Alloplastic adjuncts in breast reconstruction
Introduction
Breast cancer is the most common cancer in women, accounting for 29% of newly diagnosed cancers, and with a lifetime risk of one in eight for females in the United States (1). Numerous options and technical variations exist for post-mastectomy breast reconstruction, and can be categorized into autologous versus alloplastic, immediate versus delayed, as well as single versus two-staged. An estimated one-half to two-thirds of women who undergo a mastectomy will proceed to have an alloplastic reconstruction (2).
Acellular dermal matrices (ADMs) have been used since the 1990s in the areas of burns, head and neck, abdominal wall, hand, nasal as well as lower extremity reconstruction (3-10). These materials are allegedly immunologically inert, and act as biological scaffolds for re-epithelialization, neovascularization and fibroblast infiltration. Duncan first published the use of ADMs in breast surgery in 2001, in which AlloDerm® was utilized in revisional aesthetic surgery to correct implant rippling (11). However, it first used in breast reconstruction in 2005, where Breuing and Warren described the use of AlloDerm® as an inferior sling in single stage (direct to implant) post-mastectomy reconstructions (5). In the same year, Rietjens et al. described the use of a synthetic non-absorbable mesh (Mersilene), to recruit upper abdominal skin for additional soft-tissue coverage of the implant, as well as to recreate the infra-mammary fold (12). Since then, the types and number of alloplastic adjuncts have increased, including ADMs derived from human, bovine and porcine dermis, as well as synthetic meshes. These products vary significantly in their processing, level of sterility, biomechanical properties, thickness, preparation methods and cost (13-15). The use of ADMs in breast reconstruction has gained increasing popularity since its introduction, with an estimated 25% to 75% of tissue expander reconstructions utilizing ADMs (16-19).
Numerous advantages have been proposed with the use of alloplastic adjuncts, including: facilitating immediate implant reconstruction, improved implant positioning via better definition of the infra- and lateral mammary folds, shorter expansion times in tissue-expander reconstructions, improved capsular contracture rates, masking implant rippling, providing an additional layer between the prosthesis and overlying mastectomy skin, reduced rates of implant/expander migration, reduced discomfort during post-operative expansion, and protective effects in patients undergoing radiotherapy (5,20-24). However, there are also concerns regarding potential increased risks of infection, inflammatory reaction, seroma, masking tumour recurrence and significant costs (25-29).
Numerous alloplastic adjuncts exist, and these vary in material type, processing, storage, surgical preparation, level of sterility, available sizes and cost. However, there is little published data on most, posing a significant challenge to the reconstructive surgeon trying to compare and select the most suitable product. The aims of this systematic review were to identify, summarize and evaluate the outcomes of studies describing the use of alloplastic adjuncts for post-mastectomy breast reconstruction. The secondary aims were to determine their cost-effectiveness and to analyze outcomes in patients who also underwent radiotherapy.
Methods
Study identification
Multiple databases were searched independently by three authors (Cabalag MS, Miller GS and Chae MP), including: Ovid MEDLINE (1950 to present), Embase (1980 to 2015), PubMed and Cochrane Database of Systematic Reviews.
The following search terms and Boolean operators were used: (I) (“breast reconstruction” OR “post mastectomy” OR “implant reconstruction” OR “tissue expander” OR “alloplastic”) AND (II) (“acellular dermal matrix” OR “acellular dermal matrices” OR “mesh” OR “synthetic mesh” OR “biological matrix”). Additional searches were conducted using (I) AND (II) AND (“radiotherapy” OR “irradiated”), as well as (I) AND (II) AND (“cost” OR “cost-effectiveness” OR “cost analysis”).
Inclusion criteria
Inclusion criteria for studies reviewed included: (I) meta-analyzes or review articles; (II) adult patients aged 18 years or over undergoing post-mastectomy breast reconstruction; (III) alloplastic breast reconstruction (i.e., tissue-expander and/or implant-based) performed using adjuncts (ADMs and/or synthetic meshes; (IV) studies including outcome measures; (V) case series with more than ten patients; and (VI) English language.
Data extraction
A systematic review was conducted using the PRISMA 2009 statement (30). Data was extracted by three authors (Cabalag MS, Miller GS and Chae MP), and included author, year, journal, study design, level of evidence, outcome details, number of patients (if applicable), and follow-up period. Differences in data extraction were corrected via discussion.
Results
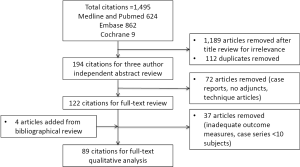
The search was conducted on April 4, 2015, resulting in 1,495 articles, managed using Endnote X7™ (Thomson Reuters, Philadelphia, PA, USA). A summary of the literature review process is shown in Figure 1. After the authors independently assessed the titles for relevance, a total of 1,189 articles were excluded, and 112 duplicates were removed. The abstracts for the remaining articles were then reviewed based on the inclusion criteria, leaving a total of 122 articles for full review. A further four articles were added based on review of bibliographies. Thirty-seven studies were eliminated after full review (inadequate outcome measures, case series <10 subjects). After full text review, analysis and data extraction was conducted for a total of 89 articles. The recommendations of this review are summarized in Table 1. Tables S1-S4 are a summary of the: systematic reviews and meta-analyses; levels III and IV studies; cost-analyzing studies; and studies focusing on synthetic meshes respectively.

Full table
Full table

Full table

Full table
Full table
Types of alloplastic adjuncts available
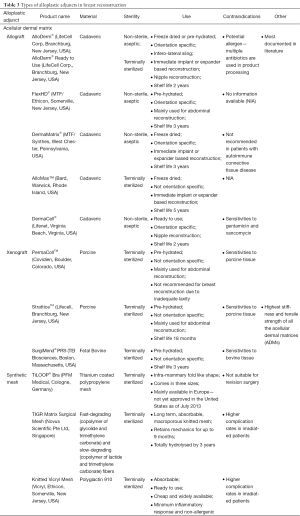
The types of alloplastic adjuncts in breast reconstruction described in the literature are listed in Table 2 and summarized in Table 3 (14). In summary, they comprise of either ADMs or synthetic meshes. Within ADMs, there are either allografts, derived from cadaveric human skin, or xenografts. There is significantly less published literature on the use of synthetic meshes in post-mastectomy reconstruction.

Full table
Full table
Allogeneic ADMs
AlloDerm® (LifeCell Corp., Branchburg, New Jersey, USA)
First introduced in 1994, AlloDerm was the first human dermis product available, and was initially used for burns reconstruction. It is a cadaveric split-thickness skin graft, in which the epidermis and cells are removed from the skin to reduce its antigenicity. It now comes in two forms: an aseptic, freeze-dried version requiring refrigerated storage and rehydration prior to use; and a newer, sterile, ready to use product. It was first used as an infero-lateral sling in breast reconstruction in 2005, but now also has a role in tissue-expander based as well as nipple reconstructions (5,31,32). Of note, AlloDerm has two distinct surfaces, and thus requires specific orientation during implantation. The dermal side of the product, characterized by the dull, rough texture, is placed against the vascularized wound bed (i.e., the mastectomy skin flaps). AlloDerm is the most extensively studied ADM in breast reconstruction, with 135 references in the PubMed database as of April 2015. Histological studies have demonstrated AlloDerm to be partially integrated into host tissue within 7 days of implantation (33).
FlexHD® (MTF/Ethicon, Somerville, New Jersey, USA)
FlexHD is a pre-hydrated, aseptic, cadaveric dermal matrix, which, similar to AlloDerm is orientation-specific. Rawlani et al. studied the use of FlexHD in 121 breast reconstructions, with complications occurring in 20 breasts (two seromas, eight partial mastectomy flap necroses and nine infections). Furthermore, when compared to the non-irradiated group, the irradiated cohort had a higher rate of complications (13.7% vs. 30.8% respectively) (34).
Allomax™ (Bard, Warwick, Rhode Island, USA)
Previously known as NeoForm®, Allomax™ is a sterile, cadaveric dermal matrix, which is non-orientation specific. Losken et al. published a study involving 22 patients and 31 breast reconstructions, reporting no cases of infection, seroma or foreign body reaction (35).
DermaMatrix® (MTF/Synthes, West Chester, Pennsylvania, USA)
DermaMatrix® is an aseptic, freeze-dried, orientation specific cadaveric allograft. Becker et al. compared DermaMatrix® with AlloDerm® in 30 patients (50 breasts) who underwent immediate expander-based breast reconstruction, in which the only statistically significant difference was a shorter duration in which the drains remained in-situ for AlloDerm®vs. DermaMatrix® (11 vs. 13 days) (36). No significant differences in complication rates (4%) were noted.
DermaCell® (Lifenet, Virginia Beach, Virginia, USA)
DermaCell® is a prehydrated, ready to use cadaveric dermal matrix, which can be stored at room temperature. The literature search revealed three articles on the use of DermaCell® in breast reconstruction, which suggested a relatively low rate of post-operative complications (14,37,38). In a recent case series of ten patients, Bullocks reported two cases of failed tissue-expander reconstructions due to chronic seromas and infection (37). In another recent case series of nine patients, Vashi et al. reported only one patient with bilateral post-mastectomy reconstruction who subsequently developed seromas and infection (38).
Xenogeneic ADMs
Strattice™ (Lifecell, Branchburg, New Jersey, USA)
Strattice™ is a pre-hydrated, terminally sterilized, porcine-derived dermal matrix.
Permacol™ (Covidien, Boulder, Colorado, USA)
Permacol™ is a pre-hydrated, terminally sterilized, porcine-derived dermal matrix. Of note, it is not recommended for breast reconstruction as it lacks adequate laxity to produce natural, ptotic lower pole coverage.
Surgimend® PRS (TEI Biosciences, Boston, Massachusetts, USA)
Surgimend® is the only product comprised of fetal bovine dermal collagen, and is terminally sterilized.
Synthetic mesh
Knitted Vicryl Mesh (Ethicon, Somerville, New Jersey, USA)
Comprised of polyglactin 910, Knitted Vicryl Mesh is cheap, ready to use and widely available. It also exhibits minimal inflammatory reaction, is non-allergenic and resistant to bacteria biofilm formation (39,40).
TiLOOP® Bra (PFM Medical, Cologne, Germany)
TiLOOP Bra is a lightweight, non-absorbable, titanium-coated polypropylene mesh, first approved for use in breast reconstruction in Europe in 2008. It is the most commonly used synthetic mesh in Germany (15). It consists of a monofilament structure and is available in three different bra-like sizes. The mesh comes in an infra-mammary fold like shape, helping to define the lower pole and preventing the implant from bottoming out. Both animal and human studies have demonstrated improved biocompatibility compared to non-titanium coated meshes, with histological evidence of incorporation during the time of expander-implant exchange (41,42). In Europe, the mesh costs €400 (43).
TIGR® Matrix Surgical Mesh (Novus Scientific Pte LTd, Singapore)
TIGR® Matrix Surgical Mesh is a long-term, absorbable synthetic mesh. It is a macroporous mesh knitted from both a fast- (copolymer of glycolide and trimethylene carbonate) and slow-degrading (copolymer of lactide and trimethylene carbonate) fibers. After 2 weeks post implantation, the mesh will become noticeably softer and flexible, with due to the degradation of the fast fibers, which becomes totally resorbed within 4 months. The slow-degrading fibers keep their mechanics for up to 9 months, and are totally hydrolysed after 3 years (13). A 10 cm × 15 cm sheet of TIGR® mesh costs USD $900. A preclinical study has demonstrated that the mesh is rapidly vascularized, demonstrates minimal inflammatory response, and is replaced by well-organized connective tissue over time (44).
Use of ADMs in post-mastectomy breast reconstruction and outcomes compared to non-ADM reconstruction
Currently, ADMs are used in both primary and revisional alloplastic breast reconstructive and aesthetic surgery. Techniques include: (I) expansion of the submuscular pocket to allow for direct-to-implant breast reconstruction (5); (II) expansion of the submuscular pocket to improve two-stage expander-to-implant breast reconstruction (31); (III) providing an interface when performing capsulotomies or capsulectomies for capsular contracture; (IV) correction of symmastia (45); (V) aid in the masking surface irregularities and rippling (23); and (VI) prevention or correction of inframammary fold malposition and ‘bottoming out’ (46).
Use of alloplastic adjuncts in single stage, direct-to-implant reconstruction
Breuing and Warren first described the use of AlloDerm® as an inferior sling in immediate, direct-to-implant post-mastectomy reconstruction (5). The technique re-establishes the lower pole of the pectoralis major muscle, creating a subpectoral-sub-AlloDerm pocket that encloses the implant. The advantages of this method include the ability for a single stage, direct-to-implant reconstruction and its associated cost benefits, the ability to control lower pole fullness by adjusting the width of the sling and providing an additional layer of tissue between skin and implant. In a recent review by Macadam and Lennox, the use of ADMs (AlloDerm®) in direct-to-implant breast reconstruction, when compared to no ADM use in two-stage reconstructions (the Mentor and Allergan core studies) (47-51), resulted in lower rates of capsular contracture (0.3% vs. 8.3-17.1%), seroma (1.2% vs. 4.9%), infection (1.4% vs. 3.2-5.7%), late revisions (8.5% vs. 27-53.3%) and implant loss (1.5% vs. 5.7-7.7%). However, a higher rate of skin flap necrosis was observed (4.7% vs. 2.3%), which may be attributable to increased skin tension due to placement of the implant (52). Of note, the rate of skin flap necrosis is comparable to expander-to-implant reconstructions without the use of ADM published in previous studies (range, 2-6%) (53-56). Similarly, Salzberg et al. demonstrated a low overall complication rate (3.9%) in a retrospective analysis of 260 patients (466 breasts) who underwent single-stage reconstruction with AlloDerm®, with a mean follow up of 29 months (57). Specific complication rates included implant loss (1.3%), flap necrosis (1.1%), hematoma (1.1%), ADM exposure (0.6%), capsular contracture (0.4%) and infection (0.2%). Irradiated breasts had a fourfold higher rate of complications. The low complication rates are also projected long-term, with no complications seen in 354 breasts with more than 1 year of follow-up. A systematic review by Jansen and Macadam further reaffirms the comparable complication rates between AlloDerm®-assisted single stage and non-ADM, two-stage reconstructions (58). Of note, to validate these findings, Zhong et al. are currently conducting a randomized controlled trial comparing direct-to-implant reconstruction with ADM to traditional two-stage non-ADM reconstruction (59).
In contrast, a meta-analysis conducted by Ho et al. revealed higher odds of infection [odds ratio (OR), 2.7; 95 percent confidence interval (95% CI), 1.1-6.4], seroma (OR, 3.9; 95% CI, 2.4-6.2) and reconstructive failure (OR, 3.0; 95% CI, 1.3-6.8) in ADM compared to non-ADM breast reconstructions. However, ADM use was associated with lower rates of capsular contracture. The meta-analysis reviewed a total of 16 studies, most of which did not differentiate between single- or two-stage reconstruction. The most common complication associated with ADM use was skin flap necrosis (10.9%; 95% CI, 8.7-13.5%), followed by seroma (6.9%; 95% CI, 5.3-8.8%), infection (5.7%; 95% CI, 4.3-7.3%), reconstructive failure (5.1%; 95% CI, 3.8-6.7%), cellulitis (2.0%; 95% CI, 1.2-3.1%), hematoma (1.3%; 95% CI, 0.6-2.4%) and capsular contracture (0.6%; 95% CI, 0.1-1.7%).
Vicryl mesh has also been used in immediate single stage reconstructions with favorable results. In a retrospective analysis by Tessler et al., 50 consecutive patients (76 reconstructions) underwent immediate implant-based reconstruction using knitted Vicryl mesh as an inferolateral sling. The overall complication rate was 6.6%, with one case (1.3%) of infection, two cases (2.6%) of mastectomy skin flap necrosis, one case (1.3%) of capsule contracture requiring revision (postradiation), one case (1.3%) of implant failure, and one case of a delayed type IV hypersensitivity reaction. Reported contour and implant positioning were excellent, with a revision rate of 3.9% (three breasts) for size enlargement. Additionally, Garganese et al. have used TiLOOP Bras in immediate implant-based reconstruction in ten patients, reporting no early complications and minimal post-operative pain (60). Klein et al. reported higher complication rates with the use of TiLOOP Bras in immediate reconstruction, with an infection, hematoma and seroma rate of 10.3%, 17.2% and 9.2% respectively (61).
Use of alloplastic adjuncts in two stage, expander-to-implant reconstruction
In 2007, Bindingnavele et al. first described the use of ADMs in a two-stage expander-to-implant reconstructions (31). Alleged advantages include increased intra-operative expansion volumes and thus reduced post-operative expansion time, avoiding the need to raise serratus anterior muscle for lateral prosthesis coverage leading to reduced post-operative pain with expansion, as well as more precise placement of the expander resulting in better lower pole projection and improved aesthetics. However, multiple studies have expressed concern regarding the increased morbidity associated with the use of ADM in two-stage reconstructions. In a series of 283 patients (415 breasts), Chun et al. demonstrated that the use of ADMs increased the odds of seroma by 4.24 times (P=0.018) and infection by 5.37 times (P=0.006), when compared to the non-ADM group (26). This was further confirmed in a meta-analysis performed by Kim et al. comparing the use of ADM (19 studies, n=2,037) and no ADM (35 studies, n=12,847) in two-stage breast reconstruction, reporting inferior outcomes in the ADM group. There were higher rates of seroma (4.8% vs. 3.5%), infection (5.3% vs. 4.7%) and mastectomy flap necrosis (6.9% vs. 4.9%) in the ADM group (62). However, the rate of reconstructive failure was comparable (3.8%). These findings were reinforced by a weighted analysis conducted by Macadam and Lennox for two-stage reconstructions using ADMs, compared to no ADMs, revealing higher rates of seroma (5.8% vs. 4.9%), infection (5.3% vs. 3.2-5.7%), and mastectomy flap necrosis (7.6% vs. 2.3%). However, there were lower rates of capsular contracture (2.6% vs. 8.3-17.1%), and late revisions (10.7% vs. 27-53%). The rate of implant extrusion was comparable (4.9% vs. 5.7-7.7%). Additionally, a meta-analysis by Hoppe et al., consisting of eight studies comparing the use of AlloDerm® in expander-implant reconstruction to traditional submuscular techniques, demonstrated a three-fold increase in the odds seroma formation (OR, 3.00; 95% CI, 1.96-4.61) and a two-fold increase in the odds of infection in the ADM group (OR, 2.33; 95% CI, 1.55-3.49) (63).
In contrast, a systematic review by Sbitany and Serletti comparing the use of ADMs in two-stage reconstruction to standard subpectoral coverage techniques revealed a comparable complication profile, but more rapid reconstruction in the ADM group. There was a significantly higher rate of seroma formation in the ADM group (8.4% vs. 4.3%, P=0.03), but the rate of infection resulting in explantation was similar (3.4% vs. 3.2%, P=0.18, in the ADM and submuscular group respectively). There were also slightly higher rates of hematoma (2.0% vs. 1.2%, P=0.09) and partial mastectomy flap necrosis (9.3% vs. 7.2%, P=0.08) in the ADM compared to the submuscular group, none of which were statistically significant. The ADM group demonstrated higher intra-operative fill volumes (mean of 68.5% of final total volume vs. 24.2%, P=0.01) and a shorter post-operative expansion period (mean of 2.4 fills to achieve final volume vs. 5.1, P=0.03) (64).
Furthermore, a multicenter, blinded randomized, controlled trial comparing the use of ADM in two-stage breast reconstruction showed no significant difference in adverse outcomes (hematoma, seroma and infection) between the ADM and non-ADM group (17% vs. 15% respectively, P=1.00) (65). Furthermore, there were no significant differences in immediate post-operative pain, pain during expansion phase, or the rate of post-operative expansion between the two groups.
A titanium-coated polypropylene mesh, TiLOOP Bra (PFM Medical, Cologne, Germany) is a widely used synthetic adjunct for post-mastectomy reconstruction in Europe. In a retrospective, multicenter analysis by Dieterich et al., 207 patients (231 breasts) underwent either single- or two-stage reconstruction using TiLOOP Bra. The overall complication rate was 29%, with major complications occurring in 13.4% of the cases requiring operative intervention. The rate of mesh removal and implant loss was 7.8% and 8.7% respectively (43). Becker et al. used TIGR® mesh in 11 patients (19 breasts) undergoing two-stage reconstruction, reporting an overall complication rate of 47.3% (one case of flap necrosis, two cases of seroma, three cases of infection/extrusion, one case of rippling, and two cases of asymmetry requiring revision) (13).
Furthermore, Haynes and Kreithen reported on the use of Vicryl mesh in 38 patients (46 breasts) who underwent two-stage reconstructions. The results suggest that Vicryl mesh may be a suitable alternative to ADMs, with an overall complication rate of 15.2% (7 breasts): 3 cases (6.5%) of infections leading to expander removal, 1 case (2.2%) of expander exposure requiring removal in a patient undergoing radiotherapy, 2 cases (4.3%) of mastectomy skin flap necrosis, and 1 case (2.2%) of seroma. However, when analyzing the non-irradiated cohort (38 breasts), the overall complication rate was 10.5% (one case of infection leading to removal of the expander, two cases of mastectomy skin flap necrosis and one case of seroma). The revision rate was 16.2% in the non-irradiated group (two for size change, three for malposition and one for capsular contracture).
Comparison of outcomes between different ADMs
With the great diversity of alloplastic adjuncts available in the market, one of the main challenges faced by reconstructive surgeons is choosing the ideal product. The ideal adjunct would be terminally sterilized, able to be stored without refrigeration, have a long shelf life, not require any preparation (e.g., rehydration or rinsing), result in minimal inflammatory reaction, not require orientation, offer good long-term durability, available in multiple sizes and thickness, as well as be affordable. The majority of published studies focus on AlloDerm®, as it was the first widely available ADM used for breast reconstruction.
Currently, Mendenhall et al. are conducting the largest prospective randomized trial comparing the outcomes after using AlloDerm® versus DermaMatrix® as an inferolateral sling in two stage expander-implant breast reconstruction in 128 patients (199 breasts). Preliminary results demonstrate a significant overall complication rate of 36.2%, with similar rates between the two groups (33.6% in the AlloDerm® and 38.8% in the DermaMatrix® group, P=0.52). In both the AlloDerm® and DermaMatrix® groups, the majority of complications were due to skin necrosis (17.8% vs. 21.4% respectively, P=0.66) and infections (13.9% vs. 16.3% respectively, P=0.29), both of which led to tissue expander losses (5% vs. 11.2% respectively, P=0.11). Of note, the rates of infection and skin necrosis are considerably higher compared to those previously reported (62,64). Complication rates (specifically infection and tissue expander loss) were significantly higher in obese patients, with the authors suggesting that ADM use should be avoided in such patients. Patients reconstructed with AlloDerm® had significantly faster expansion times (42 vs. 70 days, P<0.001).
The use of sterile AlloDerm® Ready to Use, when compared to aseptic AlloDerm®, led to reduced rates of mastectomy skin flap necrosis, seroma and infection (66,67). In contrast, although limited by sample size, a retrospective analysis comparing AlloDerm® (aseptic) with AlloDerm® Ready to Use (sterile) in implant based reconstructions, showed a higher seroma rate with the latter (68). Similarly, in a comparison between AlloDerm® and Strattice for alloplastic breast reconstruction, Glasberg and Light showed a significantly higher seroma rate with the use of AlloDerm® (21.4% vs. 6.3%, P=0.0003). All other complications were similar between the two groups (69). Other studies have shown AlloDerm® has comparable outcomes with DermMatrix, Strattice, SurgiMend, FlexHD, AlloMax and AlloDerm Ready to Use (70-75). Furthermore, Seth et al. showed no significant differences in complication rates between the use of cryopreserved or prehydrated human ADMs (PHADMs) (76).
Furthermore, Mofid et al. conducted a retrospective analysis on the use of Veritas®, a bovine pericardium xenograft, in immediate tissue expander/implant-based breast reconstructions. The overall complication rate was found to be similar, if not lower, compared to the use of AlloDerm® in previous studies (77).
Role of ADM in preventing capsular contracture
Capsular contracture is one of the most common complications in reconstructive breast surgery, with cumulative risks reported to be 12% after 1 year, and increasing to 30% at 5 years post-operatively (78). The aetiology remains unclear, although a common inflammatory pathway has been postulated, leading to increased deposition of collagen around the implant and myofibroblast migration (79-82). The use of ADMs appears to reduce the rate of capsular contracture. A meta-analysis conducted by Ho et al. revealed a pooled capsular contracture rate of 0.6%, significantly lower compared to the 3-18% rate reported in traditional two-stage reconstructions (22,23,83-85). Vardanian et al. studied the use of ADMs in immediate implant based reconstruction, and found a significantly lower rate, and risk of capsular contracture in the ADM group versus the non-ADM group (3.8% vs. 19.4% respectively; OR, 0.18; 95% CI, 0.08-0.43) (24). Basu et al. have also shown the protective effects of ADMs histologically, with intra-operative biopsies of human breast capsules and associated ADM at the time of implant exchange demonstrating decreased capsular fibrosis and fibroblast cellularity relative to controls (86). Multiple other studies have similarly demonstrated a low capsular contracture rate in patients undergoing both single- and two-stage breast reconstruction with ADM, ranging from 0-3.8% (5,24,31,34,57,87,88). Interestingly, in a primate model, Stump et al. have demonstrated the role of AlloDerm® in preventing capsular formation (89). However, further long-term follow up is necessary as the rate of capsular contracture may increase with time.
Role of ADMs in irradiated tissue
There have been mixed reports on the role of ADMs in irradiated tissue. In a study where two AlloDerm implants were placed in the backs of 41 rats that were irradiated, Komorowska-Timek et al. demonstrated that the use of AlloDerm decreased radiation-related inflammation and potentially delayed capsular formation and contraction, with the protective effects still present at 12 weeks (90). Similarly, in a retrospective review of 417 consecutive patients (592 breasts), Seth et al. demonstrated a decreased risk of all complications in irradiated breast tissue reconstructed with ADM, versus the non-ADM group (91). Non-ADM patients who received post-mastectomy radiation therapy were almost three times as likely to have a complication compared to non-irradiated patients (OR, 2.63; P=0.002). Conversely, ADM patients who received radiotherapy did not show a significant increase in the risk of complications compared to the non-irradiated group (OR, 1.90; P=0.10). Additionally, Mitchell suggested a protective effect of ADM in irradiated tissue, in a retrospective series of 103 patients (158 breasts) who underwent ADM assisted reconstruction using Strattice™ (92). Interestingly, no complications occurred in patients who received radiotherapy post reconstruction.
In contrast, Spear et al. investigated the use of AlloDerm in a prospective series of 58 immediate expander-based breast reconstructions, and found that the use of AlloDerm did not protect against the effects of radiotherapy, with an overall complication rate of 71.4% (46). Additionally, Nahabedian found a minor increase in the rates of infection, seroma and wound dehiscence in irradiated versus the non-irradiated groups (21). Twenty-three out of 100 breasts reconstructed with AlloDerm received radiotherapy, and complications included: seroma (13%), infection (8.7%), skin necroses (0%) and dehiscence (13%) versus the non-irradiated AlloDerm group: seroma (2.6%), infection (3.9%), dehiscence (1.3%) and skin necrosis (3.9%). The lack of protective effects in ADM assisted breast reconstruction is further strengthened by a recent meta-analysis conducted by Valdatta et al. (93).
Costs
Conducting cost-benefit analyzes for procedures is complex, as it requires not only the immediate costs of the procedure to be calculated, but also any additional costs that may be incurred post-operatively. Most of the cost analysis studies on the use of ADM have taken into account some, if not all of the significant outcomes associated with breast reconstruction: no complication, seroma, infection, hematoma, capsular contracture, implant exposure with loss, implant exposure with salvage, and skin flap necrosis. The majority of these studies highlight a cost advantage in conducting single stage, direct-to-implant breast reconstructions using ADMs. Using a calculator based on immediate operative costs and expected outcomes, Macadam and Lennox estimated that direct-to-implant reconstruction using ADM was cheaper than two-stage reconstruction without ADM ($11,072 vs. $15,049) (52). Similarly, de Blacam et al. estimated that direct-to-implant reconstruction with ADM was more cost-effective compared to expander-to-implant with ADM, and expander-to-implant with no ADM reconstruction ($5,432.02 vs. $11,255 vs. $10,934 respectively).
Additionally, costs will vary depending on the type and size of alloplastic adjunct used, as well as the country of interest. An inquiry in August 2011 by Cheng et al. revealed that the price of ADMs ranged from approximately USD $21.63-34.76 per centimeter squared (14). However, these prices do not reflect the charges to the patient, and some are still considered experimental and thus are not covered by insurance.
The cost of synthetic meshes is considerably cheaper, with Vicryl mesh costing under USD $200 per breast. With the use of Vicryl mesh in 76 reconstructions, Tessler et al. have reported a saving of USD $172,112 in direct material costs over 10 months (40).
Discussion
First introduced in 1995 for reconstructive burns surgery, ADMs are extracellular matrix grafts which provide a scaffold upon which the patient’s own cells can repopulate and revascularise the implanted tissue (94). Since its introduction for post-mastectomy breast reconstruction in 2005, multiple studies have detailed varied and inconsistent outcomes on the use of alloplastic adjuncts. To date, they can be classified into two main categories, ADMs that are derived from either allogeneic or xenogeneic dermis, as well as synthetic meshes. To date, there are over ten different products available (Table S1). The absence of comparative data between these products makes choosing the ideal material a significant challenge to the reconstructive surgeon. The primary aim of this systematic review was to summarize the published data available for these alloplastic adjuncts, including analyzing outcome data which available, with particular interest in its role in irradiated tissue and cost-effectiveness. Importantly, most of the published data available are on AlloDerm®.
Despite the majority of systematic reviews and meta-analyzes demonstrating inferior outcomes in ADM-assisted breast reconstructions, Macadam and Lennox suggested superior outcomes with the use of ADMs in single stage, direct-to-implant reconstructions, compared to traditional two-stage reconstructions. Reduced rates of seroma, infection, late revisions, implant loss and capsular contracture were observed (52). The direct placement of an implant may lead to a better match in the volumes of the overlying mastectomy skin flap and implant, leading to reduced rates of seroma. However, this needs to be balanced by the higher risk of skin necrosis. The use of the ADM as an inferolateral sling may allow better control of the inframammary fold, leading to improved cosmesis and lower rates of late revision. The reduced frequency of infection may be a consequence of the reduced seroma rate, as well as avoiding the need for repeated expander manipulation for filling and a second surgery for expander-implant exchange.
Based on the available systematic reviews and meta-analyzes, skin flap necrosis was the most common complication post ADM-assisted breast reconstruction, ranging from 1.1-10.9% (52,62-64,84). This is higher when compared to traditional submuscular techniques (range, 2-6%) (53-56). This increased incidence may be attributable to a number of factors, including a higher intra-operative expander fill volume leading to excessive skin tension, and inappropriate preservation of post-mastectomy skin with ADM use. However, a delicate balance needs to be achieved between adequate expander filling to maximize incorporation of the ADM to the mastectomy skin flap, without creating excessive tension. This outcome may potentially improve with increased surgeon experience. More recently, to address this issue, ADMs have been used in staged, immediate (direct-to-implant) breast reconstruction. In patients at high risk of skin flap necrosis, reconstruction using an implant and ADM sling was performed 2 weeks after the initial mastectomy, without the use of interval expanders. Initial results are promising, with no infectious or bleeding complications, and no cases of nipple malposition (95).
One of the main concerns regarding the use of ADMs is the increased risk of infection, as some are ‘aseptic’, and not terminally sterilized (i.e., a sterility assurance level of 10−6). The majority of published evidence confirms this concern, with three meta-analyzes and a systematic review pointing to increased rates of infection in ADM-assisted breast reconstruction compared to standard submuscular techniques (62,63,84,96). A possible explanation for this is that prior to being revascularised, which takes approximately 2 weeks to occur, ADMs may act as a nidus for infection (33). However, there are numerous potential confounding factors that may affect the rate of infection [e.g., patient age, smoking status, diabetes body mass index (BMI), radio- or chemotherapy]. Studies have shown that a higher BMI, higher age, larger breasts (>600 grams), presence of axillary dissection and chemo-radiation are significant risk factors for infection (26,93,97,98). Furthermore, studies may have varying definitions of infection, with a number of studies having both ‘infection’, and ‘cellulitis’, as outcomes of interest, terminally sterilized human ADMs have recently been introduced, including AlloMax and AlloDerm Ready to Use, and xenogeneic ADMs (e.g., Strattice and SurgiMend PRS) are also terminally sterilized, which may theoretically improve the infection rate. Importantly, the red breast syndrome is associated with ADM use, and may be mistaken for infection in some cases. This typically manifests as erythema limited to the region overlying the ADM, and is often self-limiting and not responsive to antibiotics. The underlying aetiology remains unclear, but may represent a delayed hypersensitivity reaction (66,99).
Furthermore, multiple studies have demonstrated a higher rate of seroma in the ADM versus the non-ADM group (62,63,84). This may be a result of a mismatch between the size of the overlying skin envelope and the underlying tissue expander volume. Additionally, seromas are also more likely to form prior to revascularization of the ADM. Further confounding factors, including surgical technique, concomitant axillary node dissection, placement and number of drains may also affect risk of seroma formation.
The use of synthetic mesh, particularly Vicryl mesh, appears to show promising outcomes as a comparable, but cheaper alternative to ADMs. However, one of the major concerns of using absorbable mesh as an inferolateral sling is implant malposition or ‘bottoming out’, in the long-term, as Vicryl mesh is normally resorbed by 3 to 4 weeks (40). The introduction of TIGR® mesh was meant to address this, but published data is scarce and despite a small sample size (19 breasts), demonstrated inferior outcomes (13). Furthermore, the use of TIGR® and Vicryl mesh may be limited to non-irradiated tissue, as the complication rate was significantly higher in irradiated patients (13,100). Further higher powered, long-term studies on the use of these synthetic meshes are needed.
Limitations
Direct comparison between alloplastic adjuncts is challenging, as there are distinct differences between ADMs and synthetic meshes, and also between different types of ADMs themselves. The definition of outcome measures in the included studies may also differ, making direct comparison challenging. For example, seromas may be classified into those that require drainage, or those that are simply observed. Additionally, a limitation inherent in most surgical outcome studies is accounting for the heterogeneity in surgeon skill and technique, which may be an important confounding factor. Related to this is the type of mastectomy performed (simple, skin sparing, nipple sparing, modified radical), and initial fill volumes in tissue expander reconstructions, as these will influence the rate of skin flap necrosis and subsequent complications. Importantly, a significant number of studies did not differentiate between single- and two-stage reconstructions, which may affect the results as these two techniques have different complication profiles. Due to the retrospective nature of the majority of included studies, the number of complications reported may be underestimated. Furthermore, there may be an element of publication bias as researchers are less likely to publish unfavorable results.
Conclusions
The majority of systematic reviews and plural of meta-analysis demonstrate increased complication rates in ADM-assisted expander-implant reconstruction compared to traditional submuscular techniques. However, the potential benefits, including superior outcomes in single-stage direct-to-implant surgery, improved cosmesis, lower costs and reduced incidences of capsular contracture, must also be considered. The reported protective effects of ADMs in irradiated tissue are inconsistent. Additionally, due to the diversity of available products, one of the main challenges is selecting the ideal material. There remains a paucity of literature comparing the long-term outcomes between the different types of alloplastic adjuncts and further studies are required to identify the superior adjunct.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta: American Cancer Society, Inc. 2013.
- Le GM, O'Malley CD, Glaser SL, et al. reast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival. Breast Cancer Res 2005;7:R184-93. [PubMed]
- Achauer BM, VanderKam VM, Celikoz B, et al. Augmentation of facial soft-tissue defects with Alloderm dermal graft. Ann Plast Surg 1998;41:503-7. [PubMed]
- Bastidas N, Ashjian PJ, Sharma S. Acellular dermal matrix for temporary coverage of exposed critical neurovascular structures in extremity wounds. Ann Plast Surg 2009;62:410-3. [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [PubMed]
- Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188-94. [PubMed]
- Chang HS, Lee D, Taban M, et al. “En-glove” lysis of lower eyelid retractors with AlloDerm and dermis-fat grafts in lower eyelid retraction surgery. Ophthal Plast Reconstr Surg 2011;27:137-41. [PubMed]
- Kokkalis ZT, Zanaros G, Weiser RW, et al. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am 2009;34:1029-36. [PubMed]
- Sajjadian A, Naghshineh N, Rubinstein R. Current status of grafts and implants in rhinoplasty: Part II. Homologous grafts and allogenic implants. Plast Reconstr Surg 2010;125:99e-109e. [PubMed]
- Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil 1996;17:124-36. [PubMed]
- Duncan DI. Correction of implant rippling using allograft dermis. Aesthet Surg J 2001;21:81-4. [PubMed]
- Rietjens M, De Lorenzi F, Venturino M, et al. The suspension technique to avoid the use of tissue expanders in breast reconstruction. Ann Plast Surg 2005;54:467-70. [PubMed]
- Becker H, Lind JG 2nd. The use of synthetic mesh in reconstructive, revision, and cosmetic breast surgery. Aesthetic Plast Surg 2013;37:914-21. [PubMed]
- Cheng A, Saint-Cyr M. Comparison of Different ADM Materials in Breast Surgery. Clin Plast Surg 2012;39:167-75. [PubMed]
- Dieterich M, Faridi A. Biological Matrices and Synthetic Meshes Used in Implant-based Breast Reconstruction - a Review of Products Available in Germany. Geburtshilfe Frauenheilkd 2013;73:1100-1106. [PubMed]
- Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [PubMed]
- JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg 2011;64:1553-61. [PubMed]
- Pannucci CJ, Antony AK, Wilkins EG. The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg 2013;132:1-10. [PubMed]
- Rezak KM, Gillette K, Samson MC, et al. Attitudes toward biological mesh in breast reconstruction: a regional survey of plastic surgeons. Plast Reconstr Surg 2010;126:92e-3e. [PubMed]
- Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg 2006;56:22-5. [PubMed]
- Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 2009;124:1743-53. [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [PubMed]
- Spear SL, Seruya M, Clemens MW, et al. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg 2011;127:1047-58. [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-410e. [PubMed]
- Buck DW 2nd, Heyer K, Wayne JD, et al. Diagnostic dilemma: acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg 2009;124:174e-6e. [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [PubMed]
- de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 2012;69:516-20. [PubMed]
- Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis. Plast Reconstr Surg 2011;127:2245-54. [PubMed]
- Parikh RP, Pappas-Politis E, Smith PD. Acellular dermal matrix masking detection of recurrent breast carcinoma: a novel complication. Aesthetic Plast Surg 2012;36:149-52. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [PubMed]
- Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1214-8. [PubMed]
- Nahabedian MY. Secondary nipple reconstruction using local flaps and AlloDerm. Plast Reconstr Surg 2005;115:2056-61. [PubMed]
- Wong AK, Schonmeyr B, Singh P, et al. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg 2008;121:1144-52. [PubMed]
- Rawlani V, Buck DW 2nd, Johnson SA, et al. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 2011;66:593-7. [PubMed]
- Losken A. Early Results Using Sterilized Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander Breast Reconstruction. Plast Reconstr Surg 2009. [Epub ahead of print]. [PubMed]
- Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 2009;123:1-6; discussion 107-8. [PubMed]
- Bullocks JM. DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg 2014;37:529-538. [PubMed]
- Vashi C. Clinical Outcomes for Breast Cancer Patients Undergoing Mastectomy and Reconstruction with Use of DermACELL, a Sterile, Room Temperature Acellular Dermal Matrix. Plast Surg Int 2014;2014:704323.
- Nyame TT, Lemon KP, Kolter R, et al. High-throughput assay for bacterial adhesion on acellular dermal matrices and synthetic surgical materials. Plast Reconstr Surg 2011;128:1061-8. [PubMed]
- Tessler O, Reish RG, Maman DY, et al. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg 2014;133:90e-9e. [PubMed]
- Dieterich M, Dieterich H, Timme S, et al. Using a titanium-coated polypropylene mesh (TiLOOP(®) Bra) for implant-based breast reconstruction: case report and histological analysis. Arch Gynecol Obstet 2012;286:273-6. [PubMed]
- Scheidbach H, Tannapfel A, Schmidt U, et al. Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. An animal experimental model. Eur Surg Res 2004;36:313-7. [PubMed]
- Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg 2013;132:8e-19e. [PubMed]
- Hjort H, Mathisen T, Alves A, et al. Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics. Hernia 2012;16:191-7. [PubMed]
- Baxter RA. Intracapsular allogenic dermal grafts for breast implant-related problems. Plast Reconstr Surg 2003;112:1692-6; discussion 1697-8.
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [PubMed]
- Bengtson BP, Van Natta BW, Murphy DK, et al. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg 2007;120:40S-48S. [PubMed]
- Cunningham B. The Mentor Study on Contour Profile Gel Silicone MemoryGel Breast Implants. Plast Reconstr Surg 2007;120:33S-39S. [PubMed]
- Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg 2007;120:19S-29S; discussion 30S-32S.
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [PubMed]
- Spear SL, Murphy DK, Slicton A, et al. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg 2007;120:8S-16S; discussion 17S-18S.
- Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75-89. [PubMed]
- Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2002;109:2265-74. [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg 2006;118:825-31. [PubMed]
- Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg 1998;101:53-63. [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [PubMed]
- Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part I. A systematic review. Plast Reconstr Surg 2011;127:2232-44. [PubMed]
- Zhong T, Temple-Oberle C, Hofer S, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials 2013;14:356. [PubMed]
- Garganese G, Fragomeni S, Cervelli D, et al. Titanized mesh for immediate prosthetic reconstruction of large/extra-large breasts. Int J Gynaecol Obstet 2012;119:S686-S7.
- Klein E, Kiechle MB, Paepke S. Analysis of immediate breast reconstruction with the use of titanized polypropylene mesh (TiLOOP® Bra). Eur J Surg Oncol 2013;39:482.
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [PubMed]
- Hoppe IC, Yueh JH, Wei CH, et al. Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty 2011;11:e40. [PubMed]
- Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg 2011;128:1162-9. [PubMed]
- McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:57S-66S. [PubMed]
- Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg 2015;74 Suppl 1:S30-2. [PubMed]
- Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg 2013;132:725-36. [PubMed]
- Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg 2013;70:497-9. [PubMed]
- Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg 2012;129:1223-33. [PubMed]
- Brooke S, Mesa J, Uluer M, et al. Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg 2012;69:347-9. [PubMed]
- Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg 2013;131:940-51. [PubMed]
- Endress R, Choi MS, Lee GK. Use of fetal bovine acellular dermal xenograft with tissue expansion for staged breast reconstruction. Ann Plast Surg 2012;68:338-41. [PubMed]
- Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg 2014;72:503-7. [PubMed]
- Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg 2013;66:323-8. [PubMed]
- Venturi ML, Mesbahi AN, Boehmler JH 4th, et al. Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg 2013;131:9e-18e. [PubMed]
- Seth AK, Persing S, Connor CM, et al. A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg 2013;70:632-5. [PubMed]
- Mofid MM, Meininger MS, Lacey MS. Veritas® bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products. Eur J Plast Surg 2012;35:717-22. [PubMed]
- Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 768-72. [PubMed]
- Adams WP Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg 2009;36:119-26. vii. [PubMed]
- Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg 2005;54:343-51. [PubMed]
- Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg 2007;120:275-84. [PubMed]
- Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006;118:1224-36. [PubMed]
- Clough KB, O'Donoghue JM, Fitoussi AD, et al. Prospective evaluation of late cosmetic results following breast reconstruction: I. Implant reconstruction. Plast Reconstr Surg 2001;107:1702-9. [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [PubMed]
- Macadam SA, Lennox PA. Acellular Dermal Matrices: Economic Considerations in Reconstructive and Aesthetic Breast Surgery. Clin Plast Surg 2012;39:187-216. [PubMed]
- Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg 2010;126:1842-7. [PubMed]
- Mofid MM. Acellular dermal matrix in cosmetic breast procedures and capsular contracture. Aesthet Surg J 2011;31:77S-84S. [PubMed]
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [PubMed]
- Stump A, Holton LH 3rd, Connor J, et al. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plast Reconstr Surg 2009;124:82-91. [PubMed]
- Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg 2009;123:807-16. [PubMed]
- Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg 2012;130:750-8. [PubMed]
- Mitchell RE. Porcine acellular dermis-assisted breast reconstruction: influence of adjuvant radiotherapy on complications and outcomes. Plast Reconstr Surg Glob Open 2013;1:e77. [PubMed]
- Valdatta L, Cattaneo AG, Pellegatta I, et al. Acellular dermal matrices and radiotherapy in breast reconstruction: a systematic review and meta-analysis of the literature. Plast Surg Int 2014;2014:472604.
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243-8. [PubMed]
- Zenn MR. Staged immediate breast reconstruction. Plast Reconstr Surg 2015;135:976-9. [PubMed]
- Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of infection rates and associated antibiotic duration in acellular dermal matrix breast reconstruction. Eplasty 2014;14:e42. [PubMed]
- Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 2010;125:1606-14. [PubMed]
- Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 2010;64:674-8. [PubMed]
- Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg 2013;71:464-70. [PubMed]
- Haynes DF, Kreithen JC. Vicryl mesh in expander/implant breast reconstruction: long-term follow-up in 38 patients. Plast Reconstr Surg 2014;134:892-9. [PubMed]