Clinical pathway for video-assisted thoracic surgery: the Hong Kong story
Introduction
Any consideration of clinical pathways is invariably complicated by the fact that they go by so many different names, and have so many different potential definitions (1,2). Clinical pathways have otherwise been known as care pathways, critical pathways, integrated care pathways, care maps, fast track management programs, enhanced recovery programs, and so on. Definitions of what constitutes a clinical pathway also vary from one institute to the next. However, the key ingredients have also most frequently been described as being:
- Formal printed documentation;
- Specified clinical application (e.g., a disease or a procedure);
- Multi-disciplinary management;
- Evidence-based;
- Interventions given step-wise according to an algorithm, and triggered by time or specific clinical criteria.
When delivered well, a clinical pathway promises to offer the patient:
- Best available clinical care as suggested by medical evidence;
- Minimization of errors (through checklisting, multi-disciplinary management, and audit);
- Consistent, objective care (not subject to individual characteristics of an individual healthcare provider on any given day) (2-9).
For the academic clinician, a well-run clinical pathway also offers the generation of reliable clinical data that can be used for vital research, because patients have been managed consistently and in a standardized manner.
Thoracic surgery is perhaps particularly suitable for the implementation of clinical pathway management (4-14). The patient undergoing any chest operation is receiving a stressful, traumatic procedure and at risk of significant morbidity (10,11,15,16). It is imperative that he/she receives meticulous, error-free care in the peri-operative. It is important that such care be delivered objectively, and not subject to the whims or fancy of a particular clinician, especially in larger surgical teams where the same surgeon may not be able to see the patient each day. On the other hand, many thoracic operations are quite systematic and routine-bound (for example, in terms of incision used, pain caused, need for chest drainage, etc.), and are hence eminently suited for standardized care post-operatively (4-9,13,14). These characteristics make thoracic surgery patients not only most requiring, but also most appropriate for the use of a clinical pathway.
In Hong Kong, minimally invasive thoracic surgery (MITS) has been practiced for over two decades, and is the standard of care for most thoracic surgical operations (11-14). Over the years, the importance of good peri-operative care to maximize the benefits of MITS has come to be appreciated. However, this appreciation did involve many lessons being learnt—all of which culminated in a bespoke clinical pathway being implemented at the University of Hong Kong for all MITS patients that produced very encouraging results. This article outlines the story of how those lessons were learned, and introduces the clinical pathway that has resulted. Readers are not asked to blindly copy the pathway described, but to understand the vital concepts behind how the Pathway was created so that they can create one specific to their own needs.
Beginnings: the Ferrari versus the jalopy
This author was very fortunate to have performed video-assisted thoracic surgery (VATS) since the 1990s (11-14). Although VATS had not reached widespread acceptance at the turn of the century, its potential advantages had already been well established by the literature (4-9). Like a thoroughbred sports car, VATS represented an exciting, glamorous prospect. It promised to lead to less pain and faster recovery for patients receiving almost any chest operation.
In 2006, this author’s expertise in VATS was one of the factors leading to an invitation to join the largest Cardiothoracic Surgery Unit in Hong Kong, with a view towards developing a VATS program there. The unit had already had a successful thoracic surgery program, and the standards of nursing care for the individual patient were excellent. Through their own initiative, the nursing staff had already produced an illustrated brochure for patients undergoing a thoracic operation that explained what they could expect day-by-day during their admission (17). It included descriptions of when patients could eat and drink, mobilize, expect to have chest drains removed, prepare for discharge, and so on. This was much appreciated by patients, and it also formed the basis for how the nurses managed patients after chest operations. This patient information document was in effect a prototype clinical pathway.
However, this author was soon worried by the slow recovery noted for patients after VATS, and on closer inspection was shocked by the content of the brochure (17). It was obviously designed for a patient who had received an open thoracotomy, and the expected recovery was agonizingly slow. For example, patients were told that they could start getting out of bed and mobilizing on day 4 after surgery, and only then ‘if they felt their own condition allowed it’. Patients were not told they could be discharged until day 8 after surgery, and even then they were advised that they may require further convalescent therapy at another hospital before going home. These were completely against what one would expect for a patient receiving VATS. Even in 2006 with multi-port VATS, patients could be expected to mobilize on the morning after surgery, and could expect to directly go home after 4–5 days (today, as explained below, one can obviously go even faster). Although the nurses were applauded for their fine work in producing this patient information brochure, the fact that they referred to it when managing all thoracic surgery patients meant that patients’ recoveries were being frequently ‘held back’.
When asked why they came up with this slow pace of recovery, the nurses replied that this was the pace they were used to when other surgeons were operating via open thoracotomy. They were simply not used to working with modern MITS, and felt intimidated by the faster pace of recovery expected. Their conservative belief was based on personal experiences and on observations of other conservative surgeons around them.
The above experience is perhaps best summarized by a parable: that of the Ferrari and the jalopy (a very old, rusty, banged up car). For patients undergoing chest surgery, VATS is like a red Ferrari. It is new, exciting, and has the ability to get you from A to B very quickly indeed. In comparison, open thoracotomy is the old jalopy: it can still get the patient from A to B, but the ride is bumpy and uncomfortable because of the old suspension, and there is the risk of breakdowns (complications) if you run the car too fast. Getting to B is just so much slower. Both the Ferrari and the jalopy nevertheless travel on the same road (clinical pathway) from A to B. If one suddenly imposes a speed limit on that road, however, and that speed limit was designed to be very low to accommodate for the failings of the jalopy, then the Ferrari will never have the opportunity to show its advantages. The old patient management was forcing the Ferrari to move at exactly the same old speed as the jalopy.
This realization brought home an essential lesson: surgery is only as good as the post-operative care received.
For many surgeons, egos suggest that performing wondrous feats of surgical skill and technical dexterity are the keys to excellent patient recovery. Many are inclined to believe that performing good MITS alone will automatically result in good outcomes (15,16,18). The parable of the Ferrari and the jalopy serves to remind us that this is far from the truth. Even if one achieves the best surgery, if the post-operative care does not take advantage of it and allow the patient to recover as quickly as he/she is capable, then the outcome will be just as slow as with open thoracotomy. The clinical pathway, in other words, is the rate-limiting step.
Clearly, in 2006, this author had to change the post-operative management. To this end, the first clinical pathway in Hong Kong dedicated to patients receiving VATS was designed (19). This was written up as a collaboration between this author and the senior nursing staff. It brought together all aspects of post-operative management, including: communication, investigations, treatment, monitoring, chest drain, analgesia, diet, mobilization, hygiene, discharge planning, and so on (20-25). On each day after surgery, interventions in each of the above categories where specifically defined, and a care map was drawn up to summarize them. The specific details for each day were listed in an exhaustive checklist which was attached onto the front patient’s case notes. The checklist included slots for each of the three shifts of nurses and staff on that day to check and sign to indicate that the task had been completed. By attaching the checklist onto the front of the patient’s notes, it was hoped that the clinical pathway would be easy to follow and would not be missed.
The basic objective of the clinical pathway was that it would achieve chest drain removal by post-operative day (POD) 2, and the patient would be discharged home by POD6. The pathway was designed for use particular with VATS patients, and as this author was performing over 85% of all thoracic procedures by VATS at that time (the rate is higher today), this meant that most patients were to be put on the pathway. In retrospect, the aim of discharge on POD6 was perhaps too slow, but this was chosen as a compromise to accommodate for the hesitancy of more conservative colleagues (another mistake).
Nevertheless, the pathway gained quick success. The learning curve proved to be very short. In an initial review of the first 30 consecutive patients receiving major lung resection who were put on the Pathway, significant differences were shown in comparison with the 73 similar patients who were consecutively operated on by the same surgeon without the pathway (19). Mean chest drain durations were lower in the pathway patients, although statistical significance was not reached (4.9 vs. 6.1 days, P=0.224). Mean post-operative lengths of stay were, however, significantly shorter with the pathway (6.8 vs. 9.1 days, P=0.005). The readmission rate was 0% in the pathway patients, compared to 5.5% in the control patients, although again significance was not quite reached (P=0.191). However, perhaps owing to the more systematic approach to wound care, minor wound complication rates were lower in the Pathway patients (3.3% vs. 19.2%, P=0.038).
These early promising results proved that experienced nursing staff can easily learn the use of a clinical pathway for VATS, with a learning curve of less than 30 cases. They also showed the early promise of using a clinical pathway, with measurable improvements in patient outcomes. Such deliverable outcomes also helped establish the use of the clinical pathway in the unit, allowing all staff to appreciate its implementation. This too is an important lesson: that success breeds success. It is imperative in the early experience to generate data that will support enthusiasm for a clinical pathway. Otherwise, staff can lose interest and the program can become neglected.
Evolution: the Journey to the West
By 2011, use of the pathway had become fairly well established within the unit. However, a confluence of several factors dictated that it needed to be changed. First, use of a portable, digital chest drainage system (Medela Thopaz, Medela AG, Baar, Switzerland) had become ubiquitous for most of this author’s patients after thoracic surgery operations. The previous 2006 pathway had not taken into account the more sophisticated settings and readouts provided by the digital system which could potentially reduce chest drain durations. Second, this author had begun to employ ‘next generation’ VATS techniques for major lung resections at this time, including Needlescopic VATS and 2-port VATS (uniportal VATS was started in 2012). Faster recovery was expected, and the compromised nature of the 2006 pathway was no longer acceptable (such as discharge on POD6). With the digital drainage and the new VATS approaches, this became the previous Ferrari-jalopy situation all over again. Thirdly, the hospital was in the midst of undergoing a hospital accreditation process, and as part of this clinical pathway became a major focus for improvement in every clinical department. As one of the earliest units in the hospital to implement a clinical pathway, the Thoracic Surgery Unit was counted on the help lead the improvement process.
Although the timing of the change to the clinical pathway was triggered by the above technical and political considerations, the need for change had actually been building slowly for some time. It was realized that the older 2006 Pathway contained some shortcomings that meant the utilization of the Pathway never reached 100% of all eligible patients. It would be educational to readers to share some of these shortcomings:
- Residents and fellows conducting morning ward rounds often dismissed the pathway as a ‘nursing thing’ and hence failed to follow it;
- Many stakeholders (e.g., anesthetists, physiotherapists, etc.) felt left out because they were not engaged in designing the pathway and did not have to complete anything in it;
- Staff were confused over who the pathway applied to (Only VATS patients? Only lung cancer patients?), and in the confusion some patients were not placed on the pathway when they should have been;
- Amidst the many papers in a patient’s case notes, the pathway clipped to the front could be missed and ignored (out of sight, out of mind).
The above considerations taught another essential lesson: clinical pathways are not static. They must evolve with changing clinical environments and demands to stay relevant and useful. Users of clinical pathways should remain aware of the need for evolution, and be prepared to modify the pathway if and when necessary.
To solve the above problems, and to meet the need for change, it was decided to rewrite the clinical pathway. However, if the 2006 version was the product of the unit’s own resources, then to improve upon it meant it was necessary to take advantage of outside experience. In this case, it was decided to visit sister hospitals in Singapore. Culturally and economically very similar to Hong Kong, Singapore has always had equivalent progress in many areas of medicine. However, in one area, Singapore had a distinct head start: the development of clinical pathways (26-28).
In travelling to Singapore, this author is reminded of the famous Chinese classical fable: the Journey to the West (29). Known around the world for introducing the legend of the Monkey King, the Journey to the West is actually based on the true journey of the Tang dynasty monk Xuanzang to India to study sacred sutras in Buddhism. Although China was justifiably proud of its own Buddhist traditions and scholarship, this epic tale is a telling reminder that one should never be too proud to learn from others. Xuanzang’s arduous odyssey brought back teachings from India that significantly advanced the understanding of Buddhism in China.
The journey to Singapore yielded an appreciation of clinical pathways that truly helped advance thoracic surgery in Hong Kong. The degree of sophistication and technological advancement associated with clinical pathways in some hospitals in Singapore is admirable, including designated clinical pathway staff to supervise and audit, optical character recognition systems to translate pathway entries into digital documentations, and so on. There are too many to list, so only a distillation of the key lessons learnt will follow.
Preparation
One of the most important elements of making a clinical pathway to work, according to our Singaporean friends, was to have the proper preparation. Even before designing the pathway, a good framework for all the subsequent work had to be set up. This preparation required the harnessing of three core elements.
A champion
It is necessary to have one ‘go to’ person who would drive the clinical pathway program. This champion’s job is not only to supervise everybody working on the Pathway, but to ensure it is constantly on everyone’s mind. The champion needs to relentlessly promote, incite and remind all persons involved about the pathway. He/she needs to check that everyone is kept motivated, and that administrators are kept supportive. He/she must be the person that everyone else recognizes as the ‘face’ of the clinical pathway program, but does not need to be the team leader. The champion needs to be the person to ask if there are any questions, and even if he/she does not have the answers, he/she should be the one who can find them out. Having a champion in the hospital that constantly pushes the agenda forward is crucial in making sure that people do not lose interest or focus.
Sympathetic leaders
The next ingredient is to ensure that the hospital leadership are supportive. In Hong Kong, we were fortunate that our clinical pathway initiative coincided with the need to gain hospital accreditation by an international validating body that required a world-class pathway. The administrators were therefore most supportive. In any institute, however, it is imperative that the administrators and budget holders are convinced of the need to have such a pathway. This is where the champion plays a vital role, in ‘selling’ the Pathway program and in maintaining their support through its development. Sympathetic leaders provide financial support, permit the necessary personnel allocations, and back up the institutional rules/orders that are all required to make the pathway work.
Dedicated clinical pathway team
While the champion is the ‘spokesperson’ and the leadership are the patrons, the workhorses of the pathway are a dedicated team of doctors and nurses and allied health colleagues who must do the day-to-day work. The duties required of the team include: co-ordination of the pathway design amongst the various specialties; education of staff about the pathway; promoting and supervising its implementation; auditing and reporting on progress; and ensuring communications between all stakeholders. In Hong Kong, learning from our Singaporean friends, it was realized that a team of several doctors and nurses would be sufficient for the pathway in a cardiothoracic surgery unit in a large teaching hospital. However, as they would be required to perform these pathway-related duties on top of their usual clinical responsibilities, support from the leadership is necessary to provide them with protected, extra time and/or resources to fulfil those duties.
Design
Once the above preparation is in place, the clinical pathway is designed with three principles in mind.
Target
The group of patients to which the pathway is to apply must be clearly defined. The pathway will not work if it is over-ambitious in trying to cover ‘all’ patients. In thoracic surgery, it would be unrealistic to try to design a pathway that would cover ‘every patient that had VATS’, simply because VATS could be used for everything from lung cancer, through myasthenia gravis, to empyema decortication. ‘One size fits all’ clearly cannot apply. On the other hand, too narrow a definition of the target would make the pathway irrelevant. For example, it would be tempting to specify a pathway for Pancoast tumor resection, but if a unit only performs a couple of such cases a year, the resulting pathway would be too underused for efficient practice and may be rendered meaningless by the need for individualization in highly complex cases. Too narrow definitions for a pathway would also end up with the creation of too many pathways for each individual disease/operation, making it impossible for staff to master them. In Hong Kong, we started with a clinical pathway for VATS lung cancer resections, representing a clearly defined cohort with a large volume of patients that allowed staff to quickly adapt to the routine use of a pathway.
Detail
The amount of detail put into the pathway must also strike the correct balance. If too few intervention points and triggers are included, the pathway is meaningless and leaves too much to the subjective interpretations of individual (possibly inexperienced) staff. If too much detail is specified in terms of interventions and when they are triggered, the pathway runs the risk of turning into a clinical manual. This must be avoided. The pathway must remain simple and user-friendly, so that it can be readily followed on a day-to-day basis by all staff. In Hong Kong, as will be explained below, we opted for a pathway that was quite detailed yet with all intervention points for each day still packaged neatly onto a single sheet of paper to allow clear, simple checklist for everyone to follow easily.
Multi-disciplinary
Before the first words are penned, it is fundamental that all staff groups involved in the care of the target group of patients are assembled to co-design the pathway together. As already alluded to above, failure to involve all stake-holding staff in the design process from day one will result in lack of interest and participation further down the road. Assembling the multi-disciplinary team early allows a more realistic Pathway that accounts for practical and logistical considerations. The example in Hong Kong was that for our 2011 VATS Pathway, we had the involvement not only from thoracic surgeons and ward nurses, but also from anaesthetists, intensivists, operating room nurses, pharmacists, radiologists, physiotherapists, occupational therapists, and dieticians. The anaesthetist colleagues, for example, were able to provide input on post-operative pain management that took into account the inventory and formulary available to our unit as well as the most up-to-date concepts of peri-operative pain control. The resulting Pathway was not only better for patients, but also more readily complied with by all staff that the 2007 version.
Execution
Once a design has been drafted, the pathway can be put into practice. It must be stressed that this is a dynamic process. No pathway is ever perfect, and continuous evaluation and adjustment if indicated must always be considered. Our experience in Hong Kong showed the process of implementing the Clinical Pathway had these stages.
Pilot
The first draft of the pathway was trialled on a limited run of 50 consecutive patients. It was then defined that the experience from the first 50 cases would be scrutinized before launching the pathway properly for all patients. A pre-defined pilot trial was necessary to identify practical problems in the design and implementation, not only for patients but for the staff executing the interventions. Without a pilot trial, if the pathway had immediately been launched without restriction, any flaws or technical issues could have ground the program to a messy halt and dealt greater damage to trust amongst patients, staff and administrators. In Hong Kong, our pilot trial helped to identify some fine details that benefited from adjustments, including: specifying how consent was obtained; communication about post-operative medications; when blood tests were needed (or not needed); and so on.
Launch
Once the lessons from the pilot were assimilated, the whole pathway was formally launched by formal tutorials for the involved staff. This was immediately followed by general implementation of the pathway to all eligible patients, so as to ensure that the message from the education was put into instant practice.
Audit
The most difficult lesson from Singapore to follow was that continuous auditing is important. Auditing allows the pathway team to identify: how well the pathway is being followed; barriers to effective implementation; feedback from staff; and gaps in practice. To be useful, auditing must be done constantly and regular, scheduled audit meetings should be held. This has been the one step that in Hong Kong has proven most difficult for staff to follow because team members must make the extra effort to conduct auditing on top of their already busy clinical responsibilities.
Feedback
One important tip to help maintain motivation and interest for the pathway is to regularly provide feedback to all stakeholders regarding the results. The results of auditing should be reported, and also any findings of differences in patient outcomes due to use of the pathway. Simple clinical research demonstrating the positive impact of using the pathway can provide encouragement to staff to persevere with its use. Such reporting via the champion also helps to keep up leadership support for the pathway program.
Hong Kong clinical pathway for VATS lung resection
Based on the considerations as described above, the clinical pathway designed for VATS lung resections at our institute in 2011 can be summarized by its four main constituents as follows (30).
Care map
The first element of the clinical pathway is a care map (Figure S1). This is essentially a one-page summary or schedule of the entire pathway. It is necessary as an overview, allowing clinicians to identify the overall scheme of management and where the patient is along the pathway at any given time.
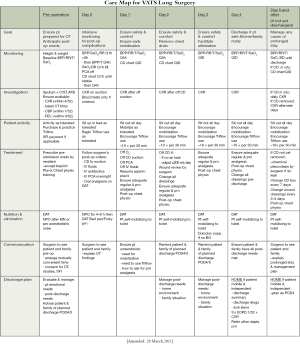
The care map looks like a school timetable. The columns represent each day on the pathway (from pre-operative to the day of discharge). The rows represent categories of the interventions given on the pathway. In our pathway, elements on the rows included: monitoring, investigation, patient activity, treatment, nutrition & elimination, communication, and discharge planning. Within each ‘box’ is a summary of the key interventions needed in that category on that day. Crucially, the top row of the care map lists the goals for each day. It is essential for the successful participation of all healthcare workers to know why those particular interventions listed in the pathway need to be followed. In short, the interventions listed are needed to allow achievement of the goals stated at the top for that day.
One key example is the goal of chest tube removal on POD2. It is known that early drain removal is important in decreasing pain and morbidity following surgery. However, to lead up to this, suction is applied on the pain of surgery to prevent atelectasis, and suction is then stopped on the first POD to quell any small air leak. If the chest X-ray (CXR) mandated on the first POD then shows a satisfactory appearance, the goal of safe drain removal on POD2 can be achieved. The care map shows how the individual steps contribute to the overall goal of drain removal 2 days after surgery.
The care map tends to be one of the most often looked at parts of the clinical pathway. It allows each healthcare worker (and even the patients themselves) to see what progress is being made and what will be coming next. Putting all the steps together shows that the interventions are not haphazard, but all geared towards achieving an endpoint: the safe passage of the patient from admission, through surgery, to discharge.
Admission data
The clinical pathway is an invaluable source of clinical data—both for audit/quality control and for clinical research. By following the pathway, outcomes can be measured for patients against a backdrop of regulated, standardized management. However, for the outcome data to make sense and be properly sorted, the background demographic and clinical data for each patient must also be input.
A key element of the clinical pathway is therefore collection of all such data on each patient (Figure S2). And there is no better time to collect such data than at each patient’s admission. That is the time when the junior doctor clerking in each patient must routinely document all the patient’s demographic and clinical details anyway.

Sadly, the admission data is also one of the parts of the clinical pathway that is most commonly neglected and poorly completed by junior doctors. For many young trainees, it seems tedious to enter so much information. This is understandable, but not acceptable. To facilitate the data collection, our pathway has incorporated these features:
- The admission data form is all on one side of A4 paper only, so that it is neat and concise and easy to fill in;
- All required data are checklisted, so that no relevant information will be missed;
- As far as possible, data entry is by simple ticking of bases or filling in of simple numbers. Entries require writing words or syntax are minimized. This not only makes it easier to fill in the form, but also makes transferring of the data into a spreadsheet/database much easier. Entry to a database of ‘0’ and ‘1’ (no tick or tick) then makes it much easier to analyse on statistical software;
- Junior doctors are advised that a properly completed admission data sheet can substitute for all/part of their admission clerking in the patient’s case notes. This gives them an incentive to fill in the sheet, as it reduces their duplicating the information writing into the case notes.
However, the more important incentive for trainees to fill in the admission data is to involve them in clinical research where the data can be used. When they see how useful the data they collect prospectively is in producing significant research results that can be presented and/or published, they become more motivated to collect good data.
Clinical pathway day-to-day
The bulk of the clinical pathway is taken up by the sheets/checklists for each day on the pathway (Figure S3).

When our pathway was designed, our institute still used paper case notes for all patients. The day-to-day sheets of the pathway were designed to fit on both sides of an A4 sheet of paper which could be inserted into the paper case notes in the corresponding place. The sheet for day 1 would be inserted where the written notes for day 1 were, and so on. This ensures that the pathway for each day was seen by all doctors, nurses and other healthcare workers looking at the case notes for that day.
On one side of the A4 sheet, the doctor’s orders are listed. The doctor looking after the patient sees the checklist and follows the listed orders, ticking and signing off on each order. The interventions are grouped basically the same as on the care map. However, the interventions are spelled out in much more detail on the day-to-day sheets.
On the other side of the A4 sheet is the list of interventions required of the nursing and allied health staff. The nurses first look at the orders prescribed by the doctor on the first side of the sheet, but then also goes through the nursing-side checklist of things to do. Again, the interventions are grouped basically the same as on the care map. There are three columns for the nurses to sign off that they have completed item on the checklist—one column for each of the three nursing shifts in 1 day.
Crucially, at the top of both the doctor and nurse sides of the sheet, the goal(s) for that day are printed prominently. As with the care map, it helps for all of the team to be able to see precisely what goal they are aiming for by carrying out the listed interventions for that day. For example, the goal on the first POD is to get the patient mobilizing. Seeing that goal helps reinforce to everyone on the team why it is important to follow the interventions of stopping suction, removing all intravenous drips, removing oxygen, and so on. It helps ensure that a whim of a staff member to ‘just leave something on for a day more’—and hence delaying mobilization—is avoided.
Our pathway was designed to complement the conventional 3-port VATS technique we still used for many patients in 2011. Some basic principles included:
- Ensure full mobilization on POD1. Hence, everything connected to the patient (with the sole exception of the chest drain) must be removed to prevent any hindrance of mobility. That includes all IV drips and oxygen. All opiates (including patient-controlled analgesia devices) must also be stopped and replaced by oral analgesia to prevent any drowsiness that may interfere with mobilization;
- Chest drains are removed on POD2 if there is no air leak. As said above, earlier drain removal reduces subsequent lasting pain. The temptation to remove drains on day 1 was avoided as the first day of mobilization may sometimes help drainage of any retained fluid inside the chest, or it may uncover small air leaks;
- If the patient’s bowels have not opened by day 3, a laxative suppository is given to prevent prolonged constipation and hard stools (which a person after a thoracic operation may find especially painful to pass);
- The anticipated discharge date is day 4. To achieve that, it is not sufficient that all the interventions in the first few days after surgery be followed. It is also important to continuously counsel the patient and family so that the discharge date is anticipated by all and is not resisted as being a ‘sudden decision to kick them out of hospital’. That is why regular counselling is emphasized throughout the pathway, including even on the day of admission.
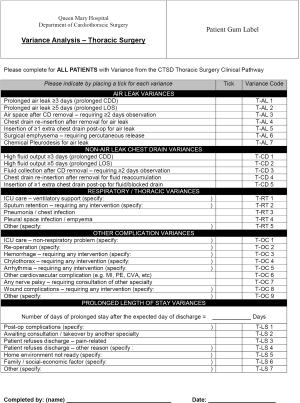
Variance
Although the pathway may appear to be a very rigid construct, it is actually designed to be flexible. Variance according to each patient’s particular circumstances and needs is permitted, thus allowing true individualization of care. However, the conditions are that: (I) variance should not be performed casually and without good reason; and (II) all such variance must be properly documented.
The reason for meticulous documentation of variance is manifold. First, it allows audit of the Pathway to identify areas for improvement. If variance is frequently encountered for a particular reason or in a specific situation, it may indicate that the original pathway was excessively strict or unrealistic in that are, for example—and that may lead to revision. Second, it generates clinical data that may be useful for clinical research. The incidence of particular complications or events that repeatedly lead to variance may be a clinically interesting observation giving insight into a condition/procedure. Third, the process of documenting variance also in effect requires the clinician to think again about whether that variance was born out of actual clinical necessity, or out of a momentary whim. Occasionally, when pondering the necessity of the variance, the clinician may realize that the variance may have been the wrong thing to do.
Documentation of variance is our pathway is in two steps:
- At the bottom of each day-to-day sheet, the doctor or nurse is asked if there has been any variance in the orders/interventions for that day (Figure S3). If so, the doctor is asked to consider whether that variance is minor and the patient should otherwise continue on the pathway on the next day, or whether the variance is so substantial that that patient is to be removed from the pathway altogether for that admission. If it is the nursing staff that records a variance, he/she is asked to notify the care manager (usually the pathway team member in charge of nursing aspects);
- If variance for that day has been confirmed as above, the doctor or care manager then completes a variance analysis sheet (Figure S4). This lists out all the common (or anticipated to be common) reasons for variance. The doctor or care manager simply has to tick the reason for the variance in this case. The variance analysis sheet is taken by the pathway team and the reason recorded to the departmental database for future audit/research.
Results
As already described above, the initial experience with our first VATS Clinical Pathway in 2006 already demonstrated benefits over traditional perioperative management (19). For patients put on the pathway, there was a trend for reduced chest drain durations and there was a significant reduction in post-operative lengths of stay (6.8 vs. 9.1 days, P=0.005). Despite the earlier discharge, the readmission rate was 0% in the pathway patients, compared to 5.5% in the control patients, although statistical significance was not reached (P=0.191). Minor wound complication rates were also lower in the Pathway patients (3.3% vs. 19.2%, P=0.038). These results showed the potential of using a clinical pathway to improve patient outcomes. They also showed that such improvements could be readily quantified.
As our pathway was evolved to the 2011 version, the next step was not only to see the improvement over the 2006 version in terms of crude patient benefit. Instead, we wanted to see if the 2011 version could address one of the key shortcomings of the 2006 version: a low rate of compliance. We also wanted to see what the effect of good compliance with a pathway meant for patients.
To understand these, we undertook a retrospective review of a prospectively collected database of all patients undergoing VATS for therapeutic major lung resections in our institute (30). As opposed to our earlier study on the 2006 pathway which focused on early experience, this later study looked at patients from 2014—well past the learning curve of the 2011 pathway. This allowed a better assessment of a mature, established pathway.
A total of 136 patients fulfilled the inclusion and exclusion criteria for this study. These included 76 males (56%) and 60 females (44%). The median age of the cohort was 61 years (range, 14–84 years). There was no mortality in this series, and minor complications occurred in 29 patients (21%). Post-operative prolonged air leakage for 5 days or more was the most common of these minor complications (18 patients, 13% of the cohort).
In summary, our study noted three key findings.
Adherence to the clinical pathway
Surprisingly, only 6.6% of all patients could adhere strictly to the clinical pathway throughout the entire duration of their in-hospital stay. Furthermore, 61% of all patients adhered to the clinical pathway for less than 50% of their post-operative in-hospital stay. In other words, despite the improvements made for the 2011 version, it remained difficult to ensure high levels of compliance to the pathway.
The likeliest explanation for the low compliance rate was that our pathway was simply too detailed. With increasing numbers and specificity of interventions on the pathway, the likelihood that they are not applicable to a few patients increases. Consequently, the likelihood of some variance (non-compliance) increases. The other obvious explanation was that we had a strict definition for ‘compliance’. A single variance meant that the pathway was considered to have ‘not been complied with’ for that patient for that entire day.
Predictors of non-adherence
Three factors that were found to be associated with non-adherence to the clinical pathway. Firstly, patient gender made a difference. Adherence for less than 50% of the post-operative in-hospital stay was noted in 68% of male patients, compared to 52% of female patients (P=0.047). Secondly, smoking history made a difference. Adherence for less than 50% of the post-operative in-hospital stay was noted in 74% of patients with a smoking history, compared to 52% of never smokers (P=0.011). Thirdly, the pain score immediately after surgery made a difference. If the pain score was 0 immediately after surgery, 59% of these pain-free patients could maintain adherence to the clinical pathway for 50% or more of their subsequent in-hospital stay. However, if the pains had any degree of pain immediately after surgery, adherence for 50% or more of their stay fell to only 34% (P=0.016). Apart from the above, no other demographic or clinical was found to be associated with non-adherence in our study.
For patient gender and smoking history, there is relatively little that can be done practically to improve compliance—other than simply expending more personnel effort in ensuring compliance. However, for the pain element, the lesson learned was that better intra-operative pain control could influence compliance with the pathway. If patients woke with less pain, then potentially we could maximize the chances of good compliance thereafter during their postoperative stay.
Consequences of adherence
Better adherence to the Clinical Pathway was associated with better outcomes.
The 53 patients who could adhere to the clinical pathway for 50% or more of the post-operative in-hospital stay experienced shorter mean chest drain durations (3.2±1.7 vs. 5.1±5.0 days, P=0.002). Those patients also had shorter mean lengths of postoperative stay (4.6±1.8 vs. 8.0±6.6 days, P
Amongst the 54 patients with a history of smoking, the risk of developing any complications after surgery was 35% if adherence to the clinical pathway was maintained for less than 50% of the time. However, this fell to only 8% if adherence was maintained for 50% or more of the time (P=0.023).
These results emphasized the clinical relevance of adhering well to the clinical pathway. Good compliance is indeed associated with demonstrably faster recovery and fewer complications.
Conclusions
The expected advantages of using a clinical pathway were already described in the Introduction. Our experience in Hong Kong has already shown that such benefits are deliverable, even in the early experience, and especially if the pathway compliance is good (19,30).
However, the story of how we established the clinical pathway in our practice has perhaps taught lessons that are at least as important. Amongst these are:
- A clinical pathway is not just an end in itself, but is an essential means to an end, translating the full benefits of surgery into measurable outcome improvements;
- A good pathway is not static: it requires constant reassessment and revision in response to evolving clinical demands and expectations;
- The key to success is meticulous preparation, team building, and multi-disciplinary design;
- Mechanisms for data collection are important to allow audit, variance analysis, and clinical research—which in turn propel the acceptance and development of the pathway.
It is hoped that this narration of the development of a clinical pathway for thoracic surgery in Hong Kong may provide incentive and ideas for other colleagues considering a similar program in their own institutes. The process is not always easy, but the result in terms of patient outcomes is invariably worth the effort.
Acknowledgements
The author wishes to thank all surgery and anesthesiology colleagues at the University of Hong Kong, and especially all Nursing Staff of the Queen Mary Hospital Cardiothoracic Surgery Unit in Hong Kong for their selfless efforts which has allowed the implementation of the clinical pathway in our unit. Particular thanks go to Ms CM Lau, Ms BP Tsui, and Dr. PS Yu for their fundamental contributions as core members of the Clinical Pathway Team.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- De Bleser L, Depreitere R, De Waele K, et al. Defining pathways. J Nurs Manag 2006;14:553-63. [PubMed]
- Kinsman L, Rotter T, James E, et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med 2010;8:31. [PubMed]
- Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010.CD006632. [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [PubMed]
- Preventza O, Hui HZ, Hramiec J. Fast track video-assisted thoracic surgery. Am Surg 2002;68:309-11. [PubMed]
- McKenna RJ Jr, Mahtabifard A, Pickens A, et al. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg 2007;84:1663-7; discussion 1667-8.
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Jones NL, Edmonds L, Ghosh S, et al. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia 2013;68:179-89. [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [PubMed]
- Sihoe AD, Yim AP. Video-Assisted Pulmonary Resections. In: Patterson GA, Cooper JD, Deslauriers J, et al. editors. Thoracic Surgery, 3rd ed. Philadelphia: Elsevier, 2008:970-88.
- Sihoe AD, Yim AP. VATS as a diagnostic tool. In: Shields TW, Locicero J, Ponn RB, et al. editors. General Thoracic Surgery, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2009:313-32.
- Sihoe AD. The Evolution of VATS Lobectomy. In: Cardoso P, editor. Topics in Thoracic Surgery. Rijeka: Intech, 2011:181-210.
- Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-17. [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [PubMed]
- Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 1999;9:403-8. [PubMed]
- Nursing staff of the Grantham Hospital Cardiothoracic Surgery Unit, Hong Kong. Education information for patients before thoraco-pulmonary surgery [Monograph]. Grantham Hospital, Hong Kong, 2006.
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [PubMed]
- Sihoe AD, Cheng LC, Das SR. Protocol-based post-operative management following lung cancer surgery. Presented at: Hospital Authority Convention 2008, May 2008, Hong Kong.
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771-80. [PubMed]
- Sihoe AD, Lee TW, Wan IY, et al. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur J Cardiothorac Surg 2006;29:795-9. [PubMed]
- Sihoe AD, Manlulu AV, Lee TW, et al. Pre-emptive local anesthesia for needlescopic video-assisted thoracic surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2007;31:103-8. [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [PubMed]
- Sihoe AD, Cheng LC. Surgery for primary pneumothorax: new ideas, new paradigms. http://www.hkma.org/english/cme/onlinecme/cme201406main.htm
- Cheah TS. The impact of clinical guidelines and clinical pathways on medical practice: effectiveness and medico-legal aspects. Ann Acad Med Singapore 1998;27:533-9. [PubMed]
- Cheah J. Development and implementation of a clinical pathway programme in an acute care general hospital in Singapore. Int J Qual Health Care 2000;12:403-12. [PubMed]
- Wu CX, Tan WS, See RC, et al. A matched-group study protocol to evaluate the implementation of an Integrated Care Pathway programme for chronic obstructive pulmonary disease in Singapore. BMJ Open 2015;5:e005655. [PubMed]
- Wu Ch'eng-en, Monkey, trans. Arthur Waley, [1943]. New York: Grove Press, 1984.
- Sihoe AD, Yu PS, Kam TH, et al. Adherence to a Fast Track Clinical Pathway for Video Assisted Thoracoscopic Surgery: Clinical Importance and Predictors. Available online: http://meetings.ismics.org/abstracts/2015/T10.cgi


