Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research
Introduction
Cancer is a systemic disease that initially presents local manifestations (1) and later advances in a multistep process with various hallmarks including rapid proliferation, resisting cell death, neoangiogenesis, local invasion, remote metastasis, etc. (2). Due to diverse pathologic features, genomic variations and patient outcomes in clinic oncology, no individual animal models can completely mimic this complex progress. In order to simulate different human cancer pathologies and address various research questions, there have been numerous animal cancer models including (I) transplanted tumors, (II) genetically engineered models and (III) environmentally caused cancers such as chemically induced malignancies.
Models of transplantable tumor are generated by transplantation of living cancer cell suspension or solid tumor tissue from a donor to another animal, which can be further classified as either xenograft or allograft based on whether the tumor donor and the host belong to different species or the same species of close genome (3). In the xenograft, the host animals are usually immune-compromised and tolerable to cross-species cancer cells (3). In contrast, allograft refers to the tumor transplantation strictly between the individuals from the same species. In both, the transplants can be orthotropic, subcutaneous or intravascular in order to study different stages of the tumor progression (4).
The genetically engineered model (GEM) for cancer refers to an animal strain with manipulated genomic alterations, specifically involving the overexpression of an oncogene or the loss of a tumor suppressor gene function (5). The GEMs can be simply divided into transgenic and endogenous ones (6). Such models allow the investigation of the functions of certain genes or pathways during tumor development or progression (7). Despite their sophistication in preparation, some available GEMs may accurately simulate the pathophysiology and molecular mechanism of certain human cancers (6).
Environmentally induced cancer models refer to particular cancer types developed in animals that have been exposed to certain environmental risk factor such as carcinogenic chemicals, radiation, viruses, microbial flora or even physical stimuli (8). Some GEMs are also applied in combination with the environmental exposures. Such primary cancer models can simulate the originality, evolution and consequence of clinical cancer process from the early initiation, through midterm progression and clinical onset until late exacerbation, and are ideally used to investigate the etiology, prevention, diagnosis and treatment of cancer in all stages. Furthermore, the primary tumor lesions also possess the histological complexity and heterogeneity comparable to the actual human malignancies (3).
Among the environmentally induced cancers, the chemically induced cancer model is generated by certain synthetic chemical compounds that are exposed to the body via ingestion, inhalation, injection, dermal absorption or other ways. Here, we highlight the chemically induced primary cancer models due to their diverse advantages including non- or less invasiveness, abundant tumor burden, full spectrum of cancer types, and reproducible process. Moreover, due to the very similar carcinogenesis process, chemically induced cancers mimic the human cancer occurrence from the initiating stage on.
Mechanisms of primary cancer development
Chemical carcinogenesis usually undergoes three-steps, namely initiation, promotion and progression (Figure 1). The plausible cellular and molecular mechanisms involve interactions or covalent binding of carcinogens with intracellular DNA, RNA and proteins, resulting in gene-mutational alterations (9).
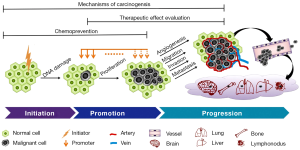
Tumor initiation, promotion and progression
Initiation is the first step in cancer development. Initiators are the chemicals that are often not reactive with DNA, but altered by drug-metabolizing enzymes in the body and are then able to cause changes in DNA (mutations) often after a covalent binding (7). Many initiators are specific to particular species or tissue types. Initiation is irreversible, i.e., once a particular cell has been affected by an initiator it is susceptible to promotion. Since initiation causes permanent genetic change, any daughter cells from the division of the mutated cell will also carry the mutation. There is a linear relationship between the dose of initiator and the quantity of produced tumor cells, i.e., the more exposure the higher risk of carcinogenesis.
Promotion is the second step that occurs on those cells already mutated by an initiator (10). The promoters refer to the compounds that promote the proliferation of the cell into a large number of daughter cells containing the mutation created by the initiator. Promoters take effect only when the organism has been previously exposed to an initiator. Unlike initiators, promoters do not covalently bind to macromolecules or DNA within the cell, but many bind to receptors on the cell surface to affect intra-cellular pathways that increase cell proliferation (11). Two categories of promoters exist: specific promoters that interact with receptors on or in target cells and nonspecific promoters that alter gene expression without involving a known receptor. Tumor growth thus promoted is dose-dependent with a threshold and a maximum effect of promoters, i.e., very low doses will not promote tumor development and extremely high doses will not produce more risk. Promoters do not necessarily cause cancer on their own, but increase the clonal expansion of initiated cells, and ultimately leads to malignancy (11).
The third step progression refers to the serial transformations from a benign tumor to a neoplasm and to malignancy. Progression is associated with karyotypic changes since most advanced tumors show aneuploidy with the wrong number of chromosomes. This karyotypic change is coupled with an increased growth rate, invasiveness, metastasis and alterations in biochemistry and morphology due to the continuing mutations or genetic instability (12). Once this step is triggered, progression is irreversible.
In the practical animal models of malignancy, a two-stage carcinogenesis strategy is frequently employed to shorten the cancer development period by treating animals with an organ-specific cancer initiator followed by a promoter (13).
Genotoxic and non-genotoxic carcinogens
The mechanisms of action of carcinogens can be simply divided into genotoxic (GTX) and non-genotoxic (NGTX) ones. GTX events may damage DNA or chromosome by direct interactions (14), while in the NGTX procedure carcinogens are capable of eliciting more diverse cellular effects largely via biotransformation, which subsequently cause multiple cellular signal transduction alterations (12,15).
GTX carcinogens, acting as electrophiles, can covalently bind to and interact with DNA which induces DNA adducts and mutations (16,17). Thus, GTX carcinogens may be considered as the tumor initiators. For example, benzo(a)pyrene (BaP) is able to form bulky BaP-DNA adducts and basic sites, or alternatively cause DNA damage by the reactive oxygen species (ROS) and metabolites produced during metabolic processing (18). From carcinogen risk assessment, GTX can be further divided into three groups: initiators with clear DNA-reactive function, borderline initiators, and weak initiators genotoxic through secondary mechanisms (12).
As opposed to GTX carcinogens, NGTX carcinogens do not affect DNA directly but target a series of physiological processes modulating cell growth, division, chronic inflammation (19), immunosuppression, ROS, steroid hormone receptor (SHR) activation and epigenetic silencing (20) by the generation of their metabolites catalyzed by cytochrome P450 (CYP) (12,21). Commonly, NGTX carcinogens, acting as promoters, function through several different modes including pro-mitogenesis by phenobarbital (PB) (22), receptor-interacting protein-mediated pathways (19) such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (23,24) and asbestos (25), gap junction intercellular communication (26) or cytotoxicity like chloroform. A classic example is 2,2,4-trimethylpentane (TMP), which is a classical promoter of renal cancer in male rat. As a representative nephrotoxic isoparaffinic component of unleaded gasoline, the metabolites of TMP can reversibly bind to alpha 2u-globulin, which decreases the catabolism of alpha 2u-globulin in the kidney and results in chronic lysosomal overload, cell death and cell compensatory proliferation leading to kidney cancer (27).
Complete and incomplete carcinogens
In terms of the participation in different carcinogenesis phases, carcinogens can be also regarded as complete or incomplete carcinogens.
The complete carcinogens have the capability to induce cancer without the participation of an additional tumor-promoting agent, which means the complete carcinogens function as both initiators and promoters simultaneously under proper dosage and exposure time (10,28), taking polycyclic aromatic hydrocarbons (PAH) as an example (23).
The incomplete carcinogens refer to the mutagenic chemicals either instigating DNA damage irreversibly or accelerating benign lesion growth, but not capable of initiating malignancy (28).
Organ-specific tumor induction by chemicals
Although there are different administrating types of carcinogens including oral, dermal, inhalation or even intraperitoneal routes, carcinogens tend to target certain particular peripheral tissues via blood circulation (29-32). More than 80% of tested chemical carcinogens were reported to be positive in at least the following 8 most frequent targeting organs mainly in different mammal species: the liver, lung, mammary gland, stomach, vascular system, kidney, hematopoietic system and urinary bladder (33). The biological basic mechanisms of underlying organ specificity are variable.
One possible explanation is that the cytochrome P450 (CYP) expression profiles in response to carcinogens in various tissue types differ markedly (34), which is critical to determine what particular tissue develops tumor (21,23). In the promotion stage, the CYP system triggers the carcinogenesis by catalyzing the activation of promoters from procarcinogen status, resulting in formation of DNA adducts (35,36). Therefore, the preference and regulation of CYP in different substrates are central to the occurrences of chemically induced malignancies in different organs (21,29,37).
For other chemical carcinogens, their sex hormone activities were also reported to contribute to the tissue specific properties. For example, in breast cancer, estrogen is a well-known risk factor. The breast carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) works as the agonists of estrogen receptor alpha (ERα) and affects miRNA specifically in mammary gland during the initiation and progression of breast cancer (38).
Classifications of chemical carcinogens
A wide variety of chemical carcinogens have been proven capable of inducing cancers in experimental animals after prolonged or excessive exposures, comprising cooked meat derived carcinogens, food additives, industrial chemicals and environmental pollutants, tobacco smoke carcinogens, antineoplastic agents, naturally occurring compounds and synthetic carcinogens (Table 1) (16,23,49).
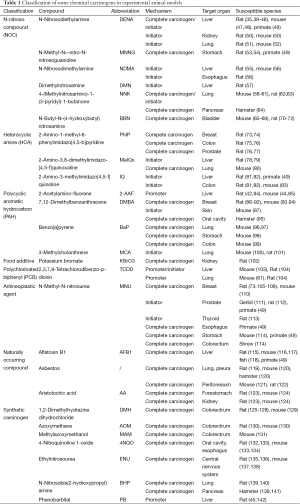
Full table
N-Nitroso compounds
N-nitroso compounds (NOCs) have initially been brought into attention for their carcinogenicity most likely from preserved food (143,144), drinking water contamination (145,146) and tobacco smoking (147).
Some NOCs like N-nitrosodiethylamine (DENA) and N-methyl-N-nitro-N-nitrosoguanidine (MNNG), can be endogenously converted in our stomach and bowel from nitrites that are usually added to preserve processed meats like bacon, ham, sausage (143,144).
DENA is a powerful and consistent hepatocarcinogen that is able to induce multifocal primary liver tumors such as hepatocellular carcinoma (HCC) superimposed on varying degrees of liver cirrhosis not only in rodents (39,148) but in monkey as well (49). Importantly, DENA-induced hepatocarcinogenesis can highly simulate the histopathological evolution of clinical liver cancers (149). DENA is usually administrated to male Sprague Dawley (SD) or Wistar rats (150-300 g) in the drinking water at 100 ppm (40), or by frequent gavage feeding at 10 mg/kg body weight (41,150). However, this classical liver cancer model has been proven time consuming, as the appearance of recognizable tumor lesions in most cases occurs after a quite long latent period, normally 16-32 weeks after the first DENA exposure. In addition, DENA also acts as initiator for renal and pulmonary cancers in rodents (50-53).
MNNG is a well-known gastric carcinogen in rodents and primates (49,54,151).
N-nitrosodimethylamine (NDMA) acts as a initiator of hepatocarcinogenesis (56) and esophageal carcinogenesis (57) in rodents, while dimethylnitrosamine (DMN), the NDMA’s precursor, is used as a mutagen for liver cancer in rats (152).
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its major metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) are powerful determinants of pulmonary cancer incidence, and biomarkers indicating the exposure of tobacco smoke (147). For the experimental model, female A/J mouse is a more sensitive strain to study lung cancer (58-60,62,63,153-155). Practically, mice treated with a single intraperitoneal injection of NNK (100 mg/kg body weight) developed lung cancer by 30 weeks at a 100% tumor incidence (61,156). Furthermore, exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) combined with NNK could significantly increase lung cancer development (61). Besides, hamsters developed pancreatic cancer as well upon NNK exposure, but with a lower organ-specificity (64).
Exposure of rodents to N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) reproducibly induces high-grade and invasive squamous cell urinary bladder cancers (65-67,70-72), which resembles the development of the most human bladder cancer based on histopathology (157). For mice, after 2-6 weeks treatment of BBN at 500 ppm in the drinking water, mutagenesis in urothelial cells was greater than the spontaneous mutation background and that in the smooth muscle cells of the urinary bladder (68); after 20 weeks of BBN treatment at the same concentration, bladder cancer developed in all treated mice (66). For models in both F334 and Wistar rats, bladder cancers were highly induced during 6-8 months after 8- to 14-week single exposure of BBN at 500 ppm in the drinking water (70). In a two-stage model, phenylethyl isothiocyanate (PEITC) was reported to promote the development of transitional cell carcinomas at 1,000 ppm in diet from the 4th to 8th week combined with BBN administration (13).
Heterocyclic amines
Red meats, cooked at high temperature and for long periods of time, tend to promote several carcinogenic compounds such as heterocyclic amines (HCAs) (143,158,159).
HCAs represent a group of mutagenic compounds which are able to increases the risk of cancer in various organs in rodents (16,23,49). Three HCAs are frequently used in animal cancer models, i.e., 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) (49).
PhIP is the most abundant HCA subtype, which has complete carcinogenicity of DNA damaging and mutagenic activities as an initiator, and estrogenic activity as a promoter as well. Exposure of rats to PhIP can cause cancers in breast (73), colorectum (75,76) and prostate (76,77).
MeIQx exposure leads to increased liver and lung cancer risks in rodents mainly as a genotoxic carcinogen during the initiation phase (78-80).
Similarly, IQ is a genotoxic carcinogen in rodents to induce liver and colon tumors (81-83), and in primate as well to induce liver cancer (49).
Polycyclic aromatic hydrocarbons
Similarly, polycyclic aromatic hydrocarbons (PAHs) are also generated during meat grilling and roasting (143,158). They include 2-acetylamino-fluorene (2-AAF), 7,12-dimethylbenzanthracene (DMBA), benz[a]anthracene (BaA), benzo[a]pyrene (BaP), and 3-methylcholanthrene (MCA), all with high carcinogenic properties (160-162).
2-AAF has been largely used as a promoter in the 8-week Solt-Farber protocol for multistep experimental hepatocarcinogenesis in rats. Briefly, initiation is triggered by a single intraperitoneal injection of DENA, normally at a dose of 200 mg/kg body weight; the initiated hepatocytes are then promoted by either feeding rats a daily diet containing 2-AAF or by gavage of 4-6 single doses (normally 20 mg/kg body weight/day) of 2-AAF on consecutive days at 1-2 weeks after DENA initiation; afterwards rats are subjected to a 2/3 partial hepatectomy to stimulate mitosis of selected initiated hepatocytes (42-44,84,163,164). Immunohistochemical staining of the placental glutathione s-transferase (GST-P) is frequently used as an accurate marker enzyme to detect liver preneoplastic focal lesions in liver cancer and indicates the endpoint of evaluation of certain chemopreventive agents (42,43,164). In mice models, 2-AAF was also found to induce liver mutations (85,165).
As a complete carcinogen, DMBA is more efficient than the other PAHs in inducing breast cancer, which is routinely used in female SD rats at the age of around 50 days (86,87). Previous studies have proved that a single dose of 50-100 mg/kg of DMBA by gavage is able to cause mammary tumors at the incidence of 100% (87). Furthermore, the developed cancerous lumps are morphologically heterogeneous and hormone dependent (86). However, in the mouse skin squamous carcinogenetic model, DMBA is used as an initiator combined with the promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) (166).
BaP is a main suggested indicator of PAH, which causes cancers usually in the lung, stomach, colon and other organs and tissues (98,99). Meanwhile, their chlorinated compounds, e.g., Cl-BaA, have also been reported showing the organ-specific distribution and even greater accumulation compared with their parents, and presenting carcinogenic impacts in various organs like the liver (167).
MCA serves as an initiator of lung cancer in rodents, usually followed by the promoter butylated hydroxytoluene (BHT) (100,168).
Potassium bromate
Potassium bromate (KBrO3) used to be a flour improver widely used as a food additive in the bread-making process, while bromate is also a disinfection byproduct formed in drinking water (169). KBrO3 acts as a complete carcinogen in renal cancer development in rat models (102).
Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are a class of dioxin-like carcinogens, which are formed as the industrial by-products and known as the widespread environmental contaminants. The best studied member is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (104).
TCDD has no direct genotoxicity but acts as a multi-site, multi-species tumor promoter, which might be mediated either by the agonist-specific activation of hydrocarbon receptor (AhR) that leads to the transcription of cytochrome P450 enzymes and induces oxidative stress, or by the alterations of Akt and ERK apoptosis signaling pathways which causes attenuation of apoptosis (61,103,104,170).
N-Methyl-N-nitrosourea
N-Methyl-N-nitrosourea (MNU) was originally designed as a chemotherapeutic alkylating compound, but later proven to exert direct mammary carcinogenic effects (171), a single dose of which has been shown to induce breast cancer in SD rats (105,106). Notably, although both MUN and DMBA-induced mammary cancers are generally hormone-dependent (4), MUN-induced mammary cancers tend to be locally aggressive and spontaneously metastasized, which are quite different from DMBA-induced breast tumors, and thus suitable for study of malignant progression or the advanced stage of mammary cancers (172). Besides, MNU is also able to enhance various cancers in experimental animals such as esophageal, gastric and colorectal malignancies (49,111,114), and initiate prostate and thyroid cancers (49,112,113,173).
Naturally occurring compounds
- flatoxins are a group of mycotoxins naturally produced from Aspergillus flavus or Aspergillus parasiticus as secondary fungal metabolites. They frequently contaminate foods and impose a global problem (174). Aflatoxin B1 (AFB1) is a well-known hepatocarcinogen acting with potent mutagenicity and cytotoxicity in experimental models (49,115-118,175-177);
- Asbestos fibers consist of several distinct types occurring in soils and rocks, which used to be heavily used in the industry. Asbestos has been identified to produce oxygen derivatives in cytotoxicity and DNA double strand breaks extensively (178,179). In the experimental murine models, asbestos has been used to develop pleural and peritoneal malignant mesotheliomas by intratracheal instillation and intraperitoneal injection receptively (119-122,125);
- Aristolochic acids (AAs) are major components extracted from Aristolochia plants, which used to be an ancient herbal medicine, but have been identified as a strong nephrotoxin for the first time by Belgian scientists in 1991 (180-182). In rodents, AA is a potent carcinogen. Long term oral administration of AA both in rats and mice leads to multiple tumors largely in forestomach and kidney (123,124).
Synthetic carcinogens
- 1,2-Dimethylhydrazine dihydrochloride (DMH) and its inter-metabolites azoxymethane (AOM) and methylazoxymethanol (MAM) are a group of synthetic compounds with the general structure of cycasin, which are highly specific complete carcinogens for rodent colorectal tumor models (126-132,183);
- 4-Nitroquinoline 1-oxide (4NQO) is a manufactured derivative of quinolone for research purposes, possessing a heterocyclic aromatic structure. As a complete carcinogen, 4NQO is able to induce all the stages of oral squamous cell carcinoma, which has been identified to highly mimic the histological and molecular features in human oral cancers (133,184);
- Ethylnitrosourea (ENU) is a highly potent mutagen first reported by Bill Russell et al. in 1979, which is largely used as neurotropic carcinogen to generate glioma rat models (135,136). Additionally, infant Big Blue transgenic mice are susceptive to ENU-induced hepatic mutation (185); and exposure to ENU markedly increases mammary tumor multiplicity and incidence in mice defective in Apc tumor suppressor gene (137);
- N-Nitrosobis(2-hydroxypropyl)amine (BHP) is a hydroxylated dipropylnitrosamine first synthesized by Krüger et al. in 1974 (139). The complete carcinogenetic effects of BHP alter due to the routes of treatment and the host species as well. For instance, BHP is known as a pancreatic carcinogen by subcutaneous injection in Syrian golden hamsters (140,141), but a pulmonary carcinogen by oral administration in Wistar rats (139,142);
- As a synthetic non-genotoxic carcinogen or promoter, phenobarbital (PB) compound is extensively used in rodents to enhance hepatic and renal carcinogenesis (35,45,186).
Experimental applications
The above-specified chemicals only exemplify a little bit of the immense known and unknown carcinogens, of which the experimental applications are still preliminary and empirical, leaving rooms for further exploitations. Nevertheless, primary cancer models are useful tools for performing various fundamental and translational investigations including (I) understanding biological and molecular mechanisms involved in tumor initiation, promotion and progression; (II) looking into influential and preventive factors of carcinogenesis; and (III) evaluating the diagnostic/therapeutic potentials of candidate drugs on induced models of primary tumors (Figure 1).
Related genes and pathways in carcinogenetic process
Cancer development and progression may involve multiple abnormal aspects such as sustained proliferation, resisting cell death, migration and metastasis, angiogenesis, interaction with immune system, reprogramming energy metabolism, etc. (2). By using many multistage carcinogens, the molecular changes associated with the initiation and promotion of cancer development are possible to be analyzed.
A range of genomic alterations and somatic mutations of different oncogenes and tumor suppressors have been recently found in various human malignancies, while the mutation-inducing factors remain not fully elucidated. For instance, in human lung adenomas and adenocarcinomas, the epidermal growth factor receptor (EGFR) gene alterations have been frequently reported; from the representative rodent lung cancer models generated by NNK, BHP, MeIQx, urethane, as well as X-ray, the mutation patterns of Egfr and K-ras were able to be detected and compared with equivalent human lesions to better understand the upstream regulation (156). Moreover, using a cross-species analysis of global gene expression profiles, differentially expressed genes and relative altered molecular pathways allow mining of the promising target genes for anti-cancer strategies (67).
In addition, when combined with carcinogen treatments, GEM such as p16, p19, p53, Apc, c-Ha-ras, myr-Akt, TGF-α and Kfl6 models show their unique advantages, which not only enable the assessment on effects of these genes on the tumor progression, but provide abundant information about the regulation of intracellular signaling networks (47,73,138,187-192).
Cancer preventive and influential factors for tumorigenesis
Research innovations have contributed to cancer therapeutic strategies by developing new technologies or drugs. However, according to the overview of global cancer burden issued from WHO, overall cancer incidence and mortality rates worldwide have been rising (193). Therefore, more attentions and efforts are required for research in the field of cancer prevention. Especially, as one of the most promising approaches to reduce cancer risks, chemoprevention is a method to suppress, delay or even reverse carcinogenesis during the early stage by means of specific chemical substances (84,98). In the experimental animal models, the evaluation of preventive effects of various drugs on tumor formation and progression can be achieved simply by early administration of the drug together with the carcinogen treatment (66,194) or even in advance of the carcinogen exposure (35,195).
Recently, many natural compounds extracted from medicinal plants such as resveratrol, curcumin, capsaicin, allicin, etc. have been reported to demonstrate chemopreventive activities (43,196), largely because of their potent activities against oxidative stress and toxic injuries that continuously occur during the entire carcinogenetic process including initiation, promotion and progression. For example, in the case of the chemically induced rat hepatocarcinogenesis, lutein, a kind of carotenoid existing in green leafy vegetables, inhibited hepatic preneoplastic lesions when administrated during promotion and DNA damage stage (44). Some other natural substances, e.g., aqueous extract of the dried leaf of A. compressa, a component of herbal tea in Mexico, astragalus membranaceus, a natural herbal medicine in traditional Chinese medicine for liver diseases (164), and betaine, particularly found in wheat, spinach and sugar beets (40) are also promising to delay rat hepatocarcinogenesis (195).
Preclinical evaluations of new specific contrast agents
Diagnostic imaging provides essential information for early diagnosis, precise staging, timely clinical treatment, and appropriated palliative therapies of various cancers in clinical patients. However, the main challenge lies in the relatively insufficient soft-tissue contrast between malignant lesion and surrounding tissues. In this context, different new specific contrast agents in preclinical settings have been explored, which extend the applications of different imaging modalities from anatomical structure to functional assessment or even to molecular imaging, particularly in the field of MRI, which offers superior life tissue contrast (3). In previous preclinical evaluations of MRI diagnosis in DENA-induced rat liver cancers, multifocal liver tumors were better detected and characterized by using liver specific contrast agents such as Mn-DPDP and Gd-EOB-DTPA relative to nonspecific contrast agents Gd-DTPA and Gd-DOTA (3,148).
Therapeutic strategies for primary malignancies
Chemically induced cancer models are also widely used to explore the therapeutic potencies of various treatments (39,66,86,87,166,194,197,198), owning to the ability to better mimic the complexity of clinical multistep malignant process, and to be more predictive in preclinical studies as well as basic research. For instance, in the study on the Rapamycin treatment upon the two-stage mouse skin tumor model, the cancer development was first initiated by DMBA and subsequently promoted by phorbol esters, which allowed to test the therapeutic effects of Rapamycin during the entire process of carcinogenesis, including both the early lesions around 9-10 weeks after the initiation and the advanced lesions after approximately 16-18 weeks of initiation (166).
Advantages and disadvantages
Among commonly adopted cancer models, chemically induced primary malignancies in mammal have multiple advantages including the eased procedures, abundant tumor generation and high analogy to human primary cancers seen in the clinic.
First of all, chemically induced primary cancer models are simple and economical due to the fact that carcinogens can be easily administrated via single intraperitoneal injection, continuous gavage feeding, repeated subcutaneous injection, daily drinking water or routine dietary supplement, requiring neither experienced manipulators nor complicated facilities or devices.
Secondly, carcinogen induced tumor models are productive owning to high success rate and multifocal lesions simultaneously induced in the targeted organs. Moreover, animal primary cancer models can produce a full spectrum of primary cancers, frequently with various sizes, differentiation degrees, and even tumor origins. Take DENA-initiated rodent hepatocarcinogenesis as an example, multifocal primary liver tumors on general liver cirrhosis can be successfully induced in SD rats after months of DENA gavage feeding (199). This enables the comparison between benign and malignant neoplasia, tumor lesions and paratumor tissues in the same carrier, thus contributing to reliable evaluation of therapeutic effects of newly discovered drugs, and exploratory study of diagnostic potentials of different imaging technologies (148,200).
Additionally, primary cancer models such as DENA-induced hepatocarcinogenesis (149), BBN-induced bladder cancer (157), NNK-induced lung cancer (62) and 4NQO-induced oral carcinogenesis (133) can ideally resemble the clinical course and evolution of multiple human cancers with respect to the similar morphology, histopathology characteristics and molecular changes. Furthermore, different carcinogens are able to cause different cancer subtypes with variant cancer biologic features. For instance, DMBA and MNU are two classical carcinogens frequently used to induce mammary tumors. The majority of DMBA- induced tumors are hormone dependent, while MUN-induced tumors tend to be hormone independent and aggressive (107,172). Therefore, primary cancer models also provide us with a tool with great value for better understanding of the mechanisms of cancer pathogenesis.
However, there are also some disadvantages of such models. In addition to the time-consuming progress of carcinogenesis, the major drawback is the difficulty in noninvasive tumor burden assessment in small living animals, because, like cancer patients in reality, tumors occur unpredictably and heterogeneously in terms of timing, location, number, size, differentiation and blood supply of lesions (3). Especially for the visceral tumors in the liver, lung, kidney or pancreas, it is quite difficult in detection of newly generated tumor lesions and monitor of their growth in individual organs. Consequently, this brings challenges in grouping and defining the endpoints of model building-up. In fact, for some experimental studies aiming at investigating cancer development, chemoprevention or cancer therapy of primary cancers in animals, tumor formation and therapeutic effects can only be assessed by histopathology via sacrificing the animals and removing the target organs (35,66), or via biopsy during the process as a supplemental method (200).
Workable solution: noninvasive MR imaging
Compared with the other clinical imaging techniques like computed tomography (CT) or ultrasonography (US), and nuclear imaging such as single photon emission computed tomography (SPECT) or positron emission tomography (PET), MRI and US are noninvasive and harmless modalities for the diagnosis and therapeutic evaluation of visceral tumors. However, the application of US for animals, especially rodents, is limited mainly due to the small size of animals and relative low sensitivity as a result of the low frequency of the usual echography probes. On the other hand, multiparametric MRI (MP-MRI) has been increasingly used for detection, localization, stage and even characterization of experimental models with primary cancers. Importantly, all these imaging results in small animals like rats can be simply achieved by clinical MR scanners (3).
Detection and diagnosis
Early diagnosis of multifocal visceral tumors has been a big challenge in both clinical and experimental situations. MRI can be ideally used for the morphological evaluation of primary cancers owing to the high sensitivity, resolution and, consequently, the excellent soft tissue contrasts.
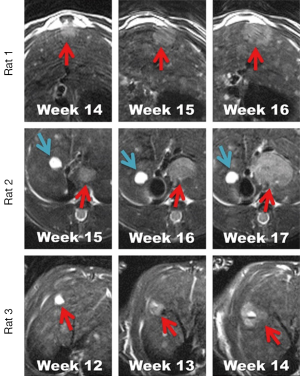
For morphological imaging, tumor lesions tend to appear hyperintense on T2 weighted imaging (T2WI), but iso- to slight hypointense on T1 weighted imaging (T1WI), which can become hyperintense by contrast-enhanced T1WI after using conventional extracellular contrast agents (CAs) like gadoterate meglumine (Gd-DOTA). According to the earlier report about the application of MRI performed in a 1.0T Magnetom SP (Siemens, Erlangen, Germany) on primary liver cancer induced by DENA, tumor lesions can be detected with a minimum diameter of 2 mm, and in numbers varying from 1 to ≥10 (199). Recently, by using a clinical 3.0T whole body MR magnet (Trio; Siemens, Erlangen, Germany) for monitoring hepatic tumor growth we also managed to observe the original small tumor lesions continuously, which enabled us to differentiate between the static benign lesion and fast-growing malignant HCC (Figure 2). Moreover, during the real progress of cancer generation in rat models, MRI could reveal complex intratumoral changes along with tumor growth, for instance tumoral hemorrhage (Figure 3), which helped us to intensively follow up the dynamic changes of local lesions, evaluate the systemic condition of tumor-bearing animals, make proper decision on the following treatment, and secure the designed experiments being successfully performed in animals with other simultaneously occurring tumor lesions.
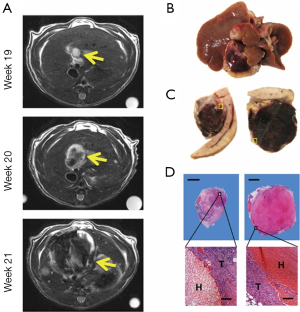
By using organ-specific CAs, MRI can provide various functional information of tumor lesions. For instance, the hepatobiliary CAs, such as mangafodipir trisodium (Mn-DPDP), gadoxetic acid disodium (Gd-EOB-DTPA) and gadobenate dimeglumine (Gd-BOPTA), can be specifically taken up by hepatocytes through corresponding transmembrane proteins and excreted into bile canaliculi through conjugated export pumps. Therefore, these organ-specific CAs may help distinguish tumor and tumor-bearing organ, relatively well differentiated and poorly differentiated lesions with or without normal biological functions (3,199).
Treatment and therapeutic monitoring
In addition to the in vivo monitoring of tumor growth before treatment, MRI, as a harmless imaging technique without ionizing radiation, is also strong at providing real-time follow-up imaging serially at different time points as frequently as every several minutes, hours or days, which enables comparing the therapeutic effects.
For evaluation of therapeutic effects, MRI also provides multi-parametric functional information (201). For instance, dynamic contrast enhanced (DCE)-MRI can help evaluate tumor vascular properties including blood flow, blood volume, vascular permeability and extravascular extracellular space, while apparent diffusion coefficient (ADC) derived from diffusion-weighted imaging (DWI) can help distinguish the lytic necrosis of less restricted diffusion caused by therapy from the non-reacted viable tumor (202-206).
Developing new diagnostics and therapeutics or theragnostics
Generally, effective anticancer therapy may cause tumor growth inhibition or even tumor necrosis. Necrosis avid contrast agents (NACAs) have been largely explored in the past decade, which is used to image physiological and biochemical processes under in vivo conditions. Hypericin is a nonporphyrin NACA, extracted from the plant Hypericum perforatum or semi-synthesized from emodin anthraquinone (207,208). In necrosis, aminophospholipids of phosphatidylethanolamine (PE) and phosphatidylserine (PS), primarily located in the intracellular membrane, are externalized from the inner leaflet to the outer leaflet of the cell membrane (209,210). Radiolabelled hypericin has been designed to target exposed PE and PS with the breakdown of cell membrane asymmetry. Using in vivo MRI and scintigraphy, the previous studies have investigated the promising therapeutic effect of the combined treatment strategy of CA4P (combretastatin A4 phosphate)/131I-Hyp (hypericin) or briefly OncoCiDia (211,212). To further expand the utility of hypericin to radionuclide imaging, the radiotracers such as 123I-Hyp and 64Cu-Bis-DOTA-Hyp have recently been synthesized, which allow detection and quantification with microSPECT and microPET/CT (60,207,208) with possibility to be studied in chemically induced tumor models.
Acknowledgements
Funding: This work has partially been supported by KU Leuven projects IOF-HB/08/009 and IOF-HB/12/018, the KU Leuven Molecular Small Animal Imaging Center MoSAIC (KUL EF/05/08) and European Union (Asia-Link CfP 2006-EuropeAid/123738/ACT/Multi-Proposal No. 128-498/111).
Footnote
Conflicts of Interest: YW Liu is sponsored by a scholarship from the center of excellence in vivo molecular imaging research (IMIR). The corresponding author is a Bayer Lecture Chair holder.
References
- Zajicek G. Cancer as a systemic disease. Med Hypotheses 1978;4:193-207. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Ni Y, Wang H, Chen F, Li J, DeKeyzer F, Feng Y, Yu J, Bosmans H, Marchal G. Tumor models and specific contrast agents for small animal imaging in oncology. Methods 2009;48:125-38. [PubMed]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer 2007;7:659-72. [PubMed]
- Doetschman T. GI GEMs: genetically engineered mouse models of gastrointestinal disease. Gastroenterology 2011;140:380-385.e2.
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer 2007;7:645-58. [PubMed]
- Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech 2008;1:78-82. [PubMed]
- Lyman GH. Risk factors for cancer. Prim Care 1992;19:465-79. [PubMed]
- Taningher M, Saccomanno G, Santi L, Grilli S, Parodi S. Quantitative predictability of carcinogenicity of the covalent binding index of chemicals to DNA: comparison of the in vivo and in vitro assays. Environ Health Perspect 1990;84:183-92. [PubMed]
- Trosko JE. Commentary: is the concept of “tumor promotion” a useful paradigm? Mol Carcinog 2001;30:131-7. [PubMed]
- Klaunig JE, Kamendulis LM, Xu Y. Epigenetic mechanisms of chemical carcinogenesis. Hum Exp Toxicol 2000;19:543-55. [PubMed]
- Oliveira PA, Colaço A, Chaves R, Guedes-Pinto H, De-La-Cruz P LF, Lopes C. Chemical carcinogenesis. An Acad Bras Cienc 2007;79:593-616. [PubMed]
- Yafune A, Taniai E, Morita R, Akane H, Kimura M, Mitsumori K, Shibutani M. Immunohistochemical cellular distribution of proteins related to M phase regulation in early proliferative lesions induced by tumor promotion in rat two-stage carcinogenesis models. Exp Toxicol Pathol 2014;66:1-11. [PubMed]
- Enoch SJ, Cronin MT. A review of the electrophilic reaction chemistry involved in covalent DNA binding. Crit Rev Toxicol 2010;40:728-48. [PubMed]
- Golan DE, Tashjian AH. Principles of pharmacology: the pathophysiologic basis of drug therapy. 3rd Edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2011.
- Williams GM. Mechanisms of chemical carcinogenesis and application to human cancer risk assessment. Toxicology 2001;166:3-10. [PubMed]
- Neumann HG. The role of DNA damage in chemical carcinogenesis of aromatic amines. J Cancer Res Clin Oncol 1986;112:100-6. [PubMed]
- Hassan AM, Alam SS, Abdel-Aziem SH, Ahmed KA. Benzo-a-pyrene induced genotoxicity and cytotoxicity in germ cells of mice: Intervention of radish and cress. J Genet Eng Biotechnol 2011;9:65-72.
- Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin 2008;29:1275-88. [PubMed]
- Chen T. The role of MicroRNA in chemical carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2010;28:89-124. [PubMed]
- Pelkonen O, Raunio H. Metabolic activation of toxins: tissue-specific expression and metabolism in target organs. Environ Health Perspect 1997;105 Suppl 4:767-74. [PubMed]
- Gonzales AJ, Christensen JG, Preston RJ, Goldsworthy TL, Tlsty TD, Fox TR. Attenuation of G1 checkpoint function by the non-genotoxic carcinogen phenobarbital. Carcinogenesis 1998;19:1173-83. [PubMed]
- Luch A. Nature and nurture - lessons from chemical carcinogenesis. Nat Rev Cancer 2005;5:113-25. [PubMed]
- Walker NJ, Tritscher AM, Sills RC, Lucier GW, Portier CJ. Hepatocarcinogenesis in female Sprague-Dawley rats following discontinuous treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 2000;54:330-7. [PubMed]
- Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, Franzoso G, Carbone M. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A 2006;103:10397-402. [PubMed]
- Trosko JE. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J Biochem Mol Biol 2003;36:43-8. [PubMed]
- Short BG, Steinhagen WH, Swenberg JA. Promoting effects of unleaded gasoline and 2,2,4-trimethylpentane on the development of atypical cell foci and renal tubular cell tumors in rats exposed to N-ethyl-N-hydroxyethylnitrosamine. Cancer Res 1989;49:6369-78. [PubMed]
- Pitot HC, Dragan YP. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J 1991;5:2280-6. [PubMed]
- Irigaray P, Belpomme D. Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis 2010;31:135-48. [PubMed]
- Bartsch H, Margison GP, Malaveille C, Camus AM, Brun G, Margison JM, Kolar GF, Wiessler M. Some aspects of metabolic activation of chemical carcinogens in relation to their organ specificity. Arch Toxicol 1977;39:51-63. [PubMed]
- Hirose M, Shirai T, Takahashi S, Ogawa K, Ito N. Organ-Specific Modification of Carcinogenesis by Antioxidants in Rats. In: Bronzetti G, Hayatsu H, Flora SD, Waters MD, Shankel DM, Associates CW&, editors. Antimutagenesis and Anticarcinogenesis Mechanisms III. New Youk: Springer US, 1993:181-8.
- Flora SD, Izzotti A, Bennicelli C. Mechanisms of Antimutagenesis and Anticarcinogenesis: Role in Primary Prevention. In: Bronzetti G, Hayatsu H, Flora SD, Waters MD, Shankel DM, Associates CW&, editors. Antimutagenesis and Anticarcinogenesis Mechanisms III. New Youk: Springer US, 1993:1-16.
- Gold LS, Slone TH, Manley NB, Bernstein L. Target organs in chronic bioassays of 533 chemical carcinogens. Environ Health Perspect 1991;93:233-46. [PubMed]
- Kawajiri K, Fujii-Kuriyama Y. P450 and human cancer. Jpn J Cancer Res 1991;82:1325-35. [PubMed]
- Chakraborty T, Chatterjee A, Rana A, Dhachinamoorthi D, Kumar P A, Chatterjee M. Carcinogen-induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: chemopreventive role of vanadium on this process. Biochim Biophys Acta 2007;1772:48-59.
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-51. [PubMed]
- Yamazaki H, Kamataki T. P450 and carcinogenesis. Nihon Yakurigaku Zasshi 2002;119:208-12. [PubMed]
- Papaioannou MD, Koufaris C, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) elicits estrogenic-like microRNA responses in breast cancer cells. Toxicol Lett 2014;229:9-16. [PubMed]
- Hanajiri K, Mitsui H, Maruyama T, Hashimoto N, Sata M, Omata M. Echographic detection of diethylnitrosamine-induced liver tumors in rats and the effect of the intratumoral injection of an inhibitor of c-Jun N-terminal kinase. J Gastroenterol Hepatol 2009;24:866-71. [PubMed]
- Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer 2009;9:261. [PubMed]
- Liu D, Zhi L, Ma M, Qiao D, Wang M, Wang Y, Jin B, Li A, Liu G, Zhang Y, Song Y, Zhang H. Primarily screening and analyzing ESTs differentially expressed in rats' primary liver cancer. Chin J Cancer Res 2013;25:71-8. [PubMed]
- Gauttier V, Pichard V, Aubert D, Kaeppel C, Schmidt M, Ferry N, Conchon S. No tumour-initiating risk associated with scAAV transduction in newborn rat liver. Gene Ther 2013;20:779-84. [PubMed]
- Kim TM, Lee HS, Shim TJ, Kim HY, Woo KS, Jeong HS, Kim DJ. Preventive effect of thiacremonone on the hepatocarcinogenesis initiated by N -nitrosodiethylamine in rats. Food Sci Biotechnol 2012;21:1277-84.
- Moreno FS, Toledo LP, de Conti A, Heidor R, Jordão A Jr, Vannucchi H, Cardozo MT, Ong TP. Lutein presents suppressing but not blocking chemopreventive activity during diethylnitrosamine-induced hepatocarcinogenesis and this involves inhibition of DNA damage. Chem Biol Interact 2007;168:221-8. [PubMed]
- Cardani R, Zavanella T. Decreased density of beta1-adrenergic receptors in preneoplastic and neoplastic liver lesions of F344 rats. Histol Histopathol 2005;20:843-50. [PubMed]
- Hayashi H, Shimamoto K, Taniai E, Ishii Y, Morita R, Suzuki K, Shibutani M, Mitsumori K. Liver tumor promoting effect of omeprazole in rats and its possible mechanism of action. J Toxicol Sci 2012;37:491-501. [PubMed]
- Tarocchi M, Hannivoort R, Hoshida Y, Lee UE, Vetter D, Narla G, Villanueva A, Oren M, Llovet JM, Friedman SL. Carcinogen-induced hepatic tumors in KLF6+/- mice recapitulate aggressive human hepatocellular carcinoma associated with p53 pathway deregulation. Hepatology 2011;54:522-31. [PubMed]
- Moto M, Okamura M, Muguruma M, Ito T, Jin M, Kashida Y, Mitsumori K. Gene expression analysis on the dicyclanil-induced hepatocellular tumors in mice. Toxicol Pathol 2006;34:744-51. [PubMed]
- Takayama S, Thorgeirsson UP, Adamson RH. Chemical carcinogenesis studies in nonhuman primates. Proc Jpn Acad Ser B Phys Biol Sci 2008;84:176-88. [PubMed]
- Vargas-Olvera CY, Sánchez-González DJ, Solano JD, Aguilar-Alonso FA, Montalvo-Muñoz F, Martínez-Martínez CM, Medina-Campos ON, Ibarra-Rubio ME. Characterization of N-diethylnitrosamine-initiated and ferric nitrilotriacetate-promoted renal cell carcinoma experimental model and effect of a tamarind seed extract against acute nephrotoxicity and carcinogenesis. Mol Cell Biochem 2012;369:105-17. [PubMed]
- Pracheta P, Sharma V, Singh L, Paliwal R, Sharma S, Yadav S, Sharma S. Chemopreventive effect of hydroethanolic extract of Euphorbia neriifolia leaves against DENA-induced renal carcinogenesis in mice. Asian Pac J Cancer Prev 2011;12:677-83. [PubMed]
- Liu WB, Ao L, Zhou ZY, Cui ZH, Zhou YH, Yuan XY, Xiang YL, Cao J, Liu JY. CpG island hypermethylation of multiple tumor suppressor genes associated with loss of their protein expression during rat lung carcinogenesis induced by 3-methylcholanthrene and diethylnitrosamine. Biochem Biophys Res Commun 2010;402:507-14. [PubMed]
- Khanduja KL, Gandhi RK, Pathania V, Syal N. Prevention of N-nitrosodiethylamine-induced lung tumorigenesis by ellagic acid and quercetin in mice. Food Chem Toxicol 1999;37:313-8. [PubMed]
- Bilici M, Cayir K, Tekin SB, Gundogdu C, Albayrak A, Suleyman B, Ozogul B, Erdemci B, Suleyman H. Effect of mirtazapine on MNNG-induced gastric adenocarcinoma in rats. Asian Pac J Cancer Prev 2012;13:4897-900. [PubMed]
- Tsutsumi M, Matsuda Y, Takada A. Role of ethanol-inducible cytochrome P-450 2E1 in the development of hepatocellular carcinoma by the chemical carcinogen, N-nitrosodimethylamine. Hepatology 1993;18:1483-9. [PubMed]
- Beebe LE, Kim YE, Amin S, Riggs CW, Kovatch RM, Anderson LM. Comparison of transplacental and neonatal initiation of mouse lung and liver tumors by N-nitrosodimethylamine (NDMA) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and promotability by a polychlorinated biphenyls mixture (Aroclor 1254). Carcinogenesis 1993;14:1545-8. [PubMed]
- Ribeiro Pinto LF, Swann PF. Opium and oesophageal cancer: effect of morphine and opium on the metabolism of N-nitrosodimethylamine and N-nitrosodiethylamine in the rat. Carcinogenesis 1997;18:365-9. [PubMed]
- Kreisel D, Gelman AE, Higashikubo R, Lin X, Vikis HG, White JM, Toth KA, Deshpande C, Carreno BM, You M, Taffner SM, Yokoyama WM, Bui JD, Schreiber RD, Krupnick AS. Strain-specific variation in murine natural killer gene complex contributes to differences in immunosurveillance for urethane-induced lung cancer. Cancer Res 2012;72:4311-7. [PubMed]
- Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Chemoprevention with acetylsalicylic acid, vitamin D and calcium reduces risk of carcinogen-induced lung tumors. Anticancer Res 2013;33:4767-70. [PubMed]
- Gordon W, Galitovskiy V, Edwards R, Andersen B, Grando SA. The tobacco carcinogen nitrosamine induces a differential gene expression response in tumour susceptible A/J and resistant C3H mouse lungs. Eur J Cancer 2013;49:725-33. [PubMed]
- Chen RJ, Siao SH, Hsu CH, Chang CY, Chang LW, Wu CH, Lin P, Wang YJ. TCDD promotes lung tumors via attenuation of apoptosis through activation of the Akt and ERK1/2 signaling pathways. PLoS One 2014;9:e99586. [PubMed]
- Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, Roberts JC. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J Biochem Mol Toxicol 2005;19:396-405. [PubMed]
- Bhatnagar S, Chaudhary N, Katare DP, Jain SK. A non-surgical method for induction of lung cancer in Wistar rats using a combination of NNK and high dietary fats. Protoplasma 2013;250:919-29. [PubMed]
- Zhang L, Weddle DL, Thomas PE, Zheng B, Castonguay A, Schuller HM, Miller MS. Low levels of expression of cytochromes P-450 in normal and cancerous fetal pancreatic tissues of hamsters treated with NNK and/or ethanol. Toxicol Sci 2000;56:313-23. [PubMed]
- Parada B, Reis F, Pinto A, Sereno J, Xavier-Cunha M, Neto P, Rocha-Pereira P, Mota A, Figueiredo A, Teixeira F. Chemopreventive Efficacy of Atorvastatin against Nitrosamine-Induced Rat Bladder Cancer: Antioxidant, Anti-Proliferative and Anti-Inflammatory Properties. Int J Mol Sci 2012;13:8482-99. [PubMed]
- Kim WJ, Lee JW, Quan C, Youn HJ, Kim HM, Bae SC. Nicotinamide inhibits growth of carcinogen induced mouse bladder tumor and human bladder tumor xenograft through up-regulation of RUNX3 and p300. J Urol 2011;185:2366-75. [PubMed]
- Lu Y, Liu P, Wen W, Grubbs CJ, Townsend RR, Malone JP, Lubet RA, You M. Cross-species comparison of orthologous gene expression in human bladder cancer and carcinogen-induced rodent models. Am J Transl Res 2010;3:8-27. [PubMed]
- He Z, Kosinska W, Zhao ZL, Wu XR, Guttenplan JB. Tissue-specific mutagenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine as the basis for urothelial carcinogenesis. Mutat Res 2012;742:92-5. [PubMed]
- Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Oral chemoprevention with acetyl salicylic Acid, vitamin D and calcium reduces the risk of tobacco carcinogen-induced bladder tumors in mice. Cancer Invest 2013;31:490-3. [PubMed]
- Sagara Y, Miyata Y, Nomata K, Hayashi T, Kanetake H. Green tea polyphenol suppresses tumor invasion and angiogenesis in N-butyl-(-4-hydroxybutyl) nitrosamine-induced bladder cancer. Cancer Epidemiol 2010;34:350-4. [PubMed]
- Lubet RA, Fischer SM, Steele VE, Juliana MM, Desmond R, Grubbs CJ. Rosiglitazone, a PPAR gamma agonist: potent promoter of hydroxybutyl(butyl)nitrosamine-induced urinary bladder cancers. Int J Cancer 2008;123:2254-9. [PubMed]
- Prasain JK, Jones K, Moore R, Barnes S, Leahy M, Roderick R, Juliana MM, Grubbs CJ. Effect of cranberry juice concentrate on chemically-induced urinary bladder cancers. Oncol Rep 2008;19:1565-70. [PubMed]
- Naiki-Ito A, Asamoto M, Hokaiwado N, Takahashi S, Yamashita H, Tsuda H, Ogawa K, Shirai T. Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res 2007;67:11353-8. [PubMed]
- Smith LP, Thomas GR. Animal models for the study of squamous cell carcinoma of the upper aerodigestive tract: a historical perspective with review of their utility and limitations. Part A. Chemically-induced de novo cancer, syngeneic animal models of HNSCC, animal models of transplanted xenogeneic human tumors. Int J Cancer 2006;118:2111-22. [PubMed]
- Wang R, Dashwood WM, Nian H, Löhr CV, Fischer KA, Tsuchiya N, Nakagama H, Ashktorab H, Dashwood RH. NADPH oxidase overexpression in human colon cancers and rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Int J Cancer 2011;128:2581-90. [PubMed]
- Tang MX, Ogawa K, Asamoto M, Chewonarin T, Suzuki S, Tanaka T, Shirai T. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr Cancer 2011;63:227-33. [PubMed]
- Tang D, Kryvenko ON, Wang Y, Trudeau S, Rundle A, Takahashi S, Shirai T, Rybicki BA. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in benign prostate and subsequent risk for prostate cancer. Int J Cancer 2013;133:961-71. [PubMed]
- Murai T, Mori S, Kang JS, Morimura K, Wanibuchi H, Totsuka Y, Fukushima S. Evidence of a threshold-effect for 2-amino-3,8-dimethylimidazo-[4,5-f]quinoxaline liver carcinogenicity in F344/DuCrj rats. Toxicol Pathol 2008;36:472-7. [PubMed]
- Mori Y, Koide A, Kobayashi Y, Furukawa F, Hirose M, Nishikawa A. Effects of cigarette smoke and a heterocyclic amine, MeIQx on cytochrome P-450, mutagenic activation of various carcinogens and glucuronidation in rat liver. Mutagenesis 2003;18:87-93. [PubMed]
- Takeuchi H, Saoo K, Yamakawa K, Matsuda Y, Yokohira M, Zeng Y, Kuno T, Totsuka Y, Takahashi M, Wakabayashi K, Imaida K. Tumorigenesis of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), but not enhancing effects of concomitant high-fat diet, on lung carcinogenesis in female A/J mice. Oncol Lett 2010;1:137-142. [PubMed]
- Wei M, Wanibuchi H, Nakae D, Tsuda H, Takahashi S, Hirose M, Totsuka Y, Tatematsu M, Fukushima S. Low-dose carcinogenicity of 2-amino-3-methylimidazo[4,5-f ]quinoline in rats: Evidence for the existence of no-effect levels and a mechanism involving p21(Cip / WAF1). Cancer Sci 2011;102:88-94. [PubMed]
- Xu M, Orner GA, Bailey GS, Stoner GD, Horio DT, Dashwood RH. Post-initiation effects of chlorophyllin and indole-3-carbinol in rats given 1,2-dimethylhydrazine or 2-amino-3-methyl- imidazo. Carcinogenesis 2001;22:309-14. [PubMed]
- Andreassen A, Møllersen L, Vikse R, Steffensen IL, Mikalsen A, Paulsen JE, Alexander J. One dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) or 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) induces tumours in Min/+ mice by truncation mutations or LOH in the Apc gene. Mutat Res 2002;517:157-66. [PubMed]
- Sehrawat A, Sultana S. Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci 2006;79:1456-65. [PubMed]
- Hoogervorst EM, van Oostrom CT, Beems RB, van Benthem J, van den Berg J, van Kreijl CF, Vos JG, de Vries A, van Steeg H. 2-AAF-induced tumor development in nucleotide excision repair-deficient mice is associated with a defect in global genome repair but not with transcription coupled repair. DNA Repair (Amst) 2005;4:3-9. [PubMed]
- Ferreira I, Ferreira J, Vollet-Filho JD, Moriyama LT, Bagnato VS, Salvadori DM, Rocha NS. Photodynamic therapy for the treatment of induced mammary tumor in rats. Lasers Med Sci 2013;28:571-7. [PubMed]
- Bhardwaj V, Ankola DD, Gupta SC, Schneider M, Lehr CM, Ravi Kumar MN. PLGA Nanoparticles Stabilized with Cationic Surfactant: Safety Studies and Application in Oral Delivery of Paclitaxel to Treat Chemical-Induced Breast Cancer in Rat. Pharmaceutical Research 2009;26:2495-503. [PubMed]
- Samuelson E, Nilsson J, Walentinsson A, Szpirer C, Behboudi A. Absence of Ras mutations in rat DMBA-induced mammary tumors. Mol Carcinog 2009;48:150-5. [PubMed]
- de Assis S, Wang M, Jin L, Bouker KB, Hilakivi-Clarke LA. Exposure to excess estradiol or leptin during pregnancy increases mammary cancer risk and prevents parity-induced protective genomic changes in rats. Cancer Prev Res (Phila) 2013;6:1194-211. [PubMed]
- Hakkak R, Al-Dwairi A, Fuchs GJ, Korourian S, Simmen FA. Dietary soy protein induces hepatic lipogenic enzyme gene expression while suppressing hepatosteatosis in obese female Zucker rats bearing DMBA-initiated mammary tumors. Genes Nutr 2012;7:549-58. [PubMed]
- Mafuvadze B, Benakanakere I, López Pérez FR, Besch-Williford C, Ellersieck MR, Hyder SM. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev Res (Phila) 2011;4:1316-24. [PubMed]
- Murray SA, Yang S, Demicco E, Ying H, Sherr DH, Hafer LJ, Rogers AE, Sonenshein GE, Xiao ZX. Increased expression of MDM2, cyclin D1, and p27Kip1 in carcinogen-induced rat mammary tumors. J Cell Biochem 2005;95:875-84. [PubMed]
- Siddiqui RA, Harvey KA, Walker C, Altenburg J, Xu Z, Terry C, Camarillo I, Jones-Hall Y, Mariash C. Characterization of synergistic anti-cancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer 2013;13:418. [PubMed]
- Wang T, Gavin HM, Arlt VM, Lawrence BP, Fenton SE, Medina D, Vorderstrasse BA. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int J Cancer 2011;128:1509-23. [PubMed]
- Mang T, Kost J, Sullivan M, Wilson BC. Autofluorescence and Photofrin-induced fluorescence imaging and spectroscopy in an animal model of oral cancer. Photodiagnosis Photodyn Ther 2006;3:168-76. [PubMed]
- Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Vinodhkumar R, Devaki T. Stabilization of pulmonary mitochondrial enzyme system by capsaicin during benzo(a)pyrene induced experimental lung cancer. Biomed Pharmacother 2008;62:390-4. [PubMed]
- Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Vinodhkumar R, Devaki T. Capsaicin modulates pulmonary antioxidant defense system during benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother Res 2008;22:529-33. [PubMed]
- Goyal PK, Verma P, Sharma P, Parmar J, Agarwal A. Evaluation of anti-cancer and anti-oxidative potential of Syzygium Cumini against benzo[a]pyrene (BaP) induced gastric carcinogenesis in mice. Asian Pac J Cancer Prev 2010;11:753-8. [PubMed]
- Diggs DL, Myers JN, Banks LD, Niaz MS, Hood DB, Roberts LJ 2nd, Ramesh A. Influence of dietary fat type on benzo(a)pyrene [B(a)P] biotransformation in a B(a)P-induced mouse model of colon cancer. J Nutr Biochem 2013;24:2051-63. [PubMed]
- Brown LM, Malkinson AM, Rannels DE, Rannels SR. Compensatory lung growth after partial pneumonectomy enhances lung tumorigenesis induced by 3-methylcholanthrene. Cancer Res 1999;59:5089-92. [PubMed]
- Malkinson AM, Koski KM, Evans WA, Festing MF. Butylated hydroxytoluene exposure is necessary to induce lung tumors in BALB mice treated with 3-methylcholanthrene. Cancer Res 1997;57:2832-4. [PubMed]
- Wei M, Hamoud AS, Yamaguchi T, Kakehashi A, Morimura K, Doi K, Kushida M, Kitano M, Wanibuchi H, Fukushima S. Potassium bromate enhances N-ethyl-N-hydroxyethylnitrosamine-induced kidney carcinogenesis only at high doses in Wistar rats: indication of the existence of an enhancement threshold. Toxicol Pathol 2009;37:983-91. [PubMed]
- Wu CH, Chen HL, Su HJ, Lee CC, Shen KT, Ho WL, Ho SY, Ho YS, Wang YJ. The topical application of 2,3,7,8-tetrachlorodibenzo-p-dioxin lacks skin tumor-promoting potency but induces hepatic injury and tumor necrosis factor-alpha expression in ICR male mice. Food Chem Toxicol 2004;42:1217-25. [PubMed]
- Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res 2006;50:897-907. [PubMed]
- Pula B, Malicka I, Pawlowska K, Paslawska U, Cegielski M, Podhorska-Okolow M, Dziegiel P, Wozniewski M. Immunohistochemical characterization of N-methyl-N-nitrosourea-induced mammary tumours of Sprague-Dawley rats. In Vivo 2013;27:793-801. [PubMed]
- Yuri T, Danbara N, Tsujita-Kyutoku M, Fukunaga K, Takada H, Inoue Y, Hada T, Tsubura A. Dietary docosahexaenoic acid suppresses N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats more effectively than eicosapentaenoic acid. Nutr Cancer 2003;45:211-7. [PubMed]
- Zysk AM, Chaney EJ, Boppart SA. Refractive index of carcinogen-induced rat mammary tumours. Phys Med Biol 2006;51:2165-77. [PubMed]
- Faustino-Rocha AI, Silva A, Gabriel J, Teixeira-Guedes CI, Lopes C, Gil da Costa R, Gama A, Ferreira R, Oliveira PA, Ginja M. Ultrasonographic, thermographic and histologic evaluation of MNU-induced mammary tumors in female Sprague-Dawley rats. Biomed Pharmacother 2013;67:771-6. [PubMed]
- Barathidasan R, Pawaiya RS, Rai RB, Dhama K. Upregulated Myc expression in N-methyl nitrosourea (MNU)- induced rat mammary tumours. Asian Pac J Cancer Prev 2013;14:4883-9. [PubMed]
- Pazos P, Lanari C, Meiss R, Charreau EH, Pasqualini CD. Mammary carcinogenesis induced by N-methyl-N-nitrosourea (MNU) and medroxyprogesterone acetate (MPA) in BALB/c mice. Breast Cancer Res Treat 1992;20:133-8. [PubMed]
- Yang J, Shikata N, Mizuoka H, Tsubura A. Colon carcinogenesis in shrews by intrarectal infusion of N-methyl-N-nitrosourea. Cancer Lett 1996;110:105-12. [PubMed]
- Gonçalves BF, de Campos SG, Zanetoni C, Scarano WR, Falleiros LR Jr, Amorim RL, Góes RM, Taboga SR. A new proposed rodent model of chemically induced prostate carcinogenesis: distinct time-course prostate cancer progression in the dorsolateral and ventral lobes. Prostate 2013;73:1202-13. [PubMed]
- Guru Kumar D, Parvathi V, Meenakshi P, Rathi MA, Gopalakrishnan VK. Anticancer activity of the ethanolic extract of Crateva nurvala bark against testosterone and MNU-induced prostate cancer in rats. Chin J Nat Med 2012;10:334-8.
- Leung WK, Wu KC, Wong CY, Cheng AS, Ching AK, Chan AW, Chong WW, Go MY, Yu J, To KF, Wang X, Chui YL, Fan DM, Sung JJ. Transgenic cyclooxygenase-2 expression and high salt enhanced susceptibility to chemical-induced gastric cancer development in mice. Carcinogenesis 2008;29:1648-54. [PubMed]
- Johnson NM, Egner PA, Baxter VK, Sporn MB, Wible RS, Sutter TR, Groopman JD, Kensler TW, Roebuck BD. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev Res (Phila) 2014;7:658-65. [PubMed]
- Sivanesan D, Begum VH. Modulatory effect of Gynandropsis gynandra L. on glucose metabolizing enzymes in aflatoxin B1-induced hepatocellular carcinoma in rats. Indian J Biochem Biophys 2007;44:477-80. [PubMed]
- Woo LL, Egner PA, Belanger CL, Wattanawaraporn R, Trudel LJ, Croy RG, Groopman JD, Essigmann JM, Wogan GN, Bouhenguel JT. Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1 mice. Toxicol Sci 2011;122:38-44. [PubMed]
- Liu SP, Li YS, Lee CM, Yen CH, Liao YJ, Huang SF, Chien CH, Chen YM. Higher susceptibility to aflatoxin B(1)-related hepatocellular carcinoma in glycine N-methyltransferase knockout mice. Int J Cancer 2011;128:511-23. [PubMed]
- Bernstein DM, Rogers RA, Sepulveda R, Donaldson K, Schuler D, Gaering S, Kunzendorf P, Chevalier J, Holm SE. The pathological response and fate in the lung and pleura of chrysotile in combination with fine particles compared to amosite asbestos following short-term inhalation exposure: interim results. Inhal Toxicol 2010;22:937-62. [PubMed]
- Yamaguchi R, Hirano T, Ootsuyama Y, Asami S, Tsurudome Y, Fukada S, Yamato H, Tsuda T, Tanaka I, Kasai H. Increased 8-hydroxyguanine in DNA and its repair activity in hamster and rat lung after intratracheal instillation of crocidolite asbestos. Jpn J Cancer Res 1999;90:505-9. [PubMed]
- Robinson C, Woo S, Walsh A, Nowak AK, Lake RA. The antioxidants vitamins A and E and selenium do not reduce the incidence of asbestos-induced disease in a mouse model of mesothelioma. Nutr Cancer 2012;64:315-22. [PubMed]
- Robinson C, Alfonso H, Woo S, Olsen N, Bill Musk AW, Robinson BW, Nowak AK, Lake RA. Effect of NSAIDS and COX-2 inhibitors on the incidence and severity of asbestos-induced malignant mesothelioma: evidence from an animal model and a human cohort. Lung Cancer 2014;86:29-34. [PubMed]
- Schmeiser HH, Janssen JW, Lyons J, Scherf HR, Pfau W, Buchmann A, Bartram CR, Wiessler M. Aristolochic acid activates ras genes in rat tumors at deoxyadenosine residues. Cancer Res 1990;50:5464-9. [PubMed]
- Wang Y, Arlt VM, Roufosse CA, McKim KL, Myers MB, Phillips DH, Parsons BL. ACB-PCR measurement of H-ras codon 61 CAA→CTA mutation provides an early indication of aristolochic acid I carcinogenic effect in tumor target tissues. Environ Mol Mutagen 2012;53:495-504. [PubMed]
- Okazaki Y, Nagai H, Chew SH, Li J, Funahashi S, Tsujimura T, Toyokuni S. CD146 and insulin-like growth factor 2 mRNA-binding protein 3 predict prognosis of asbestos-induced rat mesothelioma. Cancer Sci 2013;104:989-95. [PubMed]
- Hamiza OO, Rehman MU, Tahir M, Khan R, Khan AQ, Lateef A, Ali F, Sultana S. Amelioration of 1,2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac J Cancer Prev 2012;13:4393-402. [PubMed]
- Terai K, Sakamoto K, Goto M, Matsuda M, Kasamaki S, Shinmura K, Takita N, Kamano T. Greater development of 1,2-dimethylhydrazine-induced colon cancer in a rat model of type 2 diabetes mellitus. J Int Med Res 2006;34:385-9. [PubMed]
- Mennuni C, Ugel S, Mori F, Cipriani B, Iezzi M, Pannellini T, Lazzaro D, Ciliberto G, La Monica N, Zanovello P, Bronte V, Scarselli E. Preventive vaccination with telomerase controls tumor growth in genetically engineered and carcinogen-induced mouse models of cancer. Cancer Res 2008;68:9865-74. [PubMed]
- Sánchez Negrette M, Montenegro MA, Catuogno MS, Lértora WJ. Decrease of intestinal tumors induced by 1,2-dimethylhydrazine in rats fed with cow milk and buffalo milk. Biocell 2007;31:391-6.
- Hernández-Salazar M, Guevara-González RG, Cruz-Hernández A, Guevara-Olvera L, Bello-Pérez LA, Castaño-Tostado E, Loarca-Piña G. Flaxseed (Linum usitatissimum L.) and its total non-digestible fraction influence the expression of genes involved in azoxymethane-induced colon cancer in rats. Plant Foods Hum Nutr 2013;68:259-67. [PubMed]
- Pandurangan AK, Ananda Sadagopan SK, Dharmalingam P, Ganapasam S. Luteolin, a bioflavonoid inhibits Azoxymethane-induced colorectal cancer through activation of Nrf2 signaling. Toxicol Mech Methods 2014;24:13-20. [PubMed]
- Pratesi G, Manzotti C, Damia G, D'Incalci M. Response of chemically induced primary colon tumours of the mouse to flavone acetic acid (NSC 347 512). Br J Cancer 1988;58:144-6. [PubMed]
- Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol 2006;42:655-67. [PubMed]
- Liu YC, Ho HC, Lee MR, Lai KC, Yeh CM, Lin YM, Ho TY, Hsiang CY, Chung JG. Early induction of cytokines/cytokine receptors and Cox2, and activation of NF-κB in 4-nitroquinoline 1-oxide-induced murine oral cancer model. Toxicol Appl Pharmacol 2012;262:107-16. [PubMed]
- Bulnes S, Argandoña EG, Bengoetxea H, Leis O, Ortuzar N, Lafuente JV. The role of eNOS in vascular permeability in ENU-induced gliomas. Acta Neurochir Suppl 2010;106:277-82. [PubMed]
- Zook BC, Simmens SJ, Jones RV. Evaluation of ENU-induced gliomas in rats: nomenclature, immunochemistry, and malignancy. Toxicol Pathol 2000;28:193-201. [PubMed]
- Karabinis ME, Larson D, Barlow C, Wynshaw-Boris A, Moser AR. Heterozygosity for a mutation in Brca1 or Atm does not increase susceptibility to ENU-induced mammary tumors in Apc(Min)/+ mice. Carcinogenesis 2001;22:343-6. [PubMed]
- Morrison JP, Satoh H, Foley J, Horton JL, Dunnick JK, Kissling GE, Malarkey DE. N-ethyl-N-nitrosourea (ENU)-induced meningiomatosis and meningioma in p16(INK4a)/p19(ARF) tumor suppressor gene-deficient mice. Toxicol Pathol 2007;35:780-7. [PubMed]
- Tsujiuchi T, Nakae D, Konishi Y. Multi-step lung carcinogenesis model induced by oral administration of N-nitrosobis(2-hydroxypropyl)amine in rats. Exp Toxicol Pathol 2014;66:81-8. [PubMed]
- Ushijima T, Tsutsumi M, Sakai R, Ishizaka Y, Takaku F, Konishi Y, Takahashi M, Sugimura T, Nagao M. Ki-ras activation in pancreatic carcinomas of Syrian hamsters induced by N-nitrosobis(2-hydroxypropyl)amine. Jpn J Cancer Res 1991;82:965-8. [PubMed]
- Matsuzaki M, Natori T, Mizumoto K, Kitazawa S, Tsutsumi M, Konishi Y. Intracellular distribution of pancreatic tumor antigen purified from BHP-induced adenocarcinomas in Syrian hamsters. Pancreas 1989;4:429-35. [PubMed]
- Tsujiuchi T, Sasaki Y, Tsutsumi M, Konishi Y. Mutations of the Smad2 and Smad4 genes in lung adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine in rats. Mol Carcinog 2000;29:87-91. [PubMed]
- Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer 2008;60:131-44. [PubMed]
- Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012;106:603-7. [PubMed]
- Andrea I. Schäfer, William Mitch, Sophie Walewijk, Albert Munoz, Emma Teuten, Martin Reinhard. Chapter 7 Micropollutants in Water Recycling: A Case Study of N-Nitrosodimethylamine (NDMA) Exposure from Water versus Food. Available online: http://www.sciencedirect.com/science/article/pii/S1871271109002074
- Mitch WA, Sharp JO, Trussell RR, Valentine RL, Alvarez-Cohen L, Sedlak DL. N-Nitrosodimethylamine as a drinking water contaminant: A review 2003 Environmental Engineering Science 2003;20:389-404.
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst 1999;91:1194-210. [PubMed]
- Ni Y, Marchal G, Yu J, Mühler A, Lukito G, Baert AL. Prolonged positive contrast enhancement with Gd-EOB-DTPA in experimental liver tumors: potential value in tissue characterization. J Magn Reson Imaging 1994;4:355-63. [PubMed]
- Yang FC, Zheng SS, Jiang TA. A modified rat model for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2004;3:585-7. [PubMed]
- Song Y, Jin SJ, Cui LH, Ji XJ, Yang FG. Immunomodulatory effect of Stichopus japonicus acid mucopolysaccharide on experimental hepatocellular carcinoma in rats. Molecules 2013;18:7179-93. [PubMed]
- Arivazhagan S, Balasenthil S, Nagini S. Garlic and neem leaf extracts enhance hepatic glutathione and glutathione dependent enzymes during N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced gastric carcinogenesis in rats. Phytother Res 2000;14:291-3. [PubMed]
- Kang KW, Ha JR, Kim CW, Kim ND, Kim SG. 2-(Allylthio)pyrazine, a cancer chemopreventive agent, inhibits liver fibrosis induced by dimethylnitrosamine in rats: role of inhibition of transforming growth factor-beta1 expression. Pharmacol Toxicol 2001;89:23-9. [PubMed]
- Franklin MR, Moos PJ, El-Sayed WM, Aboul-Fadl T, Roberts JC. Pre- and post-initiation chemoprevention activity of 2-alkyl/aryl selenazolidine-4(R)-carboxylic acids against tobacco-derived nitrosamine (NNK)-induced lung tumors in the A/J mouse. Chem Biol Interact 2007;168:211-20. [PubMed]
- Abdel-Aziz HO, Takasaki I, Tabuchi Y, Nomoto K, Murai Y, Tsuneyama K, Takano Y. High-density oligonucleotide microarrays and functional network analysis reveal extended lung carcinogenesis pathway maps and multiple interacting genes in NNK [4-(methylnitrosamino)-1-(3-pyridyle)-1-butanone] induced CD1 mouse lung tumor. J Cancer Res Clin Oncol 2007;133:107-15. [PubMed]
- Chaudhary N, Bhatnagar S, Malik S, Katare DP, Jain SK. Proteomic analysis of differentially expressed proteins in lung cancer in Wistar rats using NNK as an inducer. Chem Biol Interact 2013;204:125-34. [PubMed]
- Kitahashi T, Takahashi M, Yamada Y, Oghiso Y, Yokohira M, Imaida K, Tsutsumi M, Takasuka N, Sugimura T, Wakabayashi K. Occurrence of mutations in the epidermal growth factor receptor gene in X-ray-induced rat lung tumors. Cancer Sci 2008;99:241-5. [PubMed]
- Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res 2008;68:1593-600. [PubMed]
- Ward MH, Cross AJ, Divan H, Kulldorff M, Nowell-Kadlubar S, Kadlubar FF, Sinha R. Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis 2007;28:1210-6. [PubMed]
- Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, Gammon MD, Ammerman AS, Sandler RS. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol 2003;157:434-45. [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. 2010. Available online: http://monographs.iarc.fr/ENG/Monographs/vol92/
- Europen commission. Opinion of the Scientific Committee on Food on the risks to human health of Polycyclic Aromatic Hydrocarbons in food. 2002. Available online: http://ec.europa.eu/food/fs/sc/scf/out153_en.pdf
- Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 2002;110 Suppl 3:451-88. [PubMed]
- Espandiari P, Glauert HP, Lehmler HJ, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol 2003;186:55-62. [PubMed]
- Cui R, He J, Wang B, Zhang F, Chen G, Yin S, Shen H. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol 2003;51:75-80. [PubMed]
- Jenkins GJ, Parry JM. Restriction site mutation (RSM) analysis of 2-acetylaminofluorene (2-AAF)-induced mouse liver mutations and comparison with the measurement of in vivo micronucleus induction in the bone marrows of (2-AAF)-treated mice. Teratog Carcinog Mutagen 2000;20:107-17. [PubMed]
- Amornphimoltham P, Leelahavanichkul K, Molinolo A, Patel V, Gutkind JS. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res 2008;14:8094-101. [PubMed]
- Sakakibara H, Ohura T, Kido T, Yamanaka N, Tanimura N, Shimoi K, Guruge KS. Organ-specific distribution of 7-chlorinated benz[a]anthracene and regulation of selected cytochrome P450 genes in rats. J Toxicol Sci 2013;38:137-43. [PubMed]
- Polat F, Ozdemir O, Elagoz S. Analysis of Ki-ras Exon 2 Gene Mutations in 3-Methylcholanthrene and Butylated Hydroxytoluene-Induced Rat Lung Tissues. Turk J Biol 2008;32:277-82.
- Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y. Toxicity and carcinogenicity of potassium bromate--a new renal carcinogen. Environ Health Perspect 1990;87:309-35. [PubMed]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 2000;59:65-85. [PubMed]
- Tsubura A, Lai YC, Miki H, Sasaki T, Uehara N, Yuri T, Yoshizawa K. Review: Animal models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. In Vivo 2011;25:11-22. [PubMed]
- Perše M, Cerar A, Injac R, Štrukelj B. N -methylnitrosourea Induced Breast Cancer in Rat, the Histopathology of the Resulting Tumours and its Drawbacks as a Model. Pathology & Oncology Research 2009;15:115-21. [PubMed]
- Mori M, Naito M, Watanabe H, Takeichi N, Dohi K, Ito A. Effects of sex difference, gonadectomy, and estrogen on N-methyl-N-nitrosourea induced rat thyroid tumors. Cancer Res 1990;50:7662-7. [PubMed]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev 2003;16:497-516. [PubMed]
- Smela ME, Currier SS, Bailey EA, Essigmann JM. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis 2001;22:535-45. [PubMed]
- Bbosa GS, Kitya D, Odda J, Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health 2013;5:14-34. [PubMed]
- Arana S, Dagli MLZ, Sabino M, Tabata YA, Rigolino MG, Hernández-Blázquez FJ. Evaluation of the efficacy ofhydrated sodium aluminosilicate in the prevention of aflatoxininduced hepatic cancer in rainbow trout. Pesqui Vet Bras 2011;31:751-5.
- Okayasu R, Takahashi S, Yamada S, Hei TK, Ullrich RL. Asbestos and DNA double strand breaks. Cancer Res 1999;59:298-300. [PubMed]
- Dong H, Buard A, Renier A, Lévy F, Saint-Etienne L, Jaurand MC. Role of oxygen derivatives in the cytotoxicity and DNA damage produced by asbestos on rat pleural mesothelial cells in vitro. Carcinogenesis 1994;15:1251-5. [PubMed]
- Chen CH, Dickman KG, Moriya M, Zavadil J, Sidorenko VS, Edwards KL, Gnatenko DV, Wu L, Turesky RJ, Wu XR, Pu YS, Grollman AP. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci U S A 2012;109:8241-6. [PubMed]
- Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D, Verbeelen D, Vanhaelen-Fastre R, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet 1993;341:387-91. [PubMed]
- Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis 2002;17:265-77. [PubMed]
- Perše M, Cerar A. The dimethylhydrazine induced colorectal tumours in rat - experimental colorectal carcinogenesis. Radiol Oncol 2005;39:61-70.
- Wong PN, Wilson DF. 4 Nitroquinoline 1-oxide-induced carcinogenesis in the rat palate. J Oral Pathol 1983;12:375-84. [PubMed]
- Mei N, Heflich RH, Moore MM, Chen T. Age-dependent sensitivity of Big Blue transgenic mice to the mutagenicity of N-ethyl-N-nitrosourea (ENU) in liver. Mutat Res 2005;572:14-26. [PubMed]
- Iatropoulos MJ, Wang CX, von Keutz E, Williams GM. Assessment of chronic toxicity and carcinogenicity in an accelerated cancer bioassay in rats of Nifurtimox, an antitrypanosomiasis drug. Exp Toxicol Pathol 2006;57:397-404. [PubMed]
- Hu Y, Le Leu RK, Belobrajdic D, Young GP. The potential of sphingomyelin as a chemopreventive agent in AOM-induced colon cancer model: wild-type and p53+/- mice. Mol Nutr Food Res 2008;52:558-66. [PubMed]
- Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R, Slotta-Huspenina J, Bader FG, Prazeres da Costa O, Neurath MF, Meining A, Kirchner T, Greten FR. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 2013;23:93-106. [PubMed]
- Gommeaux J, Cano C, Garcia S, Gironella M, Pietri S, Culcasi M, Pébusque MJ, Malissen B, Dusetti N, Iovanna J, Carrier A. Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Mol Cell Biol 2007;27:2215-28. [PubMed]
- Thliveris AT, Clipson L, White A, Waggoner J, Plesh L, Skinner BL, Zahm CD, Sullivan R, Dove WF, Newton MA, Halberg RB. Clonal structure of carcinogen-induced intestinal tumors in mice. Cancer Prev Res (Phila) 2011;4:916-23. [PubMed]
- Blanco-Aparicio C, Pérez-Gallego L, Pequeño B, Leal JF, Renner O, Carnero A. Mice expressing myrAKT1 in the mammary gland develop carcinogen-induced ER-positive mammary tumors that mimic human breast cancer. Carcinogenesis 2007;28:584-94. [PubMed]
- Wentz SC, Wu H, Yip-Schneider MT, Hennig M, Klein PJ, Sebolt-Leopold J, Schmidt CM. Targeting MEK is effective chemoprevention of hepatocellular carcinoma in TGF-alpha-transgenic mice. J Gastrointest Surg 2008;12:30-7. [PubMed]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Ravichandran N, Suresh G, Ramesh B, Manikandan R, Choi YW, Vijaiyan Siva G. Fisetin modulates mitochondrial enzymes and apoptotic signals in benzo(a)pyrene-induced lung cancer. Mol Cell Biochem 2014;390:225-34. [PubMed]
- González de Mejía E, Ramírez-Mares MV, Arce-Popoca E, Wallig M, Villa-Treviño S. Inhibition of liver carcinogenesis in Wistar rats by consumption of an aqueous extract from leaves of Ardisia compressa. Food Chem Toxicol 2004;42:509-16. [PubMed]
- Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett 2004;215:129-40. [PubMed]
- Nicastro HL, Grubbs CJ, Margaret Juliana M, Bode AM, Kim MS, Lu Y, You M, Milne GL, Boring D, Steele VE, Lubet RA. Preventive effects of NSAIDs, NO-NSAIDs, and NSAIDs plus difluoromethylornithine in a chemically induced urinary bladder cancer model. Cancer Prev Res (Phila) 2014;7:246-54. [PubMed]
- Schoop RA, Baatenburg de Jong RJ, Noteborn MH. Apoptin induces apoptosis in an oral cancer mouse model. Cancer Biol Ther 2008;7:1368-73. [PubMed]
- Ni Y, Marchal G, van Damme B, van Hecke P, Michiels J, Zhang X, Yu J, Baert AL. Magnetic resonance imaging, microangiography, and histology in a rat model of primary liver cancer. Invest Radiol 1992;27:689-97. [PubMed]
- Lemmer ER, Vessey CJ, Gelderblom WC, Shephard EG, Van Schalkwyk DJ, Van Wijk RA, Marasas WF, Kirsch RE, Hall Pde L. Fumonisin B1-induced hepatocellular and cholangiocellular tumors in male Fischer 344 rats: potentiating effects of 2-acetylaminofluorene on oval cell proliferation and neoplastic development in a discontinued feeding study. Carcinogenesis 2004;25:1257-64. [PubMed]
- Wang H, Marchal G, Ni Y. Multiparametric MRI biomarkers for measuring vascular disrupting effect on cancer. World J Radiol 2011;3:1-16. [PubMed]
- Wang H, Van de Putte M, Chen F, De Keyzer F, Jin L, Yu J, Marchal G, de Witte P, Ni Y. Murine liver implantation of radiation-induced fibrosarcoma: characterization with MR imaging, microangiography and histopathology. Eur Radiol 2008;18:1422-30. [PubMed]
- Wang H, Sun X, Chen F, De Keyzer F, Yu J, Landuyt W, Vandecaveye V, Peeters R, Bosmans H, Hermans R, Marchal G, Ni Y. Treatment of rodent liver tumor with combretastatin a4 phosphate: noninvasive therapeutic evaluation using multiparametric magnetic resonance imaging in correlation with microangiography and histology. Invest Radiol 2009;44:44-53. [PubMed]
- Wang H, Li J, Chen F, De Keyzer F, Yu J, Feng Y, Nuyts J, Marchal G, Ni Y. Morphological, functional and metabolic imaging biomarkers: assessment of vascular-disrupting effect on rodent liver tumours. Eur Radiol 2010;20:2013-26. [PubMed]
- Sun X, Wang H, Chen F, De Keyzer F, Yu J, Jiang Y, Feng Y, Li J, Marchal G, Ni Y. Diffusion-weighted MRI of hepatic tumor in rats: comparison between in vivo and postmortem imaging acquisitions. J Magn Reson Imaging 2009;29:621-8. [PubMed]
- Wang H, Cona MM, Chen F, Yu J, Feng Y, Li J, Keyzer FD, Marchal G, Ni Y. Comparison of two vascular-disrupting agents at a clinically relevant dose in rodent liver tumors with multiparametric magnetic resonance imaging biomarkers. Anticancer Drugs 2012;23:12-21. [PubMed]
- Ni Y, Huyghe D, Verbeke K, de Witte PA, Nuyts J, Mortelmans L, Chen F, Marchal G, Verbruggen AM, Bormans GM. First preclinical evaluation of mono-[123I]iodohypericin as a necrosis-avid tracer agent. Eur J Nucl Med Mol Imaging 2006;33:595-601. [PubMed]
- Fonge H, Vunckx K, Wang H, Feng Y, Mortelmans L, Nuyts J, Bormans G, Verbruggen A, Ni Y. Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin microSPECT. Eur Heart J 2008;29:260-9. [PubMed]
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997;89:1121-32. [PubMed]
- Marconescu A, Thorpe PE. Coincident exposure of phosphatidylethanolamine and anionic phospholipids on the surface of irradiated cells. Biochim Biophys Acta 2008;1778:2217-24.
- Li J, Sun Z, Zhang J, Shao H, Cona MM, Wang H, Marysael T, Chen F, Prinsen K, Zhou L, Huang D, Nuyts J, Yu J, Meng B, Bormans G, Fang Z, de Witte P, Li Y, Verbruggen A, Wang X, Mortelmans L, Xu K, Marchal G, Ni Y. A dual-targeting anticancer approach: soil and seed principle. Radiology 2011;260:799-807. [PubMed]
- Li J, Cona MM, Chen F, Feng Y, Zhou L, Yu J, Nuyts J, de Witte P, Zhang J, Himmelreich U, Verbruggen A, Ni Y. Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics 2012;2:1010-9. [PubMed]


