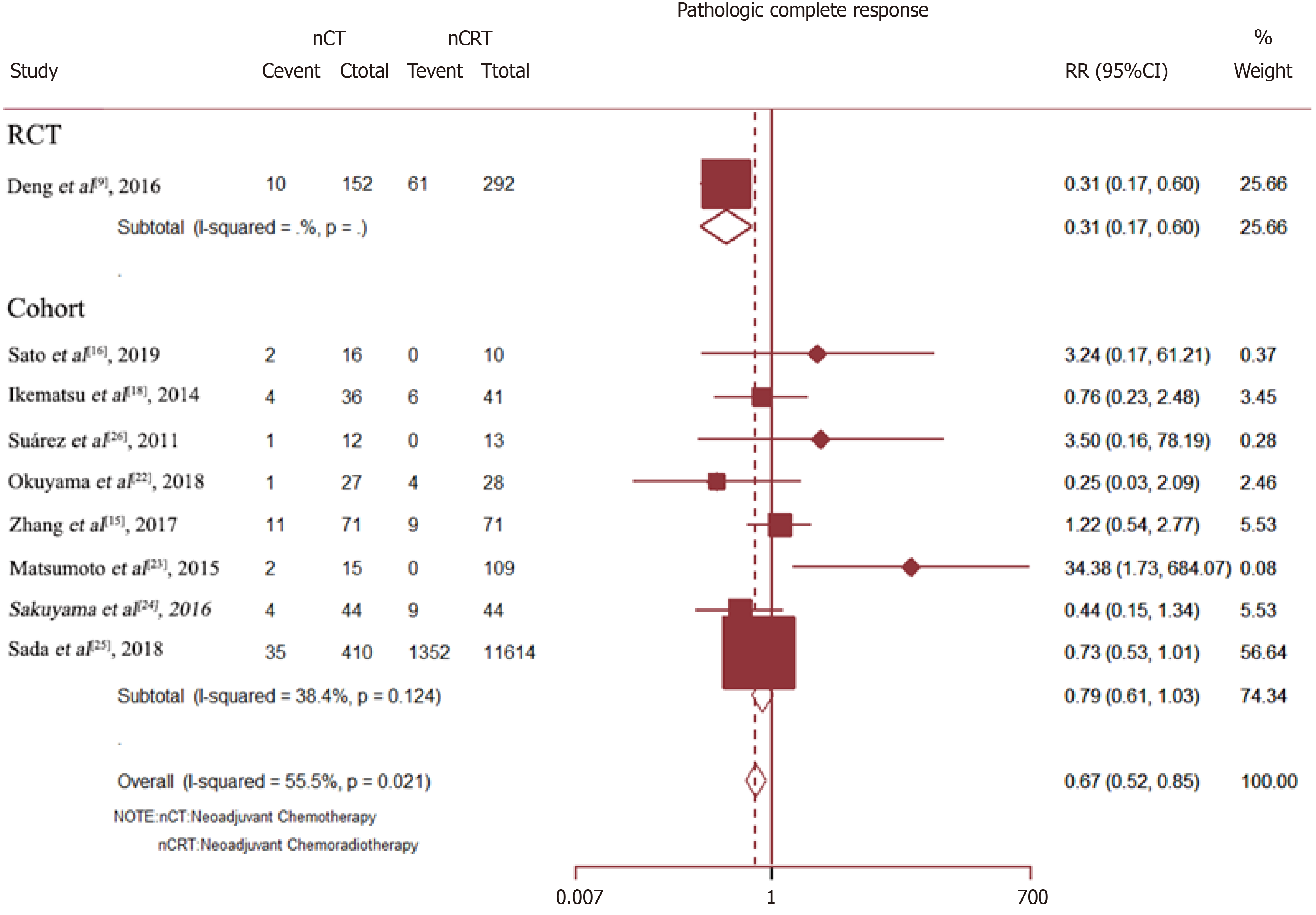
The pCR rate was available for nine studies with a total of 13005 patients. The results showed that as compared with Neo-CT, Neo-CRT was associated with a significant benefit in the pCR rate (RR = 0.67, 95%CI = 0.52–0.85; P = 0.001). However, for all cohort studies, there was no difference in the pCR rate between Neo-CT and Neo-CRT (RR = 0.79, 95%CI = 0.61–1.03; P = 0.086) (Figure 6).
Sensitivity analysis determined high heterogeneity among the pCR rates (I2 = 56.9% P = 0.000). Subsequently, exclusion of the study by Deng et al[9] resulted in higher heterogeneity. Finally, there was no significant difference in the pCR rate (I2 =38.4%) between the Neo-CT and Neo-CRT groups (Figure 7).
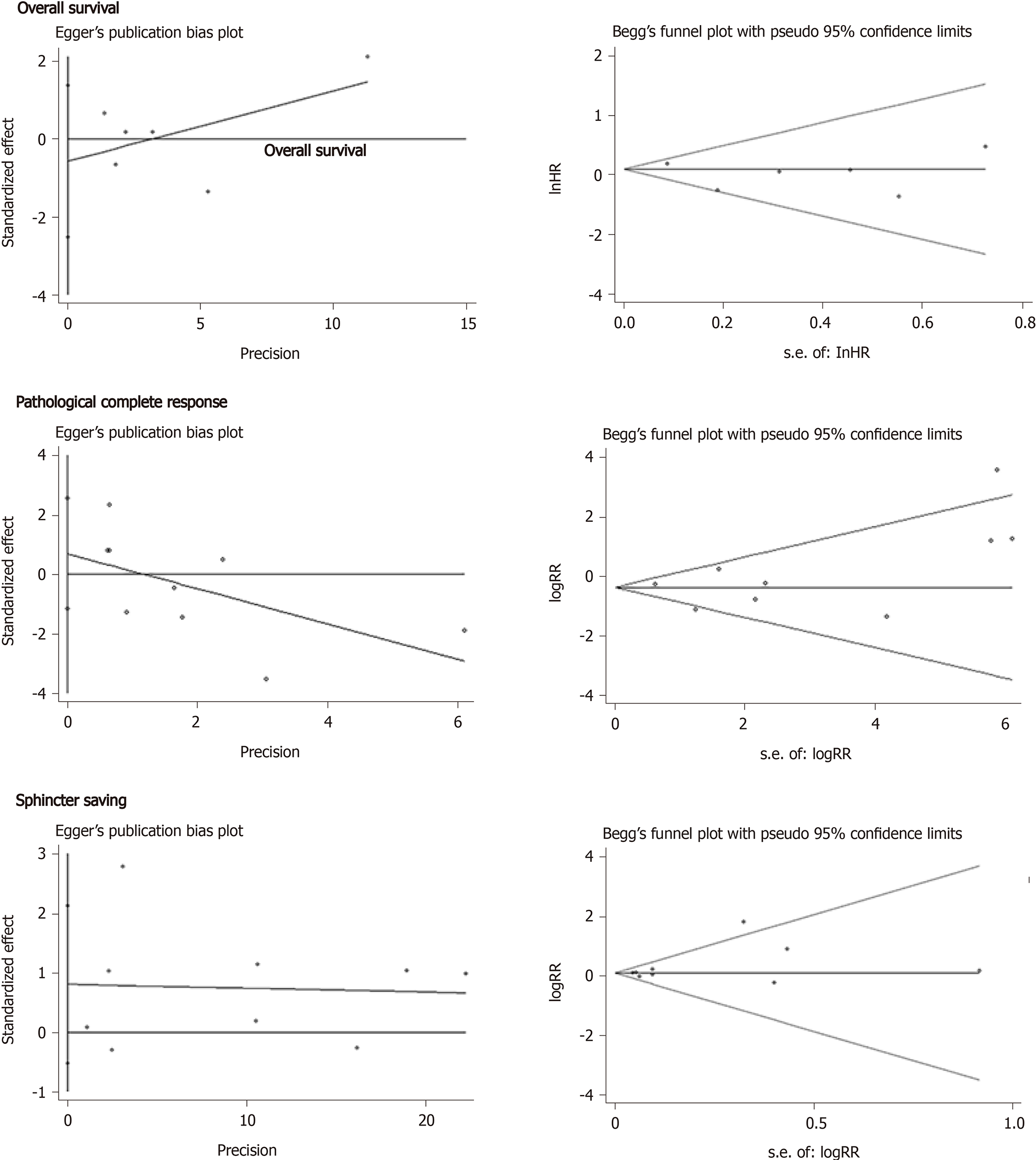
As is shown in Figure 7, there was no significant publication bias in regard to OS (Begg, Pr > |z| = 1.000; Egger, P > |t| = 0.460), pCR (Begg, Pr > |z| = 0.348; Egger, P > |t| = 0.399), and the sphincter preservation rate (Begg, Pr > |z| = 0.602; Egger, P > |t| = 0.191).
A Swedish trial conducted in 1997 demonstrated that Neo-CRT improved not only local control, but also OS in Western countries[28]. Neo-CRT followed by TME has been adopted as an indispensable treatment regimen for LARC. However, despite the low local relapse rate associated with this treatment regimen, systemic recurrence remains a significant problem, occurring in 30%–40% of patients, along with late morbidities of the bowel, bladder, and sexual function[29]. More Japanese and Chinese physicians prefer a preoperative aggressive chemotherapy regimen to suppress distant metastasis and reduce the toxicity of CRT, followed by lateral lymph node dissection, especially for poor risk cases of LARC[30]. However, the suitability of Neo-CT without radiation for LARC remains controversial.
To the best of our knowledge, this is the first meta-analysis to compare the efficacy and safety of Neo-CT without radiation vs Neo-CRT for RC patients. This study included 19 randomized control trials or cohort studies published up to September 30, 2020. The results showed that Neo-CT could decrease the incidences of anastomotic fistula and temporary colostomy and increase the sphincter preservation rate. Meanwhile, there was no significant difference in OS, the tumor downstaging rate, overall complications, and urinary complications. Although Neo-CT was associated with a higher rate of pCR after merging all trials, there was no significant difference in the pCR rate between the Neo-CT and Neo-CRT groups in all cohort studies.
Most studies of Neo-CRT treatment have demonstrated pCR rates of up to 30%[31-33]. For example, the FOWARC study reported a higher pCR rate for fluorouracil alone vs preoperative mFOLFOX6 concurrent with radiotherapy (27.5% vs 14.0%, respectively)[9]. However, in the present study, there was no significant difference in the pCR rate between the Neo-CT and Neo-CRT groups in all cohort studies (9.5% vs 11.6%, respectively, P = 0.086), likely because of the chemotherapy regimens.
In contrast, previous randomized trials investigating the addition of weekly oxaliplatin to fluoropyrimidine-based preoperative CRT regimens reported increased acute toxicity without substantial improvements in pCR rates[34,35]. A possible explanation for these results was that patients who received a full dose of mFOLFOX6 chemotherapy concurrent with full-dose radiation radiotherapy had a higher rate of treatment compliance than in previous negative trials. The efficacy and varying pCR rate of Neo-CT without radiation have also been associated with regimens involving target drugs and the interval to surgery after chemotherapy. Schrag et al[5] treated patients with clinical stage II-III RC with neoadjuvant FOLFOX-based chemotherapy (six cycles of FOLFOX with bevacizumab in the first four cycles) followed by TME and observed a pCR rate of 27%, a R0 resection rate of 100%, and no local failure during a median follow-up period of 18.2 mo. The phase II GEMCAD 0801 trial involving patients with stage II–III RC reported a pCR rate of 15% and a R0 resection rate of 100% after treatment with capecitabine, oxaliplatin, and bevacizumab[36]. Specifically, in the N-SOG 03 phase II trial, aggressive chemotherapy regimens (neoadjuvant oxaliplatin and capecitabine plus bevacizumab) reported a pCR rate of 13% and tumor regression rate of 37%, indicating a poor risk of these regimens for RC patients[6]. The results of these studies suggested that pelvic external radiation therapy was avoided by selecting appropriate patients with RC other than stage T4 and that the strategy of combining full-dose chemotherapy with multiple agents was more effective.
Pathological stage is still the most reliable predictor of survival of patients undergoing Neo-CRT and surgery, and the pCR status appears to be associated with a favorable prognosis. However, it remains controversial whether Neo-CRT can improve OS. When all studies were merged, there was no significant difference in OS between the two groups (HR = 1.09, 95%CI = 0.93–1.24; P = 0.19). Similarly, a Japanese randomized study reported no significant difference in the 5-year survival rate (67.2 vs 69.2%, respectively) or disease-free survival (DFS) (60.5 vs 63.0%, respectively) between the treatment and control groups[37]. Meanwhile, the results of the JIAO2015 trial showed no significant improvements in OS and DFS, although the rate of distant metastasis was reduced[5]. Some researchers believed that the DFS benefit in the CAO/ARO/AIO-04 trial was due to the adaptation of OX-based CT regimens[38,39]. Yang et al[40] reported that DFS was marginally but significantly higher in the fluorouracil-based Neo-CRT with OX group than the fluorouracil-based Neo-CRT group, although the distant metastasis rate was significantly decreased in the latter. However, there is no clear consensus regarding whether patients who achieved a pCR should undergo CRT even though the prognosis of these treatments is favorable because the supporting data are rather limited. Nonetheless, patients who achieve a pCR or a clinically significant downstaging to ypT1/T2N0 after neoadjuvant therapy usually have an excellent prognosis.
Although Neo-CRT may improve the pCR rate, the technical difficulty of this regimen is significantly greater due to radiation-induced pelvic fibrosis and the increased risks of surgical complications. Due to these concerns, surgery is often postponed beyond 6–8 wk. However, neo-CRT still leads to temporary ileostomy to prevent anastomotic fistula. Besides, a second surgery is necessary to return the stoma, which severely delays postoperative CT treatment. Moreover, neo-CRT is also considered an independent risk factor for major low anterior resection syndrome[11]. The potential adverse events of pelvic radiation in neo-CRT could have long-term impacts on the bowel, bladder, and sexual function, and can destroy bone marrow reserves, while decreasing future tolerance to chemotherapy[41]. The results of the present study indicated that Neo-CT without radiotherapy significantly decreased the incidences of anastomotic fistula (RR = 0.49, 95%CI = 0.35–0.68; P = 0.000), temporary colostomy (RR = 0.69, 95% = 0.58–0.83; P = 0.000) and increased the sphincter preservation rate (RR =1.07, 95%CI = 1.01–1.13; P = 0.029). For these reasons, an RC treatment paradigm that incorporates radiation selectively would be advantageous.
However, LARC is associated with great heterogeneity; therefore, any single treatment modality will not be optimal for all patients. Notably, the choice of treatment should be based on clinical presentations, imaging findings, and molecular biology to precisely stratify patients. Besides, the scheme should be dynamically adjusted according to the therapeutic response to realize the dual goals of prolonging patient survival and improving quality of life. Meanwhile, the choice of treatment for the target population under the guidance of biomarkers should be dynamically and individually adjusted based on the therapeutic effect. This approach will become the future development direction and objective for the precise medical treatment for RC.
There were some limitations to this meta-analysis that should be noted. Although this article provided an up-to-date synthesis of the best available evidence and most of the endpoints contained a medium or large sample size, detailed tumor staging, and different chemotherapy regimens should be further compared to validate the results. Moreover, further studies are needed to explore related endpoints, including the tumor downstaging rate, various complications, and other treatment-related adverse events. Finally, the survival endpoints should be verified in subsequent studies. All in all, owing to the negligible heterogeneity of every endpoint and the inclusion of only high-quality studies, these preliminary results suggested that the combination of full-dose Neo-CT without radiation presented a new neoadjuvant treatment option for LARC.
Neo-CT administration can lower the incidences of anastomotic fistula and temporary colostomy and increase the sphincter preservation rate compared to Neo-CRT and could provide an alternative to CRT for LARC.
Neoadjuvant chemoradiotherapy (Neo-CRT) is the current standard strategy for treatment of locally advanced rectal cancer (LARC). However, it delays the administration of optimal chemotherapy and increases toxicity.
This meta-analysis aimed to compare the feasibility and efficacy of neoadjuvant chemotherapy (Neo-CT) vs Neo-CRT for treatment of LARC.
To the best of our knowledge, this is the first meta-analysis to compare the efficacy and safety of Neo-CT without radiation vs Neo-CRT for rectal cancer patients, which included 19 randomized control trials or cohort studies published up to September 30, 2020.
The hazard ratio of overall survival and the risk ratio for the pathological complete response, the sphincter preservation rate, and treatment-related adverse events were analyzed.
The results of this study showed that Neo-CT decreased the incidences of anastomotic fistula and temporary colostomy and increased the sphincter preservation rate. Meanwhile, there was no significant difference in overall survival, the tumor downstaging rate, overall complications, urinary complications, and pathological complete response rates.
Neo-CT was effective for treatment of LARC, especially to lower the incidences of anastomotic fistula and temporary colostomy and increase the sphincter preservation rate as compared to Neo-CRT and could have the potential to provide an alternative to CRT for LARC.
All in all, owing to the negligible heterogeneity of every endpoint and the inclusion of only high-quality studies, these preliminary results suggested that the combination of full-dose Neo-CT without radiation presented a new neoadjuvant treatment option for LARC.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C, Sun C S-Editor: Liu M L-Editor: Filipodia P-Editor: Li JH
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4342] [Cited by in F6Publishing: 4228] [Article Influence: 211.4] [Reference Citation Analysis (1)] |
| 2. | Park EJ, Kang J, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Different clinical features according to the anastomotic leakage subtypes after rectal cancer surgeries: contained vs. free leakages. PLoS One. 2018;13:e0208572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hasegawa J, Nishimura J, Mizushima T, Miyake Y, Kim HM, Takemoto H, Tamagawa H, Noura S, Fujii M, Fujie Y, Kato T, Miwa H, Takemasa I, Ikeda M, Yamamoto H, Sekimoto M, Nezu R, Doki Y, Mori M. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73:1079-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, Watanabe M, Kitagawa Y. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36:1061-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, Temple LK, Paty PB, Saltz LB. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 6. | Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H, Ohmiya N, Goto H, Nagino M. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43:964-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3749] [Cited by in F6Publishing: 4558] [Article Influence: 268.1] [Reference Citation Analysis (0)] |
| 8. | Huang M, Lin J, Yu X, Chen S, Kang L, Deng Y, Zheng J, Luo Y, Wang L, Lan P, Wang J. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int J Colorectal Dis. 2016;31:1349-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 10. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Wu X, Peng J, Ren D, Wang J. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J Clin Oncol. 2019;37:3223-3233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan L, Kang L, Deng Y, Lan P, Wang L, Wang J. Impact of Long-Course Neoadjuvant Radiation on Postoperative Low Anterior Resection Syndrome and Quality of Life in Rectal Cancer: Post Hoc Analysis of a Randomized Controlled Trial. Ann Surg Oncol. 2019;26:746-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, Wang L, Wang J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum. 2016;59:934-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Fan WH, Wang FL, Lu ZH, Pan ZZ, Li LR, Gao YH, Chen G, Wu XJ, Ding PR, Zeng ZF, Wan DS. Surgery with versus without preoperative concurrent chemoradiotherapy for mid/low rectal cancer: an interim analysis of a prospective, randomized trial. Chin J Cancer. 2015;34:394-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Rouanet P, Rullier E, Lelong B, Maingon P, Tuech JJ, Pezet D, Castan F, Nougaret S; and the GRECCAR Study Group. Tailored Treatment Strategy for Locally Advanced Rectal Carcinoma Based on the Tumor Response to Induction Chemotherapy: Preliminary Results of the French Phase II Multicenter GRECCAR4 Trial. Dis Colon Rectum. 2017;60:653-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Cai Y, Hu H, Chen D, Xiao J, Wang W, Lan P, Huang M, Wang L, Wu X, Kang L, Wang J, Deng Y. Neoadjuvant treatment with mFOLFOXIRI alone vs chemoradiotherapy in locally advanced rectal cancer: A propensity score analysis from two prospective trials. Ann Oncol. 2017;28 Suppl 5:171. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Sato K, Miura T, Morohashi S, Sakamoto Y, Morohashi H, Yoshida T, Hakamada K. Comparable regional therapeutic effects between neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy for locally advanced lower rectal cancer in terms of histopathological analysis. Mol Clin Oncol. 2019;10:619-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Quezada-Diaz F, Jimenez-Rodriguez RM, Pappou EP, Joshua Smith J, Patil S, Wei I, Guillem JG, Paty PB, Nash GM, Weiser MR, Garcia-Aguilar J. Effect of Neoadjuvant Systemic Chemotherapy With or Without Chemoradiation on Bowel Function in Rectal Cancer Patients Treated With Total Mesorectal Excision. J Gastrointest Surg. 2019;23:800-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ikematsu H, Fukuoka S, Ito M, Kojima M, Imajoh M, Osera S, Odagaki T, Oono Y, Yano T, Kaneko K. Mo1920 The investigation regarding endoscopic and pathological response for neo-adjuvant chemotherapy or chemoradiotherapy in patients with low rectal cancer. Gastroenterology. 2014;146:S691-S692. [DOI] [Cited in This Article: ] |
| 19. | Sun Z, Adam MA, Kim J, Hsu SWD, Mantyh C. Effect of combined neoadjuvant chemoradiation on overall survival for patients with locally advanced rectal cancer. J Clin Oncol. 2016;34 Suppl 4:657-657. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Cassidy RJ, Liu Y, Zhong J, Gillespie TW, Landry JC. Survival impact of neoadjuvant chemotherapy alone vs neoadjuvant chemoradiation therapy for patients with T2N1, T3N0, and T3N1 rectal adenocarcinomas. Int J Radiat Oncol Biol Phys. 2016;96:E148. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Kim SH, Kim JH, Jung SH. Comparison of oncologic outcomes of metastatic rectal cancer patients with or without neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2015;30:1193-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Okuyama T, Sameshima S, Takeshita E, Yoshioka R, Yamagata Y, Ono Y, Tagaya N, Noie T, Oya M. Therapeutic effects of oxaliplatin-based neoadjuvant chemotherapy and chemoradiotherapy in patients with locally advanced rectal cancer: a single-center, retrospective cohort study. World J Surg Oncol. 2018;16:105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Matsumoto T, Hasegawa S, Zaima M, Inoue N, Sakai Y. Outcomes of Neoadjuvant Chemotherapy without Radiation for Rectal Cancer. Dig Surg. 2015;32:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Sakuyama N, Kojima M, Kawano S, Akimoto T, Saito N, Ito M, Ochiai A. Histological differences between preoperative chemoradiotherapy and chemotherapy for rectal cancer: a clinicopathological study. Pathol Int. 2016;66:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Sada YH, Tran Cao HS, Chang GJ, Artinyan A, Musher BL, Smaglo BG, Massarweh NN. Prognostic value of neoadjuvant treatment response in locally advanced rectal cancer. J Surg Res. 2018;226:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Suárez J, Amat I, Vera R, Balén E, Gómez M, Lera JM. Pathologic response of primary rectal cancer to oxaliplatin-based chemotherapy. Clin Colon Rectal Surg. 2011;24:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | He F, Yu L, Ding Y, Li ZH, Wang J, Zheng J, Chen HY, Liu S, Pang XL, Ajani JA, Wan XB. Effects of neoadjuvant chemotherapy with or without intensity-modulated radiotherapy for patients with rectal cancer. Cancer Sci. 2020;111:4205-4217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1753] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 29. | Temple WJ, Saettler EB. Locally recurrent rectal cancer: role of composite resection of extensive pelvic tumors with strategies for minimizing risk of recurrence. J Surg Oncol. 2000;73:47-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 30. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 559] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 31. | Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf). 2015;3:277-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Hall MD, Schultheiss TE, Smith DD, Fakih MG, Wong JY, Chen YJ. Effect of increasing radiation dose on pathologic complete response in rectal cancer patients treated with neoadjuvant chemoradiation therapy. Acta Oncol. 2016;55:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 34. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah JF, Mahé MA, Bécouarn Y, Dupuis O, Lledo G, Montoto-Grillot C, Conroy T. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 548] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 35. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 551] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 36. | Fernandez-Martos C, Brown G, Estevan R, Salud A, Montagut C, Maurel J, Safont MJ, Aparicio J, Feliu J, Vera R, Alonso V, Gallego J, Martin M, Pera M, Sierra E, Serra J, Delgado S, Roig JV, Santos J, Pericay C. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Colorectal Cancer Chemotherapy Study Group of Japan - The 2nd Trial. Results of a randomized trial with or without 5-FU-based preoperative chemotherapy followed by postoperative chemotherapy in resected colon and rectal carcinoma. Jpn J Clin Oncol. 2003;33:288-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 464] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 39. | Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Ströbel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T; German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 40. | Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, Yang JL, Zhou ZR, Liu SX. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: An updated systematic review and meta-analysis. Oncotarget. 2016;7:45513-45524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Morris KA, Haboubi NY. Pelvic radiation therapy: Between delight and disaster. World J Gastrointest Surg. 2015;7:279-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 68] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |