- Department of Neurosurgery, Imam Hospital, Mazandaran University of Medical Sciences, Sari, Iran
- Department of Neurosurgery, Sina Hospital, Tehran University of Medical Science, Tehran, Iran
- Department of Pathology, Sina Hospital, Tehran University of Medical Science, Tehran, Iran
Correspondence Address:
Abbas Amirjamshidi
Department of Neurosurgery, Sina Hospital, Tehran University of Medical Science, Tehran, Iran
DOI:10.4103/2152-7806.143365
Copyright: © 2014 Amiri RS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Amiri RS, Hanif H, Ahmadi A, Amirjamshidi A. Brucella-related multiple cerebral aneurysms: Report of a case and review of the literature. Surg Neurol Int 21-Oct-2014;5:152
How to cite this URL: Amiri RS, Hanif H, Ahmadi A, Amirjamshidi A. Brucella-related multiple cerebral aneurysms: Report of a case and review of the literature. Surg Neurol Int 21-Oct-2014;5:152. Available from: http://surgicalneurologyint.com/surgicalint_articles/brucella-related-multiple-cerebral-aneurysms-report-of-a-case-and-review-of-the-literature/
Abstract
Background:Mycotic cerebral aneurysms are uncommon. We intend to report the first case of multiple mycotic cerebral aneurysms due to Brucella infection that were treated surgically.
Case Description:A 34-year-old man with neurobrucellosis presented with intracerebral haemorrhage (ICH). Three mycotic aneurysms were detected in the vicinity of middle cerebral artery (MCA). Medical treatment failed to treat them and aneurysms had to be managed surgically.
Conclusion:Brucella-related cerebral mycotic aneurysm has rarely been reported. This is the first report of three mycotic aneurysms occurring in a young man with neurobrucellosis treated surgically.
Keywords: Brucella, endovascular treatment, meningitis, mycotic aneurysm
INTRODUCTION
Brucellosis is one of the most common zoonotic infections around the globe.[
The inflammatory processes involving intracranial vessels may lead to lacunar infarcts, venous thrombosis, small hemorrhages, or aneurysm formation.[
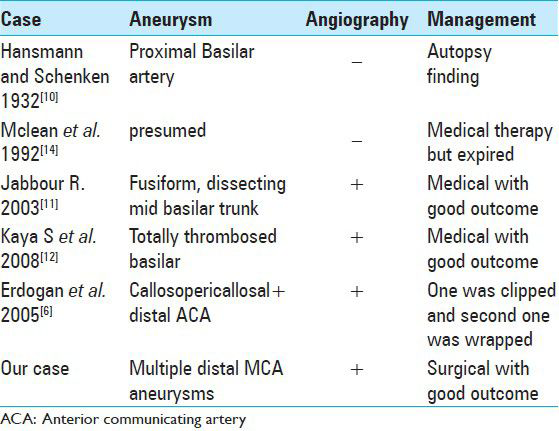
This is the first case reported in the English literature of multiple cerebral mycotic aneurysms secondary to Brucella infection that were treated surgically. The other five cases of single Brucella-related aneurysm reported are summarized in
CASE REPORT
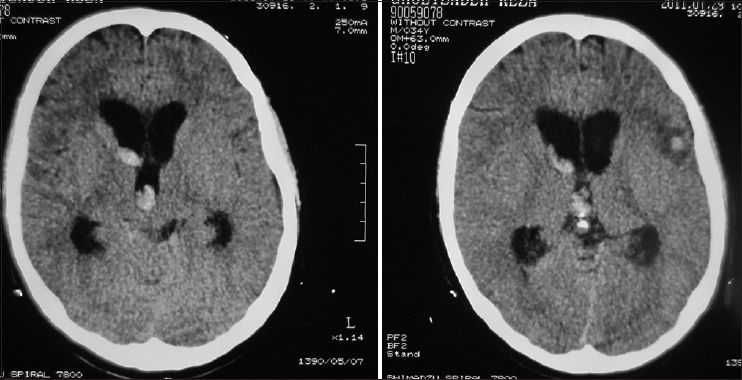
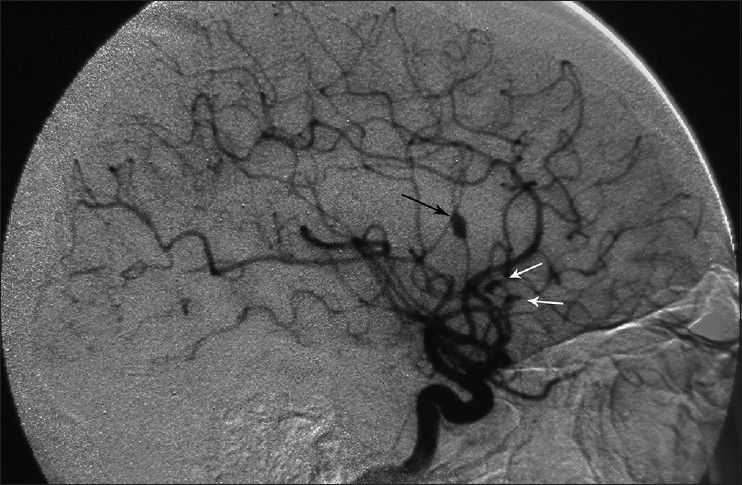
A 34-year-old male, a computer engineer by profession, was found drowsy at home and was referred to a nearby medical center. There was history of a recent business trip to Greece which took almost 6 weeks. He was suffering from headache and fever and was drowsy when admitted in the hospital [modified Rankin scale (MRS) =2]. Preliminary workups were all negative and brain computed tomography scan (CTS) showed a small left frontal opercular intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) accompanied with mild hydrocephalus [
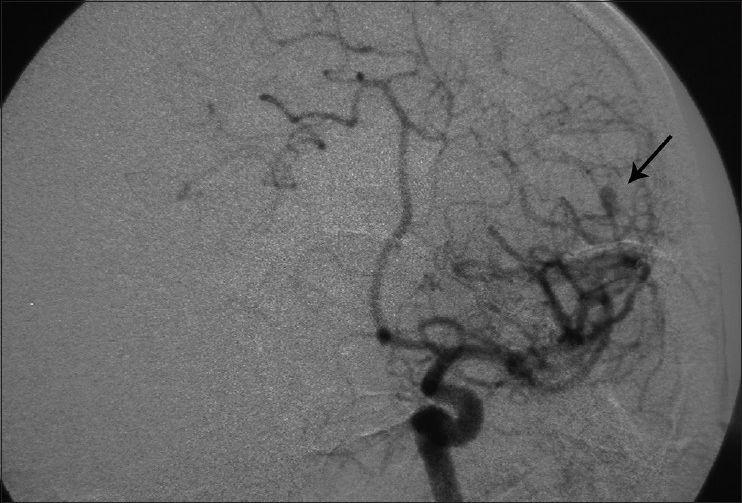
Considering the past history of fever, headache, detectable neck rigidity, and ICH, the first impression was rupture of a mycotic aneurysm. None of the aneurysms had well-identifiable neck in the angiogram. It was planned to treat the presumable mycotic lesions with a trial of wide-spectrum antibiotic therapy including Vancomycin 1 g twice/day + Rifampin 600 mg three times/day and Tazocin 4.5 g three times/day. All of the preclinical studies were negative except for the following: erythrocyte sedimentation rate (ESR) =28, C-reactive protein (CRP) =3 plus, and positive serology for brucellosis (direct Coomb's test/Brucella micro agglutination test) both in his plasma and CSF with the agglutinin titer of 1/160 and 1/80, respectively. CSF analysis was compatible with partially treated meningitis: WBC = 500/mm3, polymorphoneuclears 60%, lymphocytes = 40%, protein = 55.3mg/dl, and sugar = 56 mg/dl.
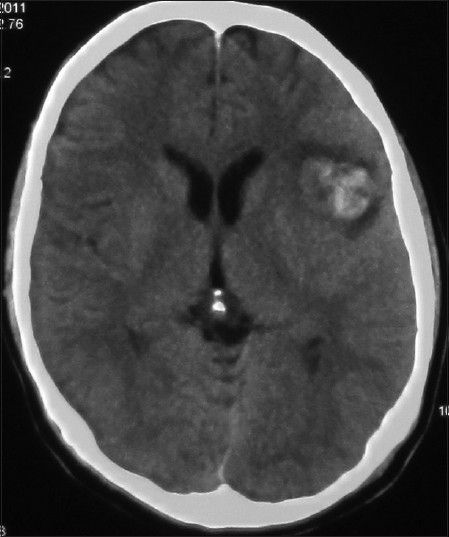
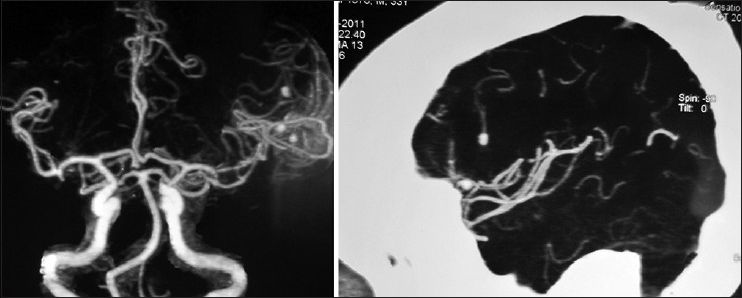
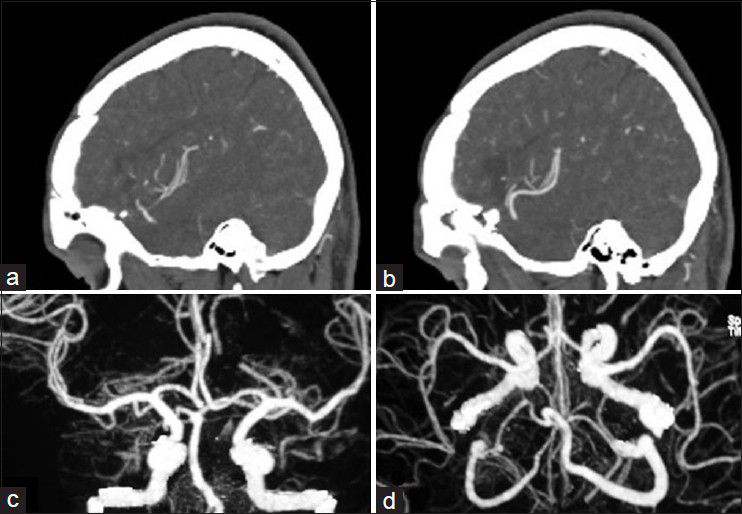
His general condition improved moderately after 3 weeks of antibiotic therapy (MRS 3), but intermittent fever persisted and the CSF picture did not change notably. A brain computed tomographic angiography (CTA) did not show any regression in the size of the distal MCA aneurysm. The other two ill-defined aneurysms noted in the previous DSA were well elucidated in the new CTA [
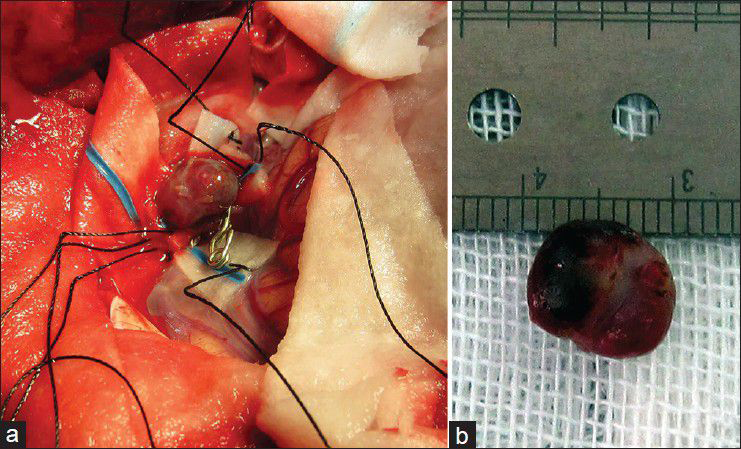
Considering the available facilities, it was decided to treat the aneurysms surgically and it was planned for either clipping-only or excision with reconstruction bypass surgery of the aneurysms according to the findings during surgery. A left posterior temporoparietal craniotomy was done after 30 days of antibiotic therapy. The dura was opened, taking care of the arachnoidal adhesions visible beneath the dura, and attached to one of the cortically exposed aneurysms. A small aneurysm was located along one of the cortical branches of M4, along the superior temporal gyrus with adhesions in the surrounding subarachnoid space. The arachnoid could be dissected sharply. The aneurysm was a small one located on a distal M4 branch, without a clippable neck. It was coagulated and excised. The second aneurysm was located along M3 segment of the frontal branch of MCA. It was dissected, clipped, and resected. The clip could be removed after micro-coagulation of the parent artery [Figure
Figure 7
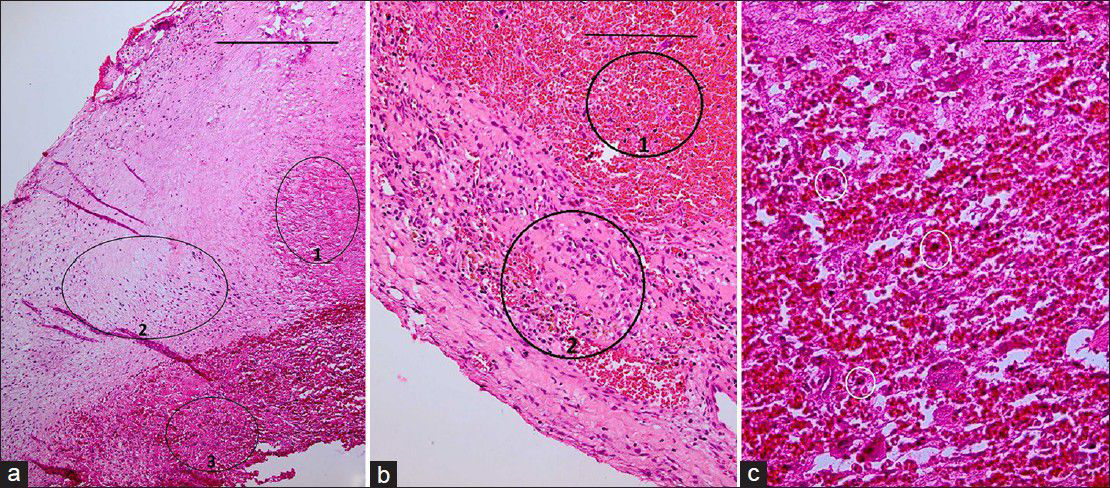
(a) H&E staining ×40 showing portions of vessel wall with necrotic (ring 1), fibrotic (ring 2), and thrombotic (ring 3) changes. Scale bar is 2 mm. (b) H and E, ×100 fibro-necrotic wall containing hemosiderin-laden macrophages (ring 2) admixed with many RBCs (ring 1). Scale bar is 1 mm. (c) H and E, ×400 showing white small rings encircling few foreign body type giant cells and inflammatory cells, interposed by thrombotic material. Periodic acid Schiff (PAS) staining (for fungi) and acid-fast bacillus (AFB) staining for mycobacteria show no specific microorganism. All these findings are compatible with “necrotizing vasculitis.” Scale bar is 100 μm
The patient's postoperative course was uneventful. Considering the positive serology for brucellosis both in plasma and CSF, the anti-brucella antibiotic regimen was continued. The patient could be discharged after 1 month with improvement of fever and stuttering (MRS 2). The anti-brucella medications were continued for another 6 weeks and negative serology tests achieved. The postoperative CTA performed after 1 year confirmed no aneurysmal dilatation along the course of MCA [
DISCUSSION
Neurobrucellosis occurs in about 5% of systemic Brucella affections, mostly manifesting as meningitis or encephalomeningitis.[
The mechanisms for involvement of CNS in brucellosis may be intracellular involvement by the organism or stimulation of the immune system.[
According to Gul et al., in their pooled analysis of 187 cases of neurobrucellosis, fever, headache, sweating, and weight loss were the predominant complaints, followed by neurological symptoms, just as what happened in our case.[
Although positive culture of Brucella organism is the gold standard test for diagnosis, only 28% of neurobrucellosis cases have positive blood cultures and 14% develop positive CSF culture.[
Only five cases of Brucella-related cerebral aneurysms have been reported, among which only one has been managed surgically [
The optimum treatment strategy for infectious aneurysm is still a matter of debate. According to several authors, antibiotic therapy for 4-6 weeks and close follow-up imaging looking for regression of the aneurysm can be the prior step in management of such aneurysms.[
Endovascular obliteration has been reported as a treatment modality for mycotic aneurysms, but with limited evidences in the literature and the possibility of complications such as rebleeding during the procedure because of the fragility of the infected vessel and also the probability of late abscess formation. This last choice has been considered only in special situations such as mycotic aneurysm occurring in the setting of severe endocarditis which could not be managed by surgical intervention.[
That was the exact algorithm undertaken in this reported case. Surgical intervention was attempted when the modality of wide-spectrum antibiotic therapy failed to manage the disease. Not only the aneurysm did not regress in size after 4 weeks of antibiotic therapy, but also two other aneurysms not well detectable in the first DSA became well distinguishable in the control imaging.
CONCLUSION
To the best of our knowledge, this is the first report in the literature on multiple mycotic cerebral aneurysms occurring in a patient with brucellosis and treated surgically.
The case reported by Hansmann et al.[
References
1. Adaletli I, Albayram S, Gurses B, Ozer H, Yilmaz MH, Gulsen F. Vasculopathic changes in the cerebral arterial system with neurobrucellosis. AJNR Am J Neuroradiol. 2006. 27: 384-6
2. Akdeniz H, Irmak H, Anlar O, Demiroz AP. Central nervous system brucellosis: Presentation, diagnosis and treatment. J Infect. 1998. 36: 297-301
3. Brust JC, Dickinson PC, Hughes JE, Holtzman RN. The diagnosis and treatment of cerebral mycotic aneurysms. Ann Neurol. 1990. 27: 238-46
4. Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, Evirgen O. Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int J Infect Dis. 2010. 14: e469-78
5. Chun JY, Smith W, Halbach VV, Higashida RT, Wilson CB, Lawton MT. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001. 48: 1203-13
6. Erdogan B, Sener L, Ozsahin K, Savas L, Caner H. An unusual case of ruptured distal anterior cerebral artery aneurysm associated with brucellosis. J Infect. 2005. 51: e79-82
7. Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, Ivanova-Georgieva R, Noureddine M, Plata A. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation. 2013. 127: 2272-84
8. Gross BA, Puri AS. Endovascular treatment of infectious intracranial aneurysms. Neurosurg Rev. 2013. 36: 11-9
9. Gul HC, Erdem H, Bek S. Overview of neurobrucellosis: A pooled analysis of 187 cases. Int J Infect Dis. 2009. 13: e339-43
10. Hansmann GH, Schenken JR. Melitensis Meningo-Encephalitis: Mycotic Aneurysm Due to Brucella Melitensis Var. Porcine. Am J Pathol. 1932. 8: 435-44
11. Jabbour R, Khalifeh R, Al Kutoubi A, Atweh S. Dissecting aneurysm of the basilar trunk in a young man. Arch Neurol. 2003. 60: 1016-8
12. Kaya S, Velioglu M, Colak A, Kutlay M, Demircan MN, Tekin T. Brucella-related cerebral aneurysms/subarachnoidal hemorrhage: A short review featuring a case report. Neurosurg Rev. 2008. 31: 337-41
13. Khayata MH, Aymard A, Casasco A, Herbreteau D, Woimant F, Merland JJ. Selective endovascular techniques in the treatment of cerebral mycotic aneurysms. Report of three cases. J Neurosurg. 1993. 78: 661-5
14. McLean DR, Russell N, Khan MY. Neurobrucellosis: Clinical and therapeutic features. Clin Infect Dis. 1992. 15: 582-90
15. Nakahara I, Taha MM, Higashi T, Iwamuro Y, Iwaasa M, Watanabe Y. Different modalities of treatment of intracranial mycotic aneurysms: Report of 4 cases. Surg Neurol. 2006. 66: 405-9
16. Pascual J, Combarros O, Polo JM, Berciano J. Localized CNS brucellosis: Report of 7 cases. Acta Neurol Scand. 1988. 78: 282-9