Abstract
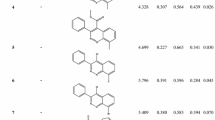
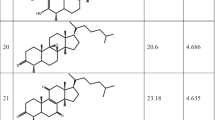
To search the minimum structural requirements for herbicidal evaluation of 5-(R1)-6-(R2)-N-(R3-phenyl)-pyrazine-2-carboxamide analogues (1–19) as new class potent herbicide, 3D-QSARs models between the substituents (R1-R3) changes of the analogues and their herbicidal activity were derived and discussed quantitatively using comparative molecular field analysis (CoMFA) and comparative molecular similarity indice analysis (CoMSIA) method. The herbicidal activity of the optimized CoMFA model I was principally dependent on steric fields. Also, it was found that the optimized CoMFA model I with the sensitivity to the perturbation (dq 2′/dr2 yy′=0.959) and the prediction (q2=0.414) produced by a progressive scrambling analyses were not dependent on chance correlation. Also, it was predicted that the herbicidal activity increases when large steric substituents were introduced to one part of ortho- and meta- position on N-phenyl ring as R3-substituent and small steric substituents to the other part.
Similar content being viewed by others
References
Akamatsu M (2002) Current state and perspectivies of 3D-QSAR. Curr Topics Med Chem 2, 1381–1394.
Bay SW and Roh JH (2001) Primary lid tuberculosis occuring at preaponeurotic portion after upper lid blepharoplasty. J Korean Ophthalmol Soc 42, 169–174.
Cha JH, Jang SH, Oh CK, and Kwon KS (2001) A case of tuberculosis verrucosa cutis. Korean J Dermatol 39, 96–98.
Choi IS, Park IN, Hong SB, Oh YM, Lim CM, Lee SD, Koh YS, Kim WS, Kim DS, Kim WD, and Shim TS (2006) Hepatotoxicity of rifampicin and pyrazinamide treatment excluding isoniazid. Tuber Respir Dis 60, 38–43.
Chung KS, Jang SC, Choi KS, and Sung ND (2006) Comparative molecular field analyses on the herbicidal activities of new 5-benzofuryl-2-[1-(alkoxyimino)alkyl]-3-hydroxycyclohex-2-en-1-one derivatives. J Korean Soc Appl Biol Chem 49, 238–242.
Clark M, Cramer III RD, Jones DM, Patterson DE, and Simeroth PE (1990) Comparative molecular field analysis (CoMFA): 2. Toward its use with 3D-structural databases. Tetrahedron Comput Methodol 3, 47–59.
Clark RD and Fox PC (2004) Statistical variation in progressive scrambling. J Comput-Aided Mol Design 18, 2821–2826.
Cramer RD, Bunce JD, and Patterson DE (1988a) Cross-validation, bootstrapping and partial least squares compared with multiple regression in conventional QSAR studies. Quant Struct Act Relat 7, 18–25.
Cramer RD, Patterson DE, and Bunce JD (1988b) Comparative molecular field analysis (CoMFA): 1. Effect of shape on the binding of steroid to carrier proteins. J Am Chem Soc 110, 5959–5967.
Dolezal M, Cmedlova P, Palek, L, Vinsova J, Kunes J, Buchta V, Jampilek J, and Kralova K (2008) Synthesis and antimycobacterial evaluation of substituted pyrazincarboxamides. Eur J Med Chem 43, 1105–1113.
Dolezal M, Palek L, Vinsova J, Buchta V, Jampilek J, and Kralova K (2006) Substituted pyrazincarboxamides: Synthesis and biological evalution. Molecules 11, 242–256.
Hansh C (1976) The structure of medicinal chemistry. J Med Chem 19, 1–6.
Hong KS, Hwang IT, Kim HR, Jeon DJ, Lee BH, Song JH, and Cho KY (2004) Inhibition of protoporphyrinogen oxidase activity and selectivity of new compound EK-5439. Korean J Pestic Sci 8, 79–87.
Juan AAS and Cho SJ (2007) 3D-QSAR study of microsomal prostaglandin E synthase (mPGES-1) inhibitors. J Mol Model 13, 601–610.
Kerr R (1994) Parallel helix bundles and ion channels: molecular modeling via simulated annealing and restrained molecular dynamics. Biophys J 67, 1501–1515.
Kim DS, Chun SJ, Jeon JJ, Lee SW, and Joe GH (2004) Synthesis and fungicidal activity of ethaboxam against Oomycetes. Pest Manag Sci 60, 1007–1012.
Kim DS, Park HC, Chun SJ, Yu SH, Choi KJ, Oh JH., Shin KH, Koh YJ, Kim BS, Hahm YI, and Chung BK (1999) Field performance of a new fungicide ethaboxam against cucumber downy mildew, potato late blight and pepper phytophthora blight in Korea. Plant Phathol J 15, 48–52.
Kim YW, Jeon WB, and Park CK (1992) Synthesis and antifungal activity of N-substituted-5-chloro-1,3-dimethylpyrazole-4-carboxamide. J Korean Agric Chem Soc 32, 87–91.
Klebe G, Abraham U, and Mietzner T (1994) Molecular similarity indices in a comp- arative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37, 4130–4146.
Lowis DR (1997) HQSAR. A new highly prediction QSAR technique. Tripos Technical Notes, Vol. 1. No. 5.
Marshall GR, Barry CD, Bosshard HE, Dammkoehler RA, and Dunn DA (1979) The Con- formational parameter in drug design; active analog approach. In Computer-assisted Drug Design, Olsen EC and Christoffersen RE (eds), No. 112, pp. 205–226. American Chemical Society, Washington DC, U.S.A.
Ra CS, Rew YS, and Cho WB (1995) Synthesis and fungicidal activity of novel 2-aminothiazole carboxamide derivatives. Korean J Med Chem 5, 72–75.
Song JY, Kim TY, Kim WB, Shong YK, Rhee YS, Suck JH, and Hong SJ (2006) Role of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the growth regulation of anaplastic thyroid cancer cells lines. J Korean Soc Endocrinol 21, 125–131.
Soung MG, Lee YJ, and Sung ND (2009) 3D-QSARs of herbicidal 2-N-phenylisoindolin-1-one analogues as a new class of potent inhibitors of protox. Bull Korean Chem Soc 30, 613–617.
Soung MG, Park KY, and Sung ND (2010a) Synthesis and 3D-QSARs analyses of herbicidal O,O-dialkyl-1-phenoxyacetoxy-1-methylphosphate analogues as a new class of potent inhibitors of pyruvate dehydrogenase. Bull Korean Chem Soc 31, 1361–1367.
Soung MG, Park KY, and Sung ND (2010b) 3D-QSAR study on the influence of alrylamino (R) substituents on herbicidal activity of thiourea analogues. Bull Korean Chem Soc 31, 1469–1473.
Suh JJ, Hong YH, and Kim BC (1987) Synthesis and anti-inflammatory activity of 4-substituted-1, 2-benzothiazine-3-carboxamide-1,1-dioxides. J Kor Pharm Sci 2, 61–65.
Sung ND and Jung HS (2005) 3D-QSAR on the herbicidal activities of new 2-(4-(6-chloro-2-benzoxazolyloxy) phenoxy-N-phenylpropionamide derivatives. J Korean Soc Appl Biol Chem 48, 252–257.
Sung ND, Jang SC, and Hwang TY (2007) 2D-QSAR and HQSAR analysis on the herbicidal activity of new O,O-dialkyl-1-phenoxyacetoxy-1-methylphosphonate analogues. Korean J Pestic Sci 11, 72–81.
Tripos S (2001) In Molecular Modeling and QSAR Software on CD-Rom (Ver. 7.3), Tripos Associates, Inc., St. Louis, MO, U.S.A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JH., Myung, PK., Kim, SJ. et al. Minimum Structural Requirements for Herbicidal Evaluation of 5-(R1)-6-(R2)-N-(R3-Phenyl)-Pyrazine-2-Carboxamide Analogues as New Class Potent Herbicide. J. Korean Soc. Appl. Biol. Chem. 53, 440–445 (2010). https://doi.org/10.3839/jksabc.2010.068
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.3839/jksabc.2010.068