- *Corresponding Author:
- S. Mustafa
Department of Chemistry, Tagore Govt. Arts and Science College, Lawspet, Puducherry-605 008, India
E-mail: mustaff_02@yahoo.co.in
| Date of Submission | 28 December 2016 |
| Date of Revision | 07 November 2017 |
| Date of Acceptance | 06 June 2018 |
| Indian J Pharm Sci 2018;80(4):772-776 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
2-(dimethylamino)-3-(5-(pyridin-2-yl)-1H-tetrazol-1-yl)naphthalene-1,4-dione (2) has been synthesized. The UV/Vis studies of 2-(dimethylamino)-3-(5-(pyridin-2-yl)-1H-tetrazol-1-yl)naphthalene-1,4-dione (2) with different metal ions were performed in aqueous dimethylformamide and were correlated with fluorescence studies of the same. The compound showed higher selectivity and sensitivity for Zn2+ ions. Thus this compound could be utilized as a turn-on probe for fluorescent imaging of Zn2+ ions in living cells.
Keywords
2,3-dichloronaphthalene-1,4-dione, pyridyl-tetrazole, fluorescence, UV/Vis studies, chemical probe, metal complex
Metals like Na+, K+ and Mg2+ play an important role in biological systems, which enables different processes like transmission of information[1-5]. d-Block metals such as zinc (Zn2+) and copper (Cu2+) play important structural and catalytic roles in neurology related diseases like neurophysiology and neuropathology. Accumulation of metals like Cd2+ in the brain leads to cancer[6]. This ion binds in high concentrations in brain systems. In case of brain injury, Zn2+ ions induce the selective neuronal cell deaths[7]. Even though the roles of various metal ions in biological systems are known, the full mechanism of the process is still unknown. Compared to conventional techniques like atomic absorption spectroscopy[8] and autoradiography[9], molecular imaging with fluorescent probes, genetically encoded sensors and hybrid probes are playing vital roles to label or sense the presence of metals[10]. In this view, various chemical probes are developed to study the fluorescence phenomena[11]. As per the existing literature, probes should not contain their excitation in UV range as they can either damage the cells or emit auto-fluorescence by biological system[12]. To overcome this defect, various groups developed different chemical probes, which are having excitation in long wavelength region[13-16].
The work reported by Raulin[17] and Frederickson et al.[18] left great encouragement for the development of chemical probes especially for the Zn2+ ions. Recently G-quadruplex protein binding probes were developed for the detection of specific biomarkers associated with various human diseases[19-22]. The use of different fluorophores like pyrazoline, cynanine and rhodamine dyes have been developed as various chemical probes to identify specific metal like Zn+2, which is the highest abundant metal after iron in the human body[23-29]. Bioinformatic researchers have also shown the importance of Zn+2 in the biological systems[30-32]. In the present work, a chemical probe was synthesized, which has an excitation in the visible region. The probe was allowed to complex various metal ions in aqueous dimethylformamide (DMF) and their UV/Vis and fluorescence properties were studied.
All reagents were purchased from Sigma-Aldrich and were used without further purification. DMF (AR grade) was used toperform analytical studies. Metal chloride salts procured from Merck were used as the source for metal ions. The solvents used in the synthesis were distilled before use. Absorption measurements were carried out using a Shimadzu UV-1800 spectrophotometer. Fluorescence spectra were recorded on a Perkin-Elmer LS55 spectrophotometer.
The slit width was 10 nm for both excitation and emission. All melting points were obtained on an Elico instrument, India (Model MP96) and were uncorrected. Mass spectra (MS) were obtained on a Pexciex API 2000 eV spectrometer; Q1MSQ1/auto injection mass spectra. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Top Spin instrument. Infrared spectra were recorded using an Alpha T OPUS spectrometer. Silica Gel 60 (60-120 mesh) was used for column chromatography.
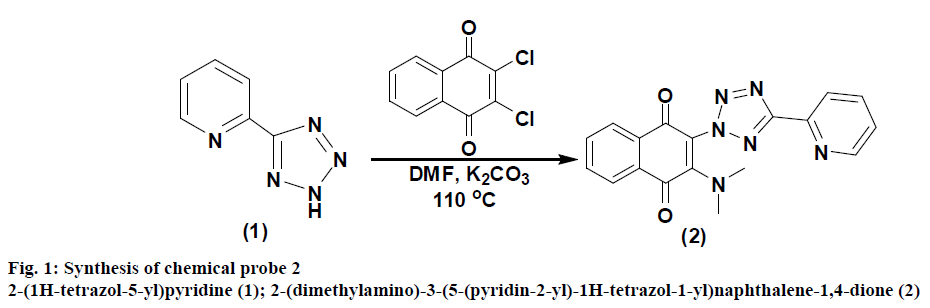
Synthesis of the compound 2-(1H-tetrazol-5-yl) pyridine (1) was carried out as per the available literature[33]. Synthesis of 2-(dimethylamino)-3- (5-(pyridin-2-yl)-1H-tetrazol-1-yl)naphthalene-1,4- dione (2) was carried out by adding to a solution of 1 (735 mg, 5 mmol) in DMF (20 ml) 2,3-dichloronaphthalene- 1,4-dione (1.1 g, 5 mmol) and K2CO3 (272 mg, 2 mmol) and the reaction mixture was stirred for 24 h at 110°. The crude mass was poured over ice and the compound was extracted with ethyl acetate (2×50 ml). The organic layer was dried over magnesium sulphate and evaporated under reduced pressure to yield the crude compound 2, which was purified by column chromatography using hexane and ethyl acetate (2:1 v/v) to afford pure compound 2 (1 g, 2.8 mmol) as a yellowish-brown solid.
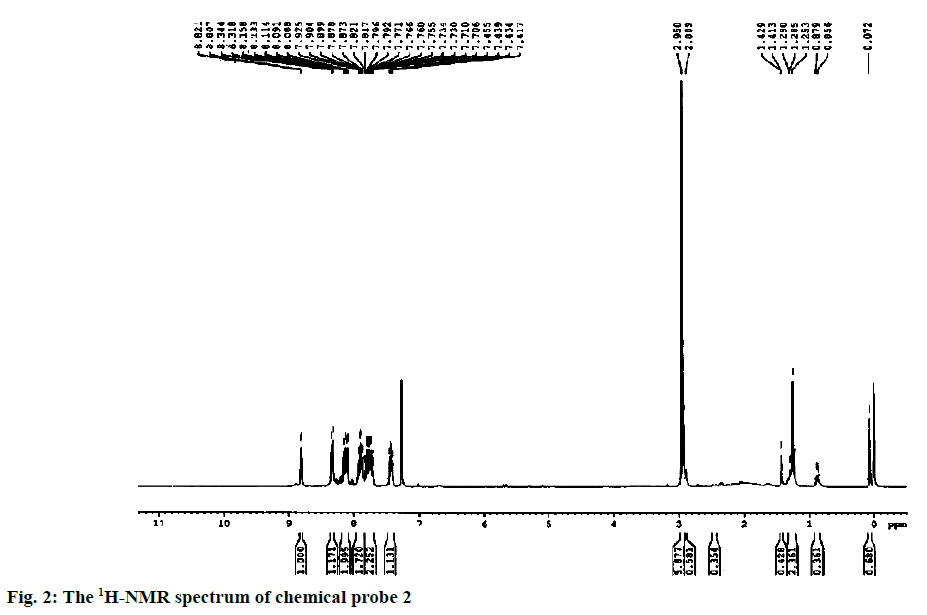
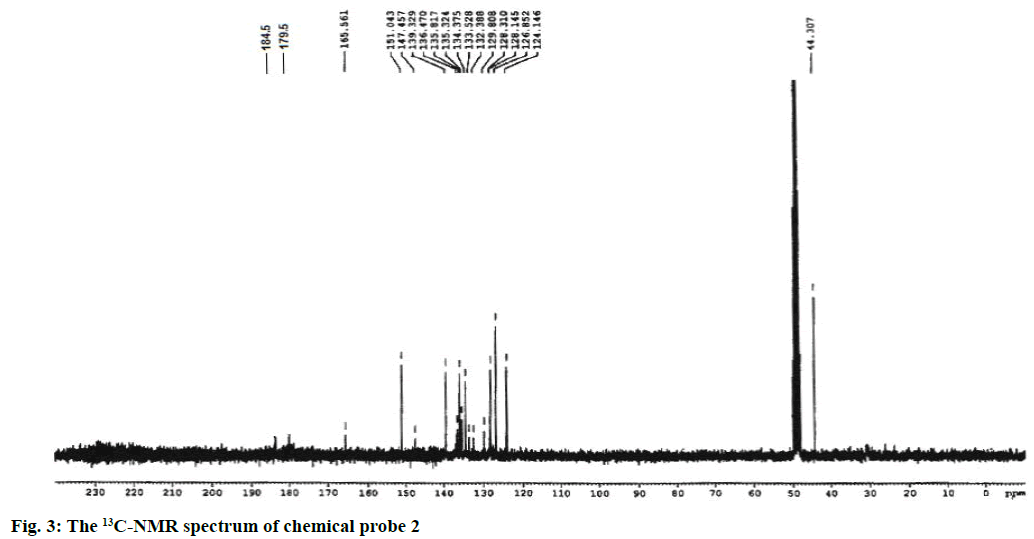
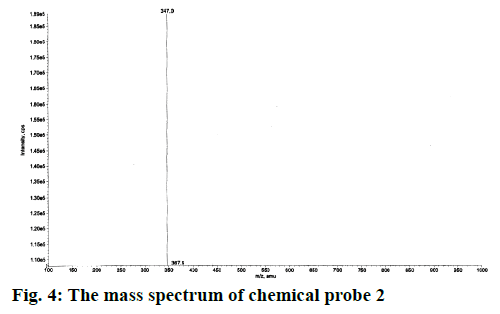
Yield of the compound 2 was 60 %; melting point: 87-90°; FTIR (KBr): ν (cm-1) 2947, 2846, 2753, 1686, 1598, 1254, 1158; 1H NMR (CDCl3, 300 MHz): δ (ppm) 8.81 (d, J=4.2 Hz, 1H, pyr-H2), 8.32 (d, J=7.8 Hz, 1H, pyr-H4), 8.18-8.08 (m, 2H, pyr-2H3,5), 7.88 (dt, J=7.8, 0.75 Hz, 1H, aromatic H1), 7.84-7.67 (m, 2H, aromatic H2,3),7.43 (dd, J=6.6, 1.5 Hz, 1H, aromatic H4), 2.95 (s, 6H, N(CH3)2);13C NMR(CD3OD, 75 MHz): δ (ppm) 184.5 (1C, C=O), 179.5 (1C, C=O), 165.5 (C-tetrazole), 151.0 (C2, pyr), 147.4 (C6, pyr), 139.3 (C4, pyr), 136.5 (C, benzo), 135.8 (C, aromatic), 135.3 (C, benzo), 134.3 (C, aromatic), 133.5 (C, aromatic), 132.4 (C, aromatic), 129.8 (C, aromatic), 128.2 (C, aromatic), 126.8 (C5, pyr), 124.1 (C3, pyr), 44.0 (aliphatic C2-N); ESI-MS (M+1)=347.
Metal chloride salts (Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+ and Mg2+) were used as the source for metal ions. Ten millimolar stock solutions of metal ions were prepared in distilled water. A quantity of 1 mM of chemical probe 2 in DMF/H2O was buffered with phosphate buffered saline (PBS, pH=7.4).
The synthetic route started from picolinonitrile by following the method available in the literature to form the tetrazole 1 and the structure was confirmed by comparing the analytical data of 1 with the reported data[29]. Tetrazole 1 on alkylation with 2,3-dichloronaphthalene-1,4-dione in dry DMF in presence of potassium carbonate at 110° afforded a single isomer by alkylation at N(2) position of tetrazole rings (Figure 1). The 1H NMR spectra of compound 2 showed characteristic pyridine peaks at δ 8.81, 8.32, 7.88 7.43 and those of naphthaquinone were at δ 8.18- 8.08, 7.84-7.67. The six protons for N,N-dimethyl group appeared at δ 2.95 as a singlet (Figure 2). The 13C NMR showed a characteristic peak at δ 165.5 (Figure 3) proved the alkylation at N2 position [29]. The absence of peak at δ 153, which was characteristic of N1 isomer is also proved the formation of N2 isomer. The ESI-MS showed (M+1) peak at 347 (Figure 4), which proved the structure of the compound.
The UV/Vis spectral studies were carried out with and without metal ions such as Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+and Mg2+. The chemical probe 2 contains naphthanone and tetrazole nitrogen atoms, so it can provide a platform for binding metal ions such as Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+ and Mg2+. The UV/Vis absorption spectra of chemical probe 2 in the presence of various metal ions Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+ and Mg2+ using their chloride salts in PBS buffer (20 mM pH 7.4) solutions (DMF:water = 2:8) are shown in Figure 5. The absorption spectrum of chemical probe 2 exhibited a broad band at 436 nm. The absorption maximum changed with the various metal ions such as Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+ and Mg2+ in aqueous DMF. Except Co2+ and Ba2+, the addition of other metal ions to chemical probe 2 showed an increase in absorption. Upon addition of Zn2+ ions, the absorption peak of chemical probe 2 at 436 nm was increased to a maximum extent compared to all other metal ions (Figure 5).
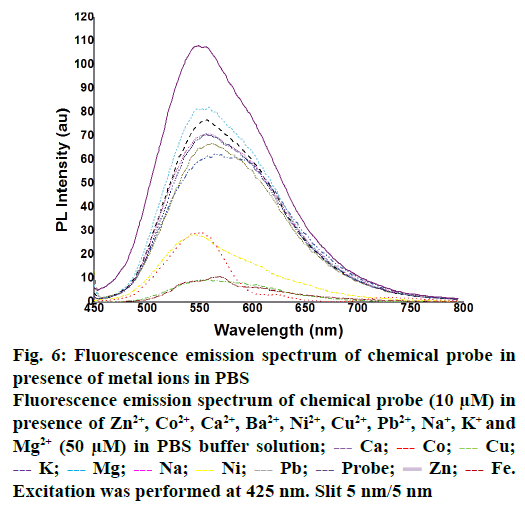
The fluorescence properties of chemical probe 2 at 436 nm were investigated in PBS buffer (20 mM pH 7.4) solution (DMF:water = 2:8). The emission maximum for all the complexes was observed at 549 nm. After addition of Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+ and K+ is not shown any significant change in emission. Whereas Zn2+ ions showed the more enhancement in the fluorescence intensity is shown in Figure 6. This observation is revealed that the formation of a complex between chemical probe 2 and Zn2+ ions and highest selectivity of chemical probe 2 to Zn2+ is disclosed. This can be assigned to π→π* transition and an intra-molecular charge transfer band from the naphthoquinone tetrazole moiety. This indicates the selectivity of compound 2 towards specific metal ion (Zn2+) with its high fluorescence emission peak. Therefore the compound 2 can be utilised as a turnon fluorescent probe for the detection of Zn2+ ions in living cells.
Figure 6: Fluorescence emission spectrum of chemical probe in presence of metal ions in PBS
Fluorescence emission spectrum of chemical probe (10 μM) in presence of Zn2+, Co2+, Ca2+, Ba2+, Ni2+, Cu2+, Pb2+, Na+, K+ and Mg2+ (50 μM) in PBS buffer solution;  Ca;
Ca;  Co;
Co;  Cu;
Cu;  K;
K;  Mg;
Mg;  Na;
Na;  Ni;
Ni;  Pb;
Pb;  Probe;
Probe;  Zn;
Zn;  Fe. Excitation was performed at 425 nm. Slit 5 nm/5 nm
Fe. Excitation was performed at 425 nm. Slit 5 nm/5 nm
Acknowledgements
Authors are thankful to the financial assistances from DST with project No.SB/FT/CS-034/2012 (SERB, New Delhi, India) and UGC with project F.No. 42-306/2013 (SR), New Delhi, India.
Conflict of interest
The authors declare no conflicts of interest.
References
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol 2000;4:184-91.
- Burdette SC, Lippard SJ. Meeting of the minds: Metalloneurochemistry. Proc Natl Acad Sci U S A 2003;100:3605-10.
- Debanne D. Information processing in the axon. Nat Rev Neurosci 2004;5:304-16.
- Clapham DE. Calcium signaling. Cell 1995;80:259-68.
- Que EL, Domaille, DW, Chang CJ. Metals in Neurobiology: Probing their chemistry and biology with molecular imaging. Chem Rev 2008;108:1517-49.
- Ciupa A, Mahon MF, De Bank PA, Caggiano L. Simple pyrazoline and pyrazole “turn on’’ fluorescent sensors selective for Cd+2 and Zn+2 in MeCN. Org Biomol Chem 2012;10:8753-7.
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci 1998;21:347-75.
- Assaf SY, Chung, SH. Release of endogenous Zn+2 from brain tissue during activity. Nature 1984;308:734-6.
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 1984;308:736-8.
- Carter KP, Young AM, Palmer AE. Fluorescent sensors for measuring metal ions in living systems. Chem Rev 2014;114:4564-601.
- Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, et al. In vivo visulationog gene expression using magnetic resonance imaging. Nat Biotechnol 2000;18:321-5.
- Mizukami S, Okada S, Kimura S, Kikuchi K. Design and synthesis of coumarin-based probes for ratiometric fluorescence imaging. Inorg Chem 2009;48:7630-8.
- Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. Novel zinc fluorescent probes excitable with visible light for biological applications. Angew Chem Int Ed Engl 2000;39:1052-4.
- Hirano T, Kikuchi K, Urano Y, Nagano T. Improvement and biological applications of fluorescent probes for zinc, AnAFs. J Am Chem Soc 2002;124:6555-62.
- Komatsu K, Kikuchi K, Kojima H, Urano, Y, Nagano T. Selective zinc sensor molecules with affinities for Zn+2, revealing dynamics and regional distribution of synaptically released Zn+2 in hippocampal. J Am Chem Soc 2005;127:10197-204.
- Nolan EM, Jaworski J, Racine ME, Sheng M, Lippard SJ. Midrange affinity fluorescent Zn (II) sensors of the inpyr family: synthesis, characterization, and biological imaging applications. Inorg Chem 2006;45:9748-57.
- Raulin J. Chemical studies on growth. Ann Sci Nat Bot Biol 1869;11:93-299.
- Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically relative zinc (bouton zinc) in the brain. J Neurosci Methods 1987;20:91-103.
- Wang M, Mao Z, Kang TS, Wong CY, Mergny JY, Leung CH, et al. Conjugating a groove-binding motif to an Ir (III) complex for the enhancement of G-quadruplex probe behaviour. Chem Sci 2016;7:2516-23.
- Leung KH, He HZ, He B, Zhong HJ, Lin S, Wang YT, et al. Lable-free luminescence switch-on detection of hepatitis C virus NS3 helicase activity using a G-quadruplex-selective probe. Chem Sci 2015;6:2166-71.
- Liu JB, Yang C, Ko CN, Vellaisamy K, Yang B, Lee MY, et al. A long life time iridium (III) complex as a selective luminescent probe for bisulfite detection in living zebrafish. Sens Actuators B Chem 2017;243:971-76.
- Lozano-Torres B, Galiana I, Rovira M, Garrido E, Chaib S, Bernardos A, et al. An OFF-ON Two-Photon Fluorescent Probe for Tracking Cell Senescence in vivo. J Am Chem Soc 2017;139:8808-11.
- Mahanand D, Houck JC. Fluormetric determination of zinc in biologic fluids. Clin Chem 1968;14:6-11.
- Walkup GK, Burdette SC, Lippard SJ, Tsien RY. A new cell-permeable fluorescent probe for Zn+2. J Am Chem Soc 2000;122:5644-45.
- Domaille DW, Que EL, Chang CJ. Synthetic fluorescent sensors for studying the cell biology of metals. Nat Chem Biol 2008;4:168-75.
- Chen H, Gao W, Zhu M, Gao H, Xue J, Li Y. A highly selective OFF-ON fluorescent sensor for zinc in aqueous solution and living cells. Chem Commun 2010;46:8389-91.
- Li Z, Zhang L, Wang L, Guo Y, Cai L, Yu M, et al. Highly sensitive and selective fluorescent sensor for Zn+2/Cu+2 and new approach for sensing Cu+2 by central metal displacement. Chem Commun 2011;47:5798-800.
- Zhang HH, Dou W, Liu WS, Tang XL, Qin WW. A 2-pyrazoline functionalized zinc complex: Available N-Ag interaction modulating its fluorescence properties. Eur J Inorg Chem 2011;2011:748-53.
- Xu Z, Liu X, Pan J, Spring DR. Coumarin-derived transformable fluorescent sensor for Zn+2. Chem Commun 2012;48:4764-6.
- Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res 2006;5:196-201.
- Pluth MD, Tomat E, Lippard SJ. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev Biochem 2011;80:333-55.
- Dean KM, Qin Y, Palmer AE. Visualizing metal ions in cells: an overview of analytical techniques, approaches, and probes. Biochim Biophys Acta 2012;9:1406-15.
- Reddy SRK, Surya SM, Shaik M, Kanuparthy PR. Copper complexes of pyridyl-tetrazole ligands with pendant amide and hydrazide arms: synthesis, characterization, DNA-binding and antioxidant properties. Transit Met Chem 2016;41:517-23.
 Probe;
Probe;  Ba;
Ba;  Ca;
Ca;  Co;
Co;  Zn;
Zn;  K;
K;  Mg;
Mg;  Cu;
Cu;  Ni;
Ni;  Pb
Pb