Abstract
Percutaneous catheter-based transluminal renal denervation (RDN) has emerged as a new approach to achieve sustained blood pressure reduction in patients with drug-resistant hypertension. Experts from ESH and ESC in their recently released position papers and consensus document have summarised the current evidence, unmet needs and practical recommendations for the application of this therapeutic strategy in clinical practice. Experts of the ESH Working Group for the interventional treatment of hypertension prepared this position paper in order to provide interventionalists with guidance through the procedure of RDN. Given that there is no established intraprocedural control of ablation success, interventionalists have to be familiar with the aspects related to the anatomy and imaging of the renal arteries, the distribution of renal sympathetic fibres, the special equipment necessary for RDN and the procedural details in order to maximise the success and minimise potential complications.
Introduction
The recently published European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines for the management of hypertension1, as well as the European and international expert statements2-5, support percutaneous catheter-based transluminal renal denervation (RDN) by delivery of radiofrequency (RF) energy as a promising novel interventional approach to reduce blood pressure (BP) in patients with drug-resistant hypertension (RHTN) (Table 1). Experts of the ESH Working Group for the interventional treatment of hypertension prepared a position paper to outline the correct interventional procedure of RDN. Given that there is no established intraprocedural control of ablation success, interventionalists have to be familiar with the aspects related to the anatomy and imaging of the renal arteries, the distribution of renal sympathetic fibres, the special equipment necessary for RDN and the procedural details in order to maximise the success and minimise the adverse effects.
Current status of RDN: the appropriate candidates
Based on the published trials and ESH/ESC guidelines, hypertensive patients are currently eligible for RDN if they have severe drug-RHTN defined by office systolic BP ≥160 mmHg (≥150 mmHg in type 2 diabetes) despite treatment with ≥3 antihypertensive drugs of different types in adequate doses, including one diuretic1-5. After referral or self-referral to a hypertension specialist or hypertension centre (preferably hypertension excellence centre) patients should undergo a thorough work-up to exclude pseudo-resistance (by performing ambulatory BP monitoring) and to check for secondary causes of hypertension1-5. To confirm treatment resistance it is mandatory to unmask contributing lifestyle factors and drug-related causes, to evaluate the patient’s adherence to antihypertensive drug regimens and lastly to optimise the pharmacological treatment (including aldosterone antagonists if considered to be safe). Although it seems logical that the procedure should only be applied to patients with a certain life expectancy, the clinical studies (Symplicity I and II, as well as EnligHTN I) have included subjects aged 18-85 years old with a mean age of about 60 years2-7. At present, there is no consensus on whether the procedure should be restricted to a certain age range. Additionally, in the aforementioned studies, only patients with renal function to some extent preserved were included (estimated glomerular filtration rate [GFR] ≥45 mL/min per 1.73 m2 using the Modification for Diet in Renal Disease formula). In recently published pilot studies, in patients with RHTN and moderate to severe chronic kidney disease (CKD) (stages 3 and 4, mean eGFR=31 ml/min/1.73 m2), renal function post-RDN was not unfavourably altered and BP reductions were comparable to those observed in the setting of RHTN without CKD8,9. These data extend the follow-up analysis of Symplicity I and II that showed no harmful effect on renal function or perfusion one to two years after RDN2-7. Moreover, the results of the Symplicity HTN-3 trial with the sham-ablation arm and ambulatory BP measurements could provide more robust evidence not only for the efficacy of the method but also for its safety.
Although cardiovascularly unstable patients, such as those with a history of a recent myocardial infarction, unstable angina pectoris, or a cerebrovascular accident within the last six months, have been excluded from the clinical trials, these patients might undergo RDN after careful consideration. Women who are pregnant, nursing, or of childbearing potential have been excluded from all RDN studies. Therefore, no evidence exists to determine the risks and benefits in this patient group.
The recommendation is to obtain renal artery imaging (by ultrasound, computed tomography angiography [CTA] or magnetic resonance angiography [MRA]) to assess renal artery anatomy for RDN. Patients with a previous renal artery intervention, evidence of renal artery atherosclerosis (defined as a diameter stenosis of >50%) or renal artery aneurysm (including fibromuscular dysplasia), main renal arteries of less than 4 mm in diameter as well as patients with multiple renal arteries of more than 2 mm in diameter were excluded from the studies2-5. Although not recommended, with a more widespread use of the technique accessory and polar arteries with a diameter >3.5 mm have also been treated10. Likewise, patients are not eligible after renal stenting due to unpredictable distribution of the delivered energy. However, case reports suggest that RDN might be feasible and successful in these cases11.
Basics of anatomic aspects of the renal artery
The renal arteries normally originate from the lateral part of the abdominal aorta at the level of the 1st and 2nd lumbar intervertebral disc space, immediately below the level of origin of the superior mesenteric artery12. The right renal artery often arises more proximally12,13. Each renal artery is directed across the crux of the diaphragm, so as to form almost a right angle with the aorta. The orifice of the right renal artery is located on the anterolateral wall of the aorta and that of the left in a more dorsolateral location. The right renal artery courses downwards toward the right kidney behind the inferior vena cava, while the left has a more horizontal, upward orientation posterior to the left renal vein. Before reaching the hilum of the kidney, each artery divides into four or five branches (segmental arteries). Each vessel gives off some small inferior suprarenal branches to the suprarenal gland, the ureter, and the surrounding cellular tissue and muscles.
RENAL ARTERY SIZE
Renal arteries are usually 4-6 cm in length and 5-7 mm in diameter. The diameters of both renal arteries are slightly smaller in females compared to males.
VARIATIONS OF THE RENAL ARTERY, ACCESSORY RENAL ARTERIES
In the majority of the population, a single renal artery supplies each kidney but, in approximately 30% of individuals, more than one artery can be found14. Extra renal arteries usually arise from the aorta or the iliac arteries and are divided into two groups: accessory and polar arteries. Accessory arteries enter the kidneys from the hilum with the main renal artery, whereas polar arteries enter the superior or inferior renal parenchyma directly from the renal cortex away from the hilum. It is unknown whether ablating main arteries in patients with multivessel anatomy is effective, although data from an observational study indicate that denervation of one vessel, the dominant one, is sufficient to induce BP reduction in treatment of RHTN15. The frequency of renal artery variations ranges between 20 and 30%12-14, while the rate of early division varies between 8 and 15%12-14. Sometimes the gonadal artery, usually arising from the aorta, originates from the renal artery, either from the main or from one of the accessory ones12. Moreover, several combinations of renal, testicular or ovarian and suprarenal arteries have also been reported. Of particular importance is the inferior suprarenal artery that is responsible for the main blood supply of the suprarenal gland12-14. This vessel originates from the renal artery, in most cases proximally, and it is advisable to avoid ablation near its ostium.
Imaging modalities of renal arteries
Knowledge of imaging modalities for renal arteries is advisable not only for the appropriate implementation of anatomic exclusion criteria for RDN, but also for preprocedural planning including appropriate guide and ablation catheter selection. The preferred method to evaluate the vascular bed supplying the kidneys optimally still remains controversial. MRA provides accurate information about the number of renal arteries, the size of the kidneys and the presence of anatomic variants16. Gadolinium-enhanced MRA has a high sensitivity of 96-100% combined with a lower specificity of 71-96% for detecting significant (>50%) stenosis in the main renal artery. MRA remains suboptimal for the detection of important lesions of distal, intrarenal and accessory renal arteries, and its applicability is limited by the high cost. Moreover, in patients with a glomerular filtration rate of less than 30 ml/min/1.73 m2, gadolinium use should be avoided due to the risk of nephrogenic systemic fibrosis. CTA with maximum intensity projection provides accurate measurements of renal artery stenosis. Accessory renal arteries are accurately detected by CTA, whereas the sensitivity and specificity in detecting renal artery stenosis are 98% and 94%, respectively. However, the radiation exposure is rather high (24/28 mGy while a standard abdominal CT count is 18/20 mGy), and there is also the risk of iodine allergy and nephrotoxicity.
By Doppler ultrasonography the ratio between the peak systolic renal artery velocity and the aortic peak systolic velocity of ≥3.5 or a peak flow >2 ml/sec in a renal artery indicates the presence of a renal artery stenosis, and an intrarenal resistance index ≥0.05 lower than in the unaffected kidney (indirect sign) is indicative of a significant unilateral renal artery stenosis17. A significant bilateral renal artery stenosis is indicated by an increase of acceleration time more than 100 m/sec17. However, renal duplex ultrasound is unable to depict the structural phenotype of the whole renal vasculature and is limited by technical as well as operator and patient-related factors18.
Location of renal sympathetic fibres
The sympathetic innervation of the kidneys is achieved through a dense network of efferent sympathetic fibres, arising from the thoracolumbar sympathetic trunk19. Afferent renal sympathetic nerves (mechanoreceptors and chemoreceptors) originate mostly from the renal pelvic wall19. In samples taken from human renal arteries, sympathetic nerve fibres were present and located mainly in the tunica adventitia and outer media of renal arteries19-21. In another post mortem study, the sympathetic nerves appeared relatively evenly distributed around the circumference of the renal artery, similar to the description of Page et al as “basket-weave plexus”22. It is thought that about 50% of renal sympathetic nerves are found within 0.5-1.0 mm, and 90.5% of all nerves exist within 2.0 mm from the lumen-intima interface. There are slightly more nerves identified in the distal artery segments compared with proximal and middle segments, and the percent of total nerves decreases as the distance from the lumen increases. However, in a recent animal study, renal artery nerves were more frequently found in the proximal segment of the renal artery and decreased gradually distally, whereas the nerve size distribution was homogenous throughout the artery length and nerve bundles were closer to the arterial wall in the distal parts22-24.
REGENERATION OF RENAL SYMPATHETIC NERVES
The durability of the effects on BP after RDN needs to be fully elucidated since renal nerve fibres may regenerate or functionally regrow25. There is experimental evidence showing that both components of renal innervation (afferent and efferent) could regrow after the denervation procedure. However, the clinical consequences of such regrowth remain uncertain, since BP reduction was sustained over a period of at least 36 months post-ablation in a published trial26.
Available systems for RDN - brief description
Several ablation systems based mainly on the delivery of RF energy with the use of catheters or balloons are currently being tested and have been or will soon be released onto the market (Table 2, Figure 1). Six ablation systems (one of which is ultrasound-based) have already gained CE approval, while the largest amount of clinical evidence with the longest follow-up exists for the Symplicity system27-32. Administration of neurotoxins by nanoparticles (ApexNano Therapeutics, Alachua, FL, USA) and the micro-infusion Bullfrog® catheter (Mercator MedSystems, Inc., San Leandro, CA, USA) along with chemical ablation with vincristine33 or alcohol are RDN methods under preclinical investigation.
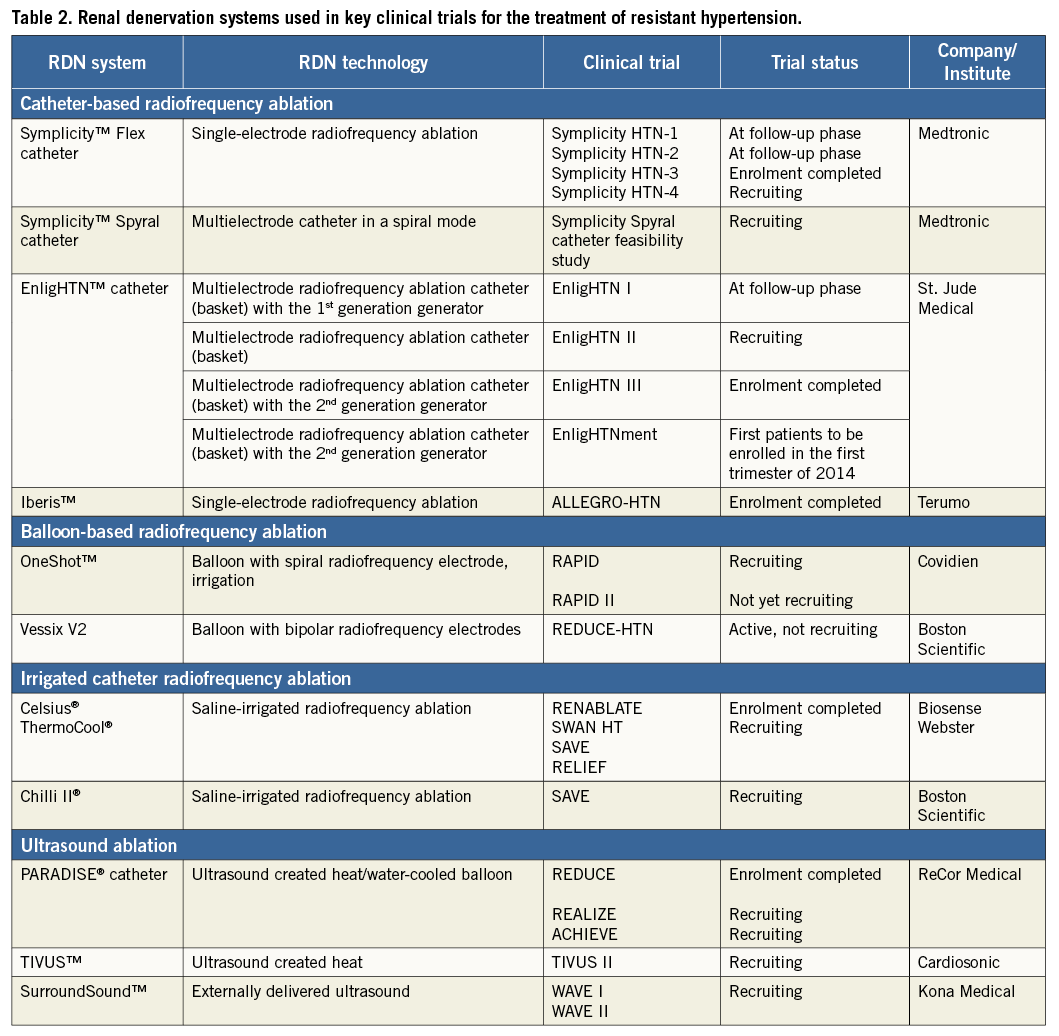
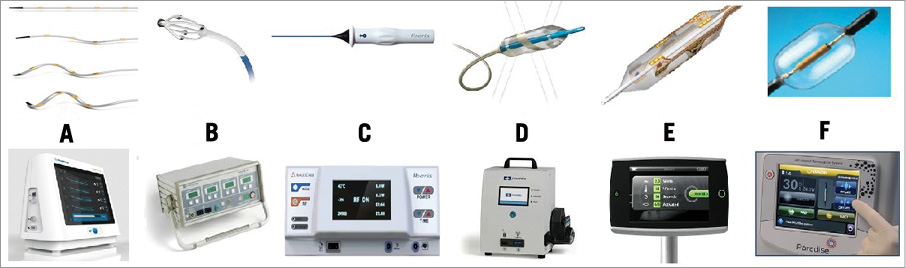
Figure 1. The available (CE mark approved) renal denervation catheters and their generators. From left to right: A) Symplicity Spyral™ (Medtronic); B) EnligHTN™ (St. Jude Medical); C) Iberis™ (Terumo); D) OneShot™ (Covidien); E) Vessix V2 (Boston Scientific); F) PARADISE® (ReCor).
THE SYMPLICITY™ SPYRAL RADIOFREQUENCY RENAL DENERVATION SYSTEM (MEDTRONIC)
The second-generation Symplicity™ Flex catheter (Medtronic, Inc., Mountain View, CA, USA) has now been further developed featuring a spiral multielectrode ablation catheter (Symplicity™ Spyral) accompanied by the Symplicity G3™ Generator (Figure 1A). The Symplicity™ Spyral catheter, which has to be introduced over a 0.014 inch guidewire, is 6 Fr guiding catheter compatible and features four electrodes at the spiral (helical) distal portion of the catheter. RF energy is applied for a one-minute ablation for all four electrodes simultaneously. All four electrodes are independently selectable. It is an exchange system and one size fits all vessel diameters up to 8 mm diameter.
THE ENLIGHTN™ RADIOFREQUENCY RENAL DENERVATION SYSTEM (ST. JUDE MEDICAL)
The EnligHTN™ radiofrequency RDN system (St. Jude Medical, St. Paul, MN, USA) is a multielectrode system with four electrodes arranged geometrically in a pre-specified position. By rotating the handle, a basket can be deployed. The catheter features include a multielectrode, non-occluding, nitinol basket (8 Fr compatible), and a deflectable, atraumatic tip (Figure 1B). The basket has two sizes: small (16 mm length, 4-6 mm vessel diameter) and large (18 mm length, 5.5-8 mm diameter).
In the first-generation device, the RF was delivered sequentially and a 90-second ablation was required. The second-generation EnligHTN generator allows simultaneous 60-second delivery of energy to all four electrodes, significantly shortening the treatment time to only two minutes for each side for the two sets of four ablations.
THE IBERIS™ RADIOFREQUENCY DENERVATION SYSTEM (TERUMO)
The Iberis™ radiofrequency generator (Terumo Medical Corp., Tokyo, Japan) uses a unipolar catheter to deliver RF energy and is provided in two lengths, 105 cm for the femoral approach and 155 cm for the radial approach. The catheter tip (10 mm) is deflectable up to 90° (Figure 1C). As opposed to the Symplicity™ Flex system and the EnligHTN™ system, no closed feedback system is implemented interrupting the RF application in case of insufficient temperature increase (target temperature >60°C) or drop in impedance (>10%), and it is the choice of the operator manually to stop the ablation cycle by activating the foot switch. RF energy is applied for two minutes up to an output power of 8 watts.
THE ONESHOT™ RENAL DENERVATION SYSTEM (COVIDIEN)
The OneShot™ renal denervation system (Covidien, Campbell, CA, USA) uses a flexible spiral monopolar electrode material painted onto a balloon catheter allowing a 360° ablation (Figure 1D). The balloon is introduced over a standard 0.014 inch guidewire and is designed to fit through a 7 Fr or 8 Fr guide catheter. Three balloon sizes of 5 mm, 6 mm and 7 mm are available for vessel diameters of 4 mm to 7 mm. Ablation is performed after a single placement and lasts 120 seconds for each artery.
THE VESSIX V2 RADIOFREQUENCY RENAL DENERVATION SYSTEM (BOSTON SCIENTIFIC)
The VessixV2 system (Boston Scientific, Natick, MA, USA) is an over-the-wire percutaneous angioplasty balloon catheter (Figure 1E) with electrical gold contacts and thermistors mounted on the exterior of the balloon catheter which is connected to an RF generator. The Vessix V2 system utilises bipolar RF energy and therefore a ground pad is not needed. The catheter is available in balloon diameters of 4 mm, 5 mm, 6 mm and 7 mm, with a balloon length of 25 mm, a therapy delivery length area of 21 mm and a total working length of approximately 90 cm. The Vessix V2 generator delivers RF energy to all electrodes simultaneously (68°C).
THE PARADISE® ULTRASOUND RENAL DENERVATION SYSTEM (RECOR)
The Paradise® catheter (ReCor Medical, Palo Alto, CA, USA) (Figure 1F) is available in four balloon sizes (5 to 8 mm) for renal artery diameters 4-8 mm. The Paradise® ultrasound passes through the vessel lumen to target renal nerves, and a cylindrical heating profile provides treatment uniformity. The Paradise® catheter includes hydro-cooling balloon technology to cool close-by tissue and to position the transducer accurately.
Description of the procedure of RDN
HANDLING PATIENTS’ EXPECTATIONS
As in the case of any intravascular intervention, patients must sign a special “informed consent” form after a detailed description of the interventional procedure. One should discuss with the patient that, according to the current data, one out of five patients is a non-responder with an office-based BP fall <10 mmHg six months after treatment34. It should be made clear that the main goal is not to reduce antihypertensive background medication but to control hypertension per se.
It is also important to explain that the BP lowering effect may be observed as early as at one month or may take up to six months after the procedure2-7. As in any interventional procedure, the possibility of vascular and access-site complications cannot be excluded35,36. Relevant changes of renal function or development of de novo renal artery stenosis have not been reported2-7.
PREPROCEDURAL MODIFICATIONS OF THERAPY
According to principles of good clinical practice, all agents which could unfavourably affect renal function (such as non-steroid anti-inflammatory drugs, high-dose diuretic therapy, metformin in diabetics) should be avoided for at least 48 hours before the procedure, and appropriate hydration for intravascular volume substitution prior to contrast medium injection is recommended. The patients are advised to continue regularly receiving their antihypertensive medications along with the concomitant treatment for associated conditions (i.e., mainly statins and aspirin or other antiplatelet agents), including the day when the procedure is scheduled.
PREPARATION IN THE CATHETERISATION LABORATORY
The patient follows the same routine preparation regarding the sterilisation of the groin and procedural monitoring (including ECG, intra-arterial BP, and oxygen saturation). Depending on the catheter system used, a dispersive electrode (ground pad) is placed on the thigh or other non-bony area out of the angiogram field.
ANTICOAGULATION - NITRATES/VASOACTIVE AGENTS
No standard protocol of medical concomitant medication has been tested, and so a wide variation from centre to centre is observed. There is general agreement that a heparin bolus of 3,000 to 5,000 units should be administered intravenously in order to obtain adequate anticoagulation with a target ACT of >250 seconds. After engagement of the main renal artery, nitroglycerine can be injected directly to the artery to avoid spasm.
There is a general recommendation that, if a patient is not already on antiplatelet treatment, aspirin 500 mg (or 300 to 600 mg clopidogrel in case of aspirin intolerance) should be started with a maintenance dose given for at least four weeks, but no hard evidence exists for this recommendation.
MANAGEMENT OF THE PAIN
Before initiation of the RDN, intravenous analgesic and sedative medications are given to handle the diffuse visceral pain during the radiofrequency delivery. The presence of an anaesthesiologist is not generally necessary but in some countries it is required. Delivery of 2 to 6 l/min of oxygen and continuous measurement of oxygen saturation are advisable. In most cases, administration of midazolam (start with 2.5 mg, continue with 1-1.5 mg bolus before energy delivery and repeat doses up to 10-15 mg in total) and fentanyl (start with 0.05-1 mg and continue with 0.05-1 mg bolus before energy delivery) or morphine (start with 5 mg and continue with 2-3 mg bolus before energy delivery) is adequate.
AORTOGRAPHY AT THE LEVEL OF RENAL ARTERIES
After introducing a 6-9 Fr sheath (depending on the selected ablation system) into the femoral artery, an aortography is performed in order to check anatomic eligibility for RDN and to select the guiding catheter. In most cases, a renal double curve (RDC1) is the best choice while, in cases with low take-off angles and downward direction of the ostium, an internal mammary artery shaped catheter (IMA) is preferable.
RENAL ANGIOGRAM
Images of the main renal arteries are recorded using a non-ionic contrast (diluted 1:1 or 1:2) in order to assess anatomy and dimensions further. Exposure to contrast should be minimised to avoid contrast-induced nephropathy, particularly in patients with relatively low glomerular filtration rate. The 20° to 30° left anterior oblique view seems to be the best for imaging of the origin of renal arteries. The anterior-posterior view usually displays the course of the renal artery better without foreshortening.
INSERTION AND MANIPULATION OF THE ABLATION CATHETER
After confirmation of suitable anatomy for RDN, the appropriate type of specially designed ablation catheter engages the ostium of the renal artery under fluoroscopic guidance to ensure back-up and to avoid disengagement during the introduction of the selected ablation device.
The distal part of the catheter is positioned proximal to the bifurcation into the main segmental arteries. Up until now, there is no method to visualise or locate renal sympathetic nerves and thus to identify the target ablation site, although there are preliminary results from animal studies using electrical intravascular stimulation37. It is important for the technical success and safety of the ablation to find the right position to ablate and always to treat distal to proximal without re-crossing previously treated sites. The ablation takes place in a similar fashion for both renal arteries.
Methodology according to RDN systems
THE SYMPLICITY SYSTEMS
THE SYMPLICITY™ FLEX RADIOFREQUENCY RENAL DENERVATION SYSTEM (MEDTRONIC)
Interventional steps: femoral approach
Once the denervation catheter through an appropriate 6 Fr guiding catheter has advanced into the distal renal artery main trunk close to its bifurcation, the catheter tip has to be deflected, pulling the lever on the handle, and impedance has to be checked. The absolute impedance value indicating a proper vessel wall apposition of the electrode ranges from 220 to 320 Ω and must be stable (±5 Ω) before starting the delivery of energy for 120 seconds. It is recommended to start the denervation procedure in an inferior orientation of the catheter tip and, by pulling and rotating the catheter tip, to perform 4-8 focal treatments with a distance of ≥5 mm between each location. The last ablation should be applied close to the renal artery origin in a superior position.
THE SYMPLICITY™ SPYRAL RADIOFREQUENCY RENAL DENERVATION SYSTEM (MEDTRONIC)
Interventional steps: femoral approach
After having positioned the appropriate renal guiding catheter, the 0.014 inch guidewire has to be introduced into the renal artery distal to the hilum. Thereafter, the Spyral catheter is advanced into the distal renal artery trunk and the guidewire is pulled back into the Spyral catheter, which now assumes its helical shape. After verifying proper wall contact of all electrodes the one-minute RF application cycle has to be started by activating the foot switch. If renal artery length is appropriate (>20 mm), the catheter can be pulled back and slightly rotated, followed by a second RF application. Finally, the guidewire is again pushed out of the catheter, resulting in straightening of the Spyral catheter.
THE ENLIGHTN™ RADIOFREQUENCY RENAL DENERVATION SYSTEM (ST. JUDE MEDICAL)
Interventional steps: femoral approach
The initial EnligHTN™ basket positioning should be proximal to the bifurcation. The basket is expanded and the generator diagnostic check for electrode contact performed. The ablation is then undertaken with simultaneous delivery of energy to four electrodes for one minute (by using the second-generation generator). For a second ablation set, the basket should be collapsed, retracted by 1 cm, and the basket rotated by ~45 degrees and expanded. Following the contact check, the second run of ablation is performed. It is recommended, if anatomically possible, to perform two sets of ablations per renal artery.
THE IBERIS™ RADIOFREQUENCY DENERVATION SYSTEM (TERUMO)
Interventional steps: radial approach
Using a dedicated radial 6 Fr introducer sheath (e.g., Glidesheath® Slender™; Terumo Corp.), a 135 cm long multipurpose catheter (Climber™; Terumo Corp.) has to be advanced and, thereafter, a hydrophilic coated 0.035 inch guidewire can be inserted into the renal artery. As a next step, the guidewire is exchanged for the Iberis™ unipolar electrode catheter which has to be positioned at the distal end of the renal artery trunk. Deflecting the flexible tip by pushing the lever on the handle should guarantee a stable vessel wall apposition of the electrode to create a spot lesion successfully using RF energy. The pattern of denervation should be helical with a 5 mm distance between two spots of ablation. The helical pattern is achieved by a manual rotation of the handle. There is no cooling mechanism during the procedure and the electrode generates a temperature up to 70°C with an 8 watts delivering energy level for a two-minute ablation point.
Interventional steps: femoral approach
The short Iberis™ catheter system is designed for transfemoral application. The interventional steps are similar to those described for the Symplicity™ Flex system.
THE ONESHOT™ RENAL DENERVATION SYSTEM (COVIDIEN)
Interventional steps: femoral approach
The correct-sized renal guiding catheter is positioned, and the balloon catheter is introduced over a 0.014 inch guidewire in the renal artery. Its position is verified using the two radiopaque markers at either end of the electrode. The balloon is inflated and the generator is activated, delivering energy for two minutes. The balloon is then deflated and the procedure is repeated in the other renal artery.
THE VESSIX V2 RADIOFREQUENCY RENAL DENERVATION SYSTEM (BOSTON SCIENTIFIC)
Interventional steps: femoral approach
The 8 Fr introducer (8 Fr Ansel 45 cm; Cook Medical, Bloomington, IN, USA) is placed near the ostium of the artery, and the appropriate V2 catheter diameter (4-7 mm) according to the renal artery diameter (3-7 mm) is selected. The V2 catheter within the renal artery is inflated to exactly 3.0 atm. The V2 generator provides treatment (~1 watt for 30 seconds), with the use of impedance to determine apposition of the balloon catheter’s electrodes to the artery wall.
THE PARADISE® ULTRASOUND RENAL DENERVATION SYSTEM (RECOR)
Interventional steps: femoral access
Through a 7 Fr sheath an appropriate guiding catheter can be placed into the renal artery origin. The Paradise® catheter is advanced and placed just proximal to the renal artery bifurcation and, if the balloon is not occlusive, it is changed to the next largest catheter size. The transducer should be in the centre of the balloon and in parallel with the artery before treating. While flushing with cooling solution the catheter is automatically inflated to 2 atm and the ultrasound energy is emitted circumferentially for 30 seconds. After initial energy delivery, the balloon catheter is automatically deflated, pulled back about 10 mm and checked for balloon occlusion of the artery using contrast injection.
FINAL RENAL ANGIOGRAM
Any signs of renal artery abnormalities (vasospasm, stenosis or dissection) should be inspected after non-ionic contrast injection. Minor irregularities at the treatment sites (ablation notches), mainly due to intimal oedema/spasm, are regular and normally disappear within hours after treatment. OCT studies in a porcine model as well as in humans have demonstrated that local and diffuse vasospasm, oedema formation and endothelial injury with thrombus formation are present acutely post-RDN although not with apparent clinical significance32,36.
REMOVAL OF THE SHEATH - DISCHARGE OF THE PATIENT
Finally, the sheath is removed, and either manual compression or commercialised closure devices can be used to achieve haemostasis at the puncture site according to the centre’s standard of care. After the procedure, the patient is transferred to a recovery unit. In some cases, transient orthostatic hypotension and bradycardia have been reported without need for any special medical support. The patient is instructed not to reduce the antihypertensive drugs by him/herself, and BP is closely monitored by the family physician and potentially also by the patient. Alteration in antihypertensive medication should only be made if clinically indicated.
ORGANISING THE RDN TEAM
The affiliation of an interventional RDN centre with a hypertension excellence centre is a major requirement to ensure appropriate selection of candidates and follow-up (Table 3). RDN centres should have adequate experience of percutaneous renal stenting, management of vascular complications and availability of duplex ultrasound, high quality CTA/MRA equipment. Interventional vascular procedures are associated with complications that in general are inversely related to operator and institutional volume. The eligible centres for RDN should have a minimal activity of 25 RDN interventions per year.

Conclusion
In summary, RDN represents a significant advancement in the treatment of RHTN. It is a straightforward, well tolerated and generally safe procedure. However, there are many unanswered questions, and there is a continuous need for further clinical trials (Table 4). RDN should be performed by physicians with sound experience in catheterisation and endovascular techniques. RDN and its technical evolutions (improved catheter design, new treatment modalities) generate considerable medical interest enriching our modern antihypertensive treatment armamentarium.

| Impact on daily practice Percutaneous catheter-based transluminal renal denervation (RDN) provides a new and effective approach for the therapy of drug-resistant hypertension. Selection of the appropriate candidates for RDN is based on careful clinical and laboratory examinations and one of the key points is the anatomic eligibility of the renal artery. Since there are numerous devices for RDN, the interventionalist in daily clinical practice should be familiar with the methodology of each system. |
Conflict of interest statement
C. Tsioufis has received a research grant and lecture honoraria from St. Jude Medical, and travel expenses from Medtronic. F. Mahfoud is supported by Deutsche Hochdruckliga and the Deutsche Gesellschaft für Kardiologie, has received scientific support from Medtronic, St. Jude, Vessix and ReCor, and has received speaker’s honoraria from Medtronic, St. Jude and Vessix. G. Mancia has received lecture honoraria from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis, Servier and Takeda. J. Redon served as a lecturer for Boehringer Ingelheim, MSD, Daiichi Sankyo, Menarini, and Takeda, and was a member of the advisory group for Daiichi Sankyo, Menarini, Takeda, and Novartis. T. Zeller reports receiving advisory board fees from Medtronic, Boston Scientific, and Gore, and lecture honoraria from Cook, Bard, Medtronic, Biotronik, ReCor, Covidien and Bayer-Medrad. R. Schmieder has received grants, lecture honoraria and consultancy fees from Medtronic. B. Damascelli has no conflicts of interest to declare.




