Abstract
Aims: Assessment of intermediate coronary lesions can be done with fractional flow reserve (FFR) and intravascular ultrasound (IVUS). There are no randomised trials and only a small registry from one centre is available but this is subject to important bias. We sought to evaluate the clinical outcomes of an FFR strategy compared with an IVUS strategy for intermediate lesion assessment.
Methods and results: We compared the outcome of patients assessed with FFR and IVUS in two centres with a differential approach. After propensity score matching 400 pairs of patients were included. Revascularisation was done when FFR was <0.75 or minimum lumen area was <4 mm2 in vessels >3 mm, and <3.5 mm2 in vessels 2.5-3 mm, along with plaque burden >50%. After FFR and IVUS, 72% and 51.2% of lesions, respectively, were left untreated (p<0.001). At one and two years no significant differences in MACE-free survival were observed in overall groups (97.7% at one year and 93.1% at two years in the FFR group and 97.7% at one year and 95.6% at two years in the IVUS group; p=0.35) and among those with deferred intervention (97.9% at one year and 94.2% at two years in the FFR group and 96.5% at one year and 93.6% at two years in the IVUS group; p=0.7).
Conclusions: IVUS and FFR may be safely used to defer revascularisation of intermediate lesions. IVUS induces a higher degree of revascularisation but much lower than previously reported and does not affect the clinical outcome.
Introduction
Adjunctive diagnostic techniques are required because of the well-known limitations of angiography to make an accurate evaluation of coronary anatomy1.
The assessment of coronary segments showing ambiguous or intermediate stenosis severity can be done with pressure-derived fractional flow reserve (FFR), which yields functional data, or with intravascular ultrasound (IVUS), which provides morphological evaluation.
Based on trials using FFR this tool has come to be the reference standard for the physiological assessment of ambiguous lesions2-7. There is a well-validated cut-off value for FFR of 0.75-0.8. IVUS has also been used in this setting based on a suggested cut-off value for the minimum lumen area (MLA) of 4 mm2 obtained from correlation with non-invasive tests for ischaemia and an excellent outcome in those with deferred intervention8-10. However, a very modest correlation between MLA and FFR has been observed in multiple studies11-17.
There are no trials comparing both strategies and there is only a registry of 167 patients from one institution which reported a 91.5% vs. 33.7% lesion-treatment rate after IVUS and FFR examination18. The selection bias, the MLA cut-off of 4 mm2 regardless of vessel size and the small sample of the study imply important limitations.
The aim of this study was to compare clinical outcomes in large groups of patients matched by means of propensity score from two institutions with a differential diagnostic approach based on IVUS or FFR. The IVUS-derived MLA cut-off was tailored to the vessel size.
Methods
In two public tertiary and university medical centres with a similar population profile, PCI volume and DES penetration, the strategy for intermediate lesion evaluation has been different in previous years: in one it was based mostly on IVUS and in the other it was based mostly on FFR.
In a six-year period in each hospital, all consecutive patients who were assessed with the reference tool IVUS or FFR to decide PCI in non-LM intermediate lesions were included. An intermediate lesion was defined as a visual angiographic diameter stenosis of 40-70%. Only de novo stenoses were assessed and left main coronary artery lesions were excluded for the purpose of this study since the latter were evaluated with IVUS in the vast majority of cases at both centres.
Angiographic analysis
Quantitative coronary angiography analysis was performed by validated and automated edge-detection software. The minimum lumen diameter was measured from the angiographic projections with the tightest stenosis. The reference diameter was measured from an angiographically normal segment proximal to the lesion or distal for ostial lesions. Angiographic projections were always performed after administration of intracoronary nitroglycerine.
IVUS imaging and online analysis
IVUS assessment was performed using solid state or phased array catheters with the s5 Imaging System console (Volcano Corp., Rancho Cordova, CA, USA). Automatic pullback was performed at 0.5 mm per second from a point at least 10 mm distal to the end of the lesion. Intracoronary nitroglycerine was administered prior to image acquisition. After imaging acquisition the lumen-intima and media-adventitia interfaces were planimetered at the target site and the following measurements were obtained: 1) MLA; 2) minimum and maximum lumen diameters; 3) external elastic membrane cross-sectional area (EEM CSA); 4) plaque plus media CSA; and 5) plaque burden (calculated as plaque plus media CSA divided by the EEM CSA). The criteria for revascularisation were a minimum lumen area (MLA) below 4 mm2 in vessels with a reference diameter >3 mm and a MLA <3.5 mm2 in vessels with reference diameter 2.5-3 mm, always with a plaque burden over 50%. The use of 4 mm2 was based on aforementioned studies reporting a correlation between IVUS-derived MLA and non-invasive tests for ischaemia8-10. The use of 3.5 m2 for smaller vessels was empirically established following the rationale of a tailored MLA cut-off related to the vessel size. This has proven to be more appropriate according to recently published studies addressing the correlation between IVUS and FFR11-17.
Pressure wire protocol
After deciding to perform a functional study of the lesion, 200 to 300 μg of nitroglycerine was administered through the catheter guidewire. The functional evaluation was performed with a 0.014 inch intracoronary pressure wire (Pressure-Wire™; St. Jude Medical Systems AB, Uppsala, Sweden, or Volcano Primewire™; Volcano Corp., Rancho Cordova, CA, USA). The guidewire was calibrated externally and then advanced to the distal end of the guiding catheter while verifying the equality of the pressure curves in the catheter and the pressure wire. The guide was advanced until the sensor was located at least 20 mm distal to the lesion being studied. The FFR was obtained by administering 300 to 500 μg of intracoronary adenosine, while taking special care to avoid wedging the catheter in the coronary ostium after bolus injection of the drug. The use of these high dosages has been shown to achieve equivalent or even greater degrees of hyperaemia compared to the intravenous route19. The beat-to-beat ratio of the mean aortic pressure at the end of the guide catheter and the pressure distal to the lesion, obtained via the pressure wire in a situation of maximum hyperaemia, was used to calculate the FFR. At least three FFR determinations were made, and the lowest FFR was used for decision making. A maximum dose of 1,200 μg of intracoronary adenosine was used as long as a lower dose did not produce a period of asystole ≥6 seconds. Lesions with an FFR ≥0.75 were not revascularised. In the case of tandem lesions, the FFR was obtained distal to both lesions. When FFR was <0.75, PCI of the more narrowed lesion was carried out (in case of equal stenosis, the longer), and afterwards a new FFR measurement was conducted for the remaining lesion.
Patients with both techniques used for assessing the same lesion were excluded. Revascularisation strategy could be either percutaneous (with drug-eluting stents or bare metal stents at the operator’s discretion) or surgical. Cardiac enzyme determination was not systematically done after the procedure except in those cases with clinical indication based on angiographic results or subsequent symptomatic and electrocardiographic evolution. All baseline clinical, angiographic and procedural characteristics from all patients were collected and clinical follow-up was conducted.
The two databases were pooled and analysed at the co-ordination centre, Hospital Universitario Marques de Valdecilla, Santander, Spain, by two investigators. The same definitions were applied for cardiac adverse events in order to guarantee a homogeneous event adjudication process.
Clinical endpoints
The primary endpoint of the study was a composite of major adverse cardiac events (MACE) including cardiac death, target lesion myocardial infarction (MI) and target lesion revascularisation (TLR) at 12 months. MACE at 24 months and the incidences of each individual event were considered as secondary endpoints. The target lesion was defined as the lesion evaluated in the index procedure with IVUS or pressure wire, with or without subsequent treatment.
The following major adverse cardiac events were defined: all-cause death and cardiac death (including any sudden death by undefined cause); myocardial infarction, defined as a typical increase and gradual fall (troponin), or as a faster increase and fall (CK-MB) of biochemical markers for myocardial necrosis along with at least one of the following: chest pain, new ST-T changes or new left bundle branch block, development of pathological Q-waves, new regional wall motion or perfusion abnormalities and finally intracoronay thrombus detection in angiography or autopsy. Target-lesion-related MI was defined as an infarction attributed to the target lesion either after angiographic confirmation or by detection of electrocardiographic or wall motion abnormalities suggesting potential involvement of the target lesion. Periprocedural MI was included in this endpoint. TLR of the deferred lesion was defined as revascularisation by means of PCI or CABG of the lesion assessed in the index procedure with IVUS or pressure wire. TLR of lesions treated after IVUS or pressure wire examination was defined as revascularisation upon a restenotic lesion, including the stent and the 5 mm of vessel adjacent to the stent.
Statistical analysis
Continuous variables are presented as mean±standard deviation. Categorical variables are expressed as percentages. Continuous variables were compared with the t-test if they followed a normal distribution and with Wilcoxon tests when they did not (assessment of type of distribution by the Kolmogorov-Smirnov test). The categorical variables were compared with chi-square test or Fisher’s exact test, according to indication. Survival free from MACE was analysed with Kaplan-Meier curves.
We performed adjustment for differences in clinical, angiographic and procedural characteristics (Table 1, Table 2) using propensity score matching. The propensity scores were estimated using logistic regression in which intracoronary diagnostic assignment was used as the outcome variable and the covariates (age, gender, hypertension, smoker, diabetes mellitus, hyperlipidaemia, previous PCI, previous MI, previous CABG, left ventricular ejection fraction, stable angina, acute coronary syndrome, MI, multivessel disease, number of lesions, target vessel, reference vessel diameter, lesion stenosis, lesion length) as predictors. To perform the propensity score matching procedure, the PS Matching custom dialogue was used in conjunction with SPSS version 19 (http://sourceforge.net/projects/psmspss/files/; Thoemmes, 2012). The PS Matching programme performs all analyses in R through the SPSS-R Plugin (version 2.10.1). This procedure involved three stages:
1)The propensity scores were estimated using logistic regression in which the assessment tool was used as the outcome variable (FFR assessment versus IVUS assessment), and the clinical and angiographic covariates as predictors.
2) Patients were matched using simple one to one nearest neighbour matching, which is based on a “greedy” matching algorithm that sorts the observations in the FFR group by their estimated propensity score. It then matches each unit sequentially to a unit in the IVUS group that has the closest propensity score. In order to exclude bad matches we imposed a caliper of 0.2 of the standard deviation of the logit of the propensity score. Units outside the area of common support (defined as the region of the distributions of estimated propensity scores in the IVUS and FFR group for which units in both groups are observed) were disregarded. This was done to improve the balance of the covariates.
3) A series of model adequacy checks was performed to check whether balance on the covariates was achieved through the matching procedure. This was done by computing the global imbalance measure and through the production of five diagnostic plots: (a) histograms of the propensity scores in both groups before and after matching, (b) a dot-plot of individual propensity scores of units in the control and treatment groups, either matched or unmatched, (c) histograms of the standardised differences of all terms (covariates, quadratic terms, interactions) before and after matching, (d) a dot-plot which displays the magnitude of the standardised differences before and after matching for each covariate, and (e) a line-plot of standardised mean differences before and after matching. A p-value <0.05 was considered as statistically significant. All statistical analyses were performed using SPSS version 19 for Windows.
Results
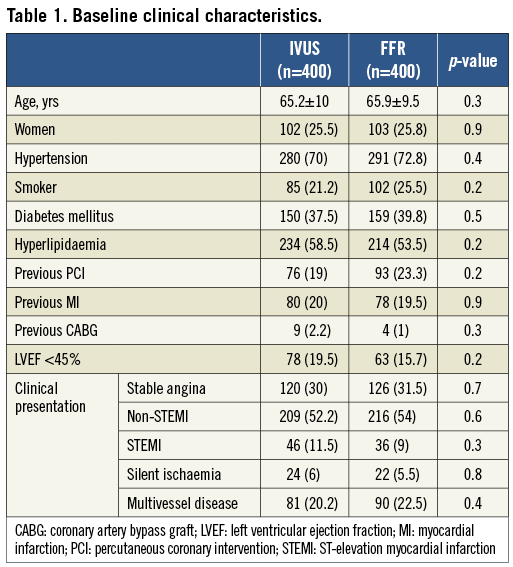
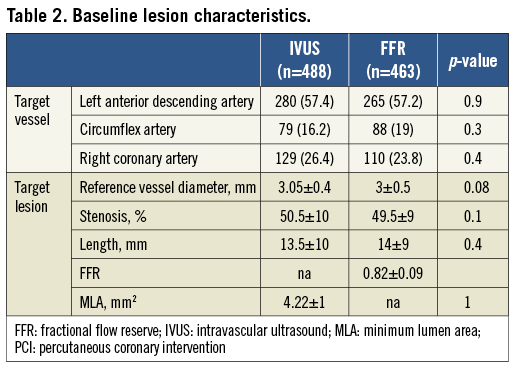
A total of 400 consecutive patients with 463 lesions were included in the FFR group and 530 consecutives patients with 653 lesions in the IVUS group. After performing the propensity score procedure we ended up with 400 patients in both groups. In the IVUS group a total of 488 lesions were assessed and in the FFR group a total of 463 lesions. The baseline clinical characteristics of both groups are presented in Table 1. Both groups were well balanced with no significant differences. The majority of patients were not evaluated in the context of stable angina. Those patients with acute coronary syndrome had IVUS or pressure wire evaluation over non-culprit intermediate lesions. In Table 2 angiographic and procedural characteristics are described which reveal no significant differences between groups. The most common target vessel was the left anterior descending artery. No significant differences were observed in the lesion profile. Similar stenosis, length and reference vessel diameter values were obtained in the FFR and IVUS groups. PCI in non-target lesions was carried out in a similar proportion (35.9% in the IVUS group and 30.4% in the FFR group; p=0.1).
The strategy followed by the operator after IVUS or FFR assessment is presented in Figure 1. The rate of patients undergoing revascularisation was significantly greater in the IVUS group as compared to the FFR group (48.8% vs. 28%; p<0.001). Otherwise, the revascularisation techniques used in both groups were similar, the percutaneous being the most commonly used. The total number of stents implanted was 1.06±1.4 in the IVUS group vs. 0.84±1.3 in the FFR group (p=0.02). The vast majority of stents implanted in both groups were drug-eluting stents either on target or non-target lesions (overall lesions treated with DES 76% in the FFR group vs. 78% in the IVUS group; p=0.6). The distribution of DES types was quite similar as well, with around two thirds of cases being treated with second-generation DES in both groups.
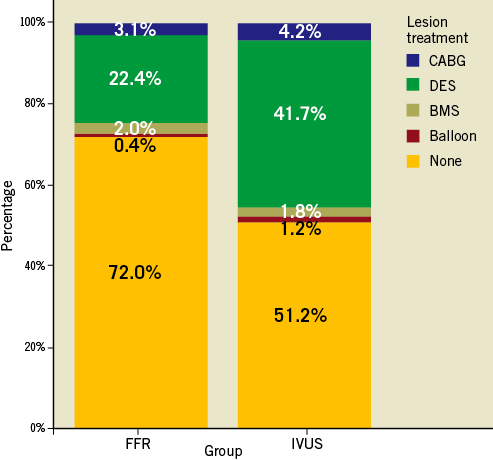
Figure 1. Treatment choice for lesions evaluated in FFR-guided vs. IVUS-guided groups.
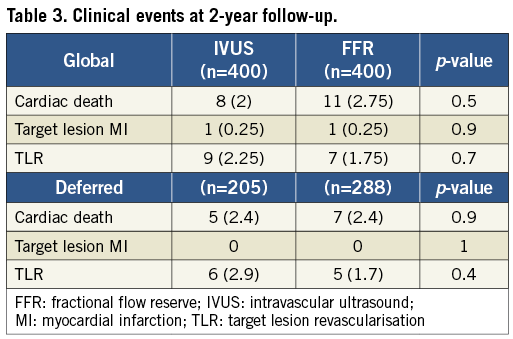
The pre-specified events reported during the two-year follow-up period are shown in Table 3. The clinical outcome of patients with deferred intervention over the index lesion is presented separately in Table 3. Event-free survival curves for the overall and deferred groups are depicted in Figure 2. At one and two-year follow-up no significant differences were observed in the composite endpoint including cardiac death, target lesion MI and TLR either in overall groups (97.7% at one year and 93.1% at two years in the FFR group and 97.7% at one year and 95.6% at two years in the IVUS group; p=0.35) or in those with deferred intervention (97.9% at one year and 94.2% at two years in the FFR group and 96.5% at one year and 93.6% at two years in the IVUS group; p=0.7). Regarding stent thrombosis, two definite cases, one in each group, were reported.
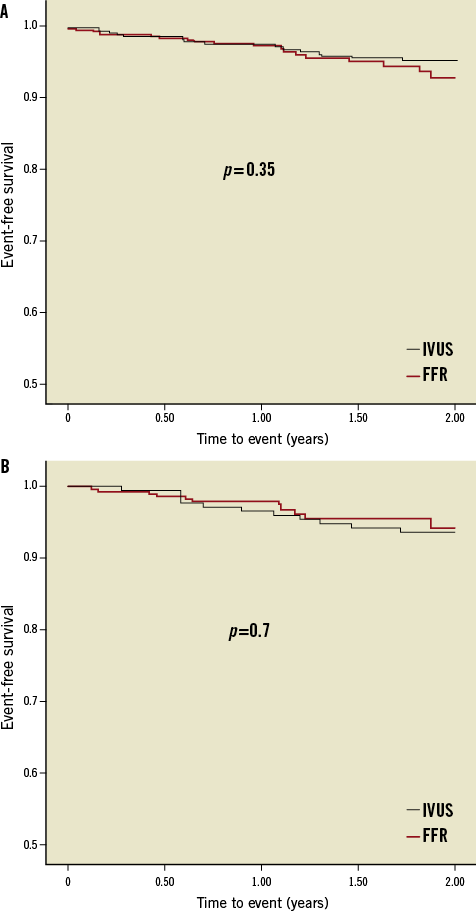
Figure 2. Kaplan-Meier event-free survival curves for the composite endpoint (cardiac death, target lesion MI and TLR). A) Overall groups. B) Deferred subgroups.
Discussion
The main findings of the present study are: 1) the assessment of non-left main intermediate coronary lesions with IVUS induced a higher degree of revascularisation compared to FFR but much lower than that previously reported; 2) a very low rate of lesion-related events in deferred cases was observed with both strategies; 3) the higher rate of revascularisation promoted using IVUS had no effect on outcomes.
The assessment and proper approach to intermediate coronary artery lesions remain challenging for interventional cardiologists. Several studies have already proven the usefulness of fractional flow reserve in the evaluation of ambiguous lesions, mostly in stable patients but also in unstable condition2-5,20. Thus, so far, this method is considered the gold standard technique. Moreover, the identification of lesions causing ischaemia is crucial to provide a clinical benefit with revascularisation to patients with stable angina as was shown in the FAME-2 trial21.
Intravascular ultrasound has been used for the same purpose based on studies demonstrating an adequate correlation with non-invasive tests for ischaemia and the excellent outcome observed in those with deferred intervention8-10. However, a very modest correlation between MLA and FFR has been observed in multiple studies11-17. One of the advantages of IVUS is to provide helpful information to guide the eventual revascularisation procedure.
To our knowledge there are very few studies that compare the clinical outcomes of these two different approaches. Nam et al conducted a clinical study of 167 consecutive patients from the same centre who were assessed with either FFR or IVUS to decide revascularisation18. They reported a strikingly remarkable difference in the rate of revascularisation (33.7% after FFR and 91.5% after IVUS; p<0.001) and a comparable one-year clinical outcome.
In our study we chose to include patients from two different institutions, one that predominantly used FFR and another that more often utilised IVUS in the study period. We presumed this design could minimise the selection bias generated when choosing one or other diagnostic tool in the same centre. Moreover, in order to reduce further this bias we decided to perform a propensity score matching. We matched each FFR patient to an IVUS patient and performed all the analysis afterwards.
In our study we have observed something that almost every invasive cardiologist has in mind, namely that when using IVUS to decide whether or not to treat a lesion you are going to treat more than if you employ FFR. This had already been pointed out in the above-mentioned study18 but, in our analysis, even though the difference was statistically significant (48.8% vs. 28%, p<0.001), it was not as wide as has been previously found (91.5% vs. 33.7%, p<0.001). Two factors could explain the remarkable differences between this and our study. First, they used an MLA of 4 mm2 as the only criterion for deciding revascularisation and, second, the populations treated in this and our study were different, in that the mean MLA in all assessed lesions were 3.1 mm2 and 4.2 mm2, respectively. The observation of lower lumen dimensions for intermediate lesions and lower MLA cut-off values in correlation with FFR in East Asians compared to Westerners has been reported previously in an international registry22.
The proposal of an MLA of 4 mm2 as a universal criterion has been responsible for the reported striking excess of revascularisation after IVUS evaluation. Although previous studies suggested this threshold after correlation with non-invasive ischaemic tests, a more recent study has found a significantly lower value23. Moreover, many studies showing a correlation between IVUS and FFR have mostly found a lower value for the best cut-off MLA (2-3 mm2), with this value depending upon the reference vessel diameter12-17. We have used IVUS criteria based on MLA depending on vessel size (4 mm2 and 3.5 mm2 as cut-off values) with the requisite of plaque burden over 50%. If 0.5 mm2 lower MLA was applied for the cut-off values (<3.5 mm2 in vessels >3 mm and <3 mm2 in vessels 2.5-3 mm) the incidence of performing PCI might be decreased from 48.8% to 33.2% which is much closer to that of the FFR-guided group.
In our study, as in others, we found a very low rate of lesion-related events in the group with deferred intervention assessed either with IVUS or with FFR. However, we also found an equivalent outcome for the global population using both tools, which indicates a neutral clinical impact of the higher revascularisation rate induced by IVUS over FFR. In fact, the use of drug-eluting stents in intermediate lesions (generally with low or mid restenotic risk) allows a very low rate of target-lesion-related events24. Nevertheless, from an economic point of view, FFR resulted in a more cost-effective approach than IVUS.
In any case, IVUS might play a role in the evaluation of intermediate lesions as an alternative to the FFR tool when this one is not available or feasible, or when some morphological information could be useful to guide the eventual revascularisation procedure (e.g., calcified, bifurcated or ostial lesions).
Limitations
It is obvious that the non-randomised nature of the study represents its main limitation. We have tried to reduce the selection bias, unavoidable in a single-centre registry, comparing cohorts from different institutions showing a differential diagnostic approach to ambiguous lesions. IVUS and pressure wire were available in both centres, but the assessment of intermediate lesions during the study period was mostly done with IVUS in one centre and with pressure wire in the other. These institutions were both public tertiary and university medical centres with highly comparable PCI volume, DES penetration, patient population and approaches to treatment.
In addition, we matched the cohorts by means of a propensity score. However, we acknowledge that propensity matching may not take into account all of the differences between the two cohorts.
The adherence to the protocol among the operators was very high in both institutions (98% with FFR and 95% with IVUS) with protocol deviations occurring in very few cases showing borderline values for MLA or FFR.
FFR was obtained using intracoronary rather than intravenous high dose adenosine. However, the centre using FFR in this study has demonstrated that an intracoronary bolus dose >300 μg can be equal to or more effective than an intravenous infusion of adenosine in achieving maximum hyperaemia when calculating the FFR19.
The angiographic data were collected retrospectively and there was no angiographic core lab in this study. Different QCA programmes and many operators were involved. Patients were selected for IVUS or FFR examination after stenosis estimation by the operator at the time of the procedure, after visual and, eventually, QCA evaluation. This is the regular way in real practice.
In the IVUS group, it is highly probable that IVUS was also used to guide the procedure which could have had a positive influence on outcomes. However, this potential advantage should be taken as an inherent part of the IVUS-based approach.
Finally, cardiac enzymes were not routinely measured after interventions, which could have led to an underreporting of electrocardiographic and clinically silent peri-postprocedural infarctions, especially in the IVUS group in which more lesions were treated.
Conclusion
The assessment of non-left main intermediate coronary lesions with IVUS and FFR is a safe strategy with a very low rate of lesion-related events in deferred cases. The use of IVUS implies a higher degree of revascularisation as compared to the FFR approach but much lower than that previously reported. This excess of revascularisation could have been further attenuated using 0.5 mm2 lower MLA cut-off values tailored to the vessel size. The higher rate of treatment induced by IVUS did not influence the outcome.
Conflict of interest statement
J. M. de la Torre Hernandez has received a research grant from Abbott Vascular and honoraria for advisory panels and presentations from Medtronic, Abbott Vascular, Boston Scientific, Biotronik, Volcano, St. Jude, Lilly and AstraZeneca. R. López-Palop has received research grants from Abbott Vascular, Terumo and Medtronic, and honoraria for advisory panels and presentations from Medtronic, Abbott Vascular, Boston Scientific, Terumo, Volcano, St. Jude, Lilly and AstraZeneca. The other authors have no conflicts of interest to declare.




