Abstract
Negative priming (NP) refers to fact that people respond more slowly and make more errors when responding to target stimuli that were previously ignored. This phenomenon has also been observed when participants respond to the location, and not only to the identity, of the stimulus. Intriguingly, while roughly the same pattern of results has been observed in the visual, auditory, and tactile modalities when it comes to identity-based NP, the same does not hold true for spatial NP: In particular, feature mismatch seems to be the sole cause of auditory spatial NP, whereas response inhibition would appear to be the sole cause of spatial NP in vision. We conducted a novel tactile variant of the spatial NP task. We investigated whether spatial NP in the tactile modality exists, and further, we investigated whether the pattern of spatial NP in the tactile modality compares with what has been documented previously in vision or audition. Tactile spatial NP was observed, and it was independent of feature mismatch, thereby reflecting a comparable pattern as visual spatial NP. We discuss spatial selection with respect to possible modality-specific processes.
Similar content being viewed by others
It is commonly agreed that if a to-be-ignored stimulus (distractor) from a prime display becomes the to-be-selected stimulus (target) in the subsequent probe display, a person’s response to the identity of this target will likely be impaired in terms of the latency and/or accuracy of their response (Dalrymple-Alford & Budayr, 1966). This robust empirical phenomenon, known as negative priming (NP; Tipper, 1985), has been observed in a wide variety of different tasks and populations (for reviews, see Fox, 1995; Tipper, 2001). By now, NP has been reported in numerous visual (e.g., Frings & Wentura, 2006; Neill, 1997; Tipper & Cranston, 1985), auditory (Leboe, Mondor, & Leboe, 2006; Mayr & Buchner, 2007), and tactile (Frings, Bader, & Spence, 2008; Frings & Spence, 2011, 2013) studies, as well as in the olfactory modality (Olsson, 1999). Moreover, there would now appear to be a consensus amongst most researchers that NP taps selective control mechanisms (although it should be noted that NP can be observed without selection in the prime display [e.g., Joordens, Betancourt, & Spalek, 2006] or in the probe display [e.g., Frings & Wentura, 2006]). Nevertheless, the exact nature of these control processes is still a topic of heated debate (e.g., Neill, 2007). In this article, we analyze for the first time a spatial variant of NP in the tactile modality. At first, we briefly discuss identity-based NP in general and then, in more detail, introduce what is already known about spatial NP in the visual and auditory modalities.
A coarse-grained taxonomy of theories of NP differentiates between inhibition- (Houghton & Tipper, 1994; Tipper, 1985) and retrieval-based (e.g., Milliken, Joordens, Merikle, & Seiffert, 1998; Neill, 1997; Rothermund, Wentura, & DeHouwer, 2005) accounts. According to retrieval theories, NP is caused by an automatic retrieval process that is initiated when participants process the probe target, the assumption being that processing the target stimulus activates memory traces associated with that particular stimulus. When the ignored distractor from the prime trial becomes the target in the probe trial (ignored-repetition [IR] condition), the last memory trace of the current target stimulus may contain information such as “distractor” or “do-not-respond” or else may be linked to the response that was given when this stimulus was present the last time. It is this information that interferes with a person’s ability to respond quickly and accurately to the current target. By contrast, according to inhibition theory, the abstract representation of the distractor stimulus is actively suppressed by mechanisms of selective attention during the processing of the prime episode. This forward-acting inhibition persists until the probe display is presented. Thus, in the IR condition, the still inhibited representation has to be activated in order for the participant to respond, and hence NP occurs. Both accounts are well supported by the empirical literature. This has led several prominent authors to conclude that both inhibitory mechanisms and retrieval processes likely contribute to NP (see Kane, May, Hasher, Rahhal, & Stoltzfus, 1997) or that certain aspects of retrieval and inhibition theories are very much the same (e.g., Tipper, 2001). In sum, although NP may not be the best experimental index if one wants to tap inhibition or retrieval processes separately (cf. Wühr & Frings, 2008), it remains an important tool with which to investigate the mechanisms underlying distractor processing (Neill, 2007).
Spatial negative priming
While most studies of NP have used identity-based tasks—that is, tasks where the participants have had to identify the target stimulus while attempting to ignore the identity of the distractor—there is also a spatial variant of the NP task. Tipper, Brehaut, and Driver (1990) were the first to analyze this phenomenon in vision. They presented target–distractor pairs from two of four possible locations; the target was specified by shape, and the participants had to press a key that corresponded spatially with the location of the target, while trying to ignore the location of the distractor. As a consequence, on IR trials, it is the repetition of an ignored location (not an ignored stimulus identity) as the target’s location in the probe that lengthens reaction times (RTs) and results in higher error rates (Chao, 2009; Milliken, Tipper, & Weaver, 1994). Generally speaking, the same theories that have been used to explain identity-based NP have also been used to explain the location-based variant of the task. According to inhibition theories, it is residual inhibition of the prime distractor location that causes the effect (Milliken, Tipper, Houghton, & Lupiáñez, 2000; Tipper et al., 1990). According to retrieval-based accounts, the probe target location on the IR trials acts as a retrieval cue and would, thereby, retrieve a “do-not-respond-tag” (Neill & Valdes, 1992). Yet for visual spatial NP, there is, in the meantime, clear evidence that it is not the location but the response to that location that is inhibited (Buckolz, Goldfarb, & Khan, 2004; Fitzgeorge, Buckolz, & Khan, 2011). In fact, although retrieval as well as inhibition theories may be able to explain spatial NP, Buckolz and colleagues (2004) established that inhibition at the level of response selection is the main cause of visuospatial NP. Using an innovative experimental procedure, they were able to disentangle the aftereffects of ignoring a specific location or response to this location in the prime display (by separating location and responses for some stimuli locations). Their results clearly showed that response inhibition was the main factor underlying visuospatial NP (see Buckolz, Edgar, Kajaste, Lok, & Khan, 2012; Guy, Buckolz, & Khan, 2006).
Yet, some years ago, Park and Kanwisher (1994) put forward an alternative theory for spatial NP. They suggested that feature mismatch might explain the impaired performance observed on the IR trials. In particular, when a visual feature is used to discriminate the target from the distractor (say shape), on the IR trials this means that the same location in prime and probe will be encoded with two different shapes, which, in turn, causes interference. For example, ignoring stimulus A at position 1 while responding to stimulus B at position 2 on the prime trial would lead, in the case of an IR trial, to probe interference, because the participants now have to respond to stimulus B at position 1. In other words, feature mismatches were always present on IR trials and never on the control trials and, hence, could completely explain visuospatial NP. However, Milliken et al. (1994) responded to this possible confound by introducing shape switches between prime and probe displays; that is, a cue signaled the target identity for the next display, and as a result, feature mismatches and priming conditions could be varied orthogonally. In fact, visuospatial NP was also observed in those trials without a feature mismatch (Milliken et al., 1994; Tipper, Weaver, & Milliken, 1995), thereby suggesting that spatial NP in vision does not hinge on feature mismatch (still, Milliken et al., 1994, observed evidence for feature mismatch). Yet, given the Buckolz and colleagues (2012; Buckolz et al., 2004) results mentioned above, one can say that feature mismatch does not play a dominant role in visuospatial NP anymore and that response inhibition is accepted as the main force underlying spatial NP in vision (Neill, 2007).
Until recently, the possible existence of spatial NP has been completely neglected in the other senses. Mayr, Hauke, and Buchner (2009) were the first to analyze the location variant of the task in the auditory modality. These researchers presented two auditory stimuli from two of four possible spatially distributed loudspeakers. In addition, a cue signaled to the participants what the identity of the target would be on the next trial. That is, just as in vision, it was possible to vary feature mismatch and priming conditions orthogonally. Surprisingly, and completely contrary to what has been observed in the visual modality, spatial auditory NP was completely dependent on feature mismatch. NP occurred when a specific position was ignored and then repeated—but only if the sound stimulus changed. On IR trials, on which, due to a switch in the identity of the target, the same stimulus was presented at the ignored and then at the repeated position, no NP was observed. Since this original study, the dependence of auditory spatial NP on feature mismatch has been replicated several times (Mayr, Buchner, Möller, & Hauke, 2011; Mayr et al., 2009; Mayr, Möller, & Buchner, 2014; Möller, Mayr, & Buchner, 2013).
Thus, one can say that the picture concerning NP in general at this point in time suggests that ignoring the identity of distractors is independent of the modality of the stimulus (Buchner, Zabal, & Mayr, 2003; Driver & Baylis, 1993) or at least that there are no qualitative differences between the modalities (although note that the evidence reported by Frings, Amendt, & Spence, 2011, suggests larger identity effects in touch). Yet the ignoring of a distractor’s location revealed modality-specific patterns in vision and audition to date. This is noteworthy for the literature on NP but may suggest, beyond this particular literature, that selection is modality specific dependent of whether one is talking about spatial selection or selection based on stimulus identity. We will turn to this in the Discussion section.
In this article, we analyze for the first time (to the best of our knowledge) spatial NP in the tactile modality. Identity-based NP in the tactile modality has been observed previously (Frings et al., 2008) and, generally speaking, has been in line with the pattern of results that had been observed previously in the visual and auditory modalities. Here, though, we introduce a tactile variant of the location-based NP task by delivering vibrotactile stimuli to two out of four possible locations. Thus, we analyze whether ignoring the location of a tactile distractor while responding to a tactile target’s location results in performance costs when, on the next trial, the target is presented at the location of the previous distractor. In other words, we analyze whether spatial NP does exist in the tactile modality. In addition, we presented a visual cue prior to each prime and probe that signaled the identity of the upcoming target. As a result, it was possible to orthogonally vary feature mismatch and priming condition. If ignoring a spatial distractor in touch is comparable to what has been documented in audition, one would expect tactile spatial NP to be completely dependent on feature mismatch. Yet, if tactile spatial NP occurs even without feature mismatch, it can be stated that feature mismatch does not play a dominant role for spatial NP in touch that would be comparable to what has been reported in vision.
Method
Participants
Twenty-one students from Trier University, 6 male and 15 female, took part in this study in return for payment (8 €). The median age of the participants was 24 years (ranging from 21 to 31 years). All of the participants reported normal or corrected-to-normal vision. The sense of touch was tested by delivering the Haptic-Figure Test (HFT; Grunwald, 2010). This test allows for the visuo-haptic ability of the participant to be assessed. In this test, the participants have to recognize elementary geometric forms (reliefs) via haptic exploration and to match them to a visual display. Due to the construction of the stimuli, the relief structure can be felt but not seen. The time taken by the participants to explore the stimuli and the number of correctly identified reliefs is measured and compared with a norm sample; according to the results of this test, all of our participants exhibited a normal sense of touch. The data from one participant had to be discarded due to his/her extremely high error rate in the NP task (fewer than 25% valid trials).
Design
The experiment itself involved a 2 (trial type: ignored repetition vs. control) × 2 (selection criterion: change vs. no change) factorial design; both factors were varied on a within–participants basis. Identity NP in the tactile modality has been observed with quite large effect sizes (J. Cohen, 1988; the variant of Cohen’s d for dependent means varied between dz = 0.7 and dz = 1.2; Frings et al., 2011; Frings et al., 2008). Given N = 21, the power to detect large-sized effects in binary comparisons of two levels of the trial type factor (i.e., dz = 0.8; α = .05, one-tailed) was 1 − β = .97 (GPower; Faul, Erdfelder, Lang, & Buchner, 2007).
Apparatus and materials
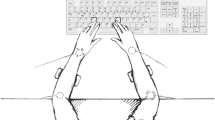
The experiment was conducted in a soundproofed, completely dark room, walls and furniture were painted black, and all sources of illumination were eliminated. The participants were seated in front of a 24-in. TFT screen with a viewing distance of approximately 60 cm. A standard PC with E-Prime (Version 2.0) was used to run the experiment and to control the presentation of the vibrotactile stimuli by means of tactors via USB 2.0. The vibrotactile stimuli were presented by means of four tactors (Model C-2, Engineering Acoustic, Inc.) that were attached to participants’ wrists and elbows on both arms by means of Velcro strips. All of the tactors were 1.17 in. in diameter and 0.30 in. thick. The optimal frequency for the operation of the tactors was at a frequency of 250 Hz at which all stimuli were delivered. Each tactor was driven independently by an individual standard amplifier (Power Amplifier Module PM40C, t.amp).
Two vibrotactile stimuli were used in this experiment. The first stimulus consisted of a continuous vibration, and the second an alternation of 100-ms vibrations with 110-ms breaks. On both the training and experimental trials, the stimuli were presented repeatedly until a response was detected.
Procedure
The participants were tested individually. The tactors were fixed on the inside of the participants’ wrists and elbows on both arms. In order to reduce the amount of noise created by the tactors (possibly touching the table) and to ensure a comfortable position, the participants rested their forearms on armrests. Additionally, white noise was presented via headphones to rule out any influence of tactile target detection due to auditory signals (attributable to the activation of the tactors). The participants’ task was to respond to the location of the target stimulus by pressing one of four possible keys and to ignore the distractor stimulus. The participants were instructed to respond to the targets presented to their left arm by pressing the “D” key with their left middle finger for targets presented at the left wrist and the “C” key with the left index finger for targets presented at the left elbow. The participants were instructed to respond to those targets presented to their right forearm by pressing the “J” key on the keyboard with the right middle finger for targets presented to the right wrist and the “N” key with the right index finger for targets presented to the right elbow. In other words, the arrangement of the stimulus locations and response keys corresponded spatially, and the participants had to press the spatially corresponding key according to the target’s location.
Each experimental trial consisted of a prime display followed by a probe display. Both displays contained two vibrotactile stimuli, presented from two of four possible locations. A green “1” or a blue “2” was presented at the center of the screen for 500 ms before the vibrotactile display was delivered. The number, as well as the color, of that cue indicated the identity of the subsequent target (a green “1” indicating that the participants should respond to the position of the continuous vibration, while a blue “2” indicated that the participants should respond to the position of the intermittent vibration). On change trials, the identity of the target switched between the prime and probe displays, whereas on the no-change trials, the identity of the target was the same in both displays. The participants’ task was to respond according to the location of the designated target stimulus with one of four possible keys on the keyboard. The four response keys were spatially mapped to the four areas on the skin that could be stimulated (see Fig. 1).
Two types of trials were presented, ignored repetition trials and control trials. Ignored repetition trials involved the presentation of the target in the probe display from the location of the preceding prime distractor, whereas the probe distractor was presented to one of the two locations that had not been stimulated during the prime display. On the control trials, the probe target and probe distractor were presented from the two remaining locations that had not been stimulated during the prime display. Each specific stimulus-location mapping in the prime display resulted in four possible probe displays for the change condition and four possible probe displays for the no-change condition (see Table 1). The orthogonal combination of stimulus-location mapping and the two experimental variables, selection criterion and trial type, resulted in 192 prime–probe sequences (48 IR trials with selection criterion changed, 48 IR trials with no change in the selection criterion, 48 control trials with selection criterion changed, and 48 control trials with no change in the selection criterion), which were presented in random order.
Each double trial was signaled by a centrally presented fixation cross, and the participants had to initiate the next prime–probe sequence by pressing the space bar. The visual cue indicating the identity of the next target was presented for 500 ms. The subsequent prime target and prime distractor were presented until a response had been detected from the participant. In case of an incorrect response to the prime target, a 200-ms feedback signal was presented; in the case of a correct response, a blank screen without any feedback was provided for 200 ms instead. Next, the cue indicating the target identity in the probe display was presented for 500 ms. Probe target and probe distractor were then presented repeatedly until a response had been detected. Once again, feedback was given only if the participant had made an incorrect response.
The experiment started with three consecutive training phases. During the first 32 training trials, just one stimulus was presented. During the next 96 training trials, both the target and distractor were presented; however, displays were not presented in prime–probe sequences in this phase. During these two training phases, feedback was given after every trial. In the third and final training phase, the participants had to work through 96 prime–probe sequences that were identical to the subsequent experimental trials (also consisting of 48 IR and 48 control trials). Each prime–probe sequence was initiated by the participant pressing the space bar. The training phase lasted for approximately 20 min. The HFT (see above) was delivered after the experiment and took about 15 min to complete.
Results
Only those trials on which a correct response had been made to both the prime and probe targets were used for the analyses of the RT data. Additionally, all of the trials with an RT shorter than 200 ms. as well as those trials with an RT having an interquartile range 1.5 above the third quartile of each participant’s individual RT distribution (Tukey, 1977). were excluded from analyses. Due to these restrictions, 33.60% of all trials were excluded from analyses (mean error rate on prime trials, 17%; on probe trials, 20%). Mean RTs and error rates are shown in Table 2.
Reaction times
A 2 (trial type: ignored repetition vs. control) × 2 (selection criterion: change vs. no change) MANOVA with Pillai’s trace as criterion revealed a significant main effect for trial type, F(1, 19) = 33.93, p < .001, η p 2 = .641. The participants responded more rapidly on the control trials than on the ignored repetition trials. NP effects, calculated as the difference between ignored repetition trials and control trials, differed significantly from zero for both conditions. In particular, in the condition in which the selection criterion did not change, a significant NP effect was observed (M = −194 ms, SD = 124 ms, dz = 1.57), t(19) = −7.0, p < .001, as well as in the condition in which it did change (M = −143 ms, SD = 173 ms, dz = 0.83), t(19) = −3.7, p = .002 (see Fig. 2). No main effect of selection criterion was found, F(1, 19) = 0.83, p = .776, η p 2 = .004, suggesting that the mean RT was not affected by the selection criterion. Most important, there was no interaction between trial type and selection criterion, F(1, 19) = 2.19, p = .155, η p 2 = .10; that is, the strength of the NP effect did not vary as a function of the selection criterion used (i.e., the tactile NP effect in the RT data was unaffected by feature mismatch).
Error rates
Only those trials on which a correct response was made in the prime display, but not to the probe target, were analyzed. The same 2 × 2 MANOVA as that reported above for the RTs was conducted for the analyses of errors. Once again, the main effect of trial type reached statistical significance, F(1, 19) = 4.65, p = .044, η p 2 = .20. The participants made more errors in the ignored repetition than in the control condition. We tested the spatial NP effect for criterion change trials and no-criterion-change trials separately. Only NP effects for the latter reached statistical significance (M = −5.1%, SD = 9.3%, dz = 0.55), t(19) = −2.46, p = .024, whereas no difference was observed when criterion change was indicated (M = −2.2%, SD = 7.9%, dz = 0.28), t(19) = −1.23, p = .233. The main effect of selection criterion did not reach statistical significance, F(1, 19) = 0.79, p = .387, η p 2 = .04, nor was the interaction between trial type and selection criterion significant, F(1, 19) = 2.50, p = .13 η p 2 = .12 (i.e., the tactile NP effect in the error rates was unaffected by feature mismatch).
Discussion
Here, we report a novel tactile variant of the spatial NP task. The results of the present study highlighted the existence of a tactile NP effect; that is, ignoring the location of a tactile distractor led to impaired performance if participants had to respond to this location shortly thereafter. Thus, spatial NP can be observed in the tactile modality. In addition, we analyzed whether this tactile NP effect was dependent on feature mismatches, as has been shown to be the case for auditory spatial NP, or whether instead this tactile NP was independent of feature mismatches, as documented in the case of visual spatial NP. The analysis of the RT data revealed significant NP effects whether or not there was a feature mismatch. In terms of the error data, we observed a significant NP effect only in the condition with feature mismatch. However, this was not significantly different from the NP effect that was seen in the condition without feature mismatches. Even if one were to increase the sample size and, thereby, the power, any possible difference between tactile spatial NP with and without feature mismatches would be of the ordinal type. That is, it might be that in the feature mismatch condition, NP might be somewhat larger than in the condition without feature mismatch (because feature mismatch may, at least in some way, contribute to spatial NP in touch, as has been reported to be the case in vision; Milliken et al., 1994). Yet the complete dependence of spatial NP on feature mismatch (as in audition) can, however, safely be ruled out. Note that we observed a large-sized NP effect in the condition without feature mismatch in the RT data that was significant at p = .002. Put simply, the pattern of data from the tactile spatial NP task closely resembled that documented previously for vision but is different from that seen in audition.
The results of the present study constitute the first demonstration of spatial NP in the tactile modality. Yet one might argue that our design had some limitations. In fact, by using only IR and control trials, clever participants might have used the prime–probe contingency in order to predict the probe target’s location; this would have diminished the NP effect. Different possibilities to hedge against this contingency have been suggested; for example, Christie and Klein (2001) used all possible combinations of prime and probe displays (i.e., target repetitions were included, as well as target-to-distractor repetitions or complete switches of distractor and target locations, etc.). Yet we would argue that strategic usage of such contingences did not play a crucial role in our experiment, for at least two reasons. First, we found quite large NP effects (which simply would have not been found if participants had used the prime–probe contingency extensively). Second, with respect to the argument that task difficulty determines whether participants have the cognitive resources for using prime–probe contingencies (Frings & Wentura, 2006), it could be said that the likelihood that participants used strategies in this quite difficult task was low. Nevertheless, future research should use more complex designs (including target location repetitions) so as to pinpoint the possible influence of inhibition of return (Christie & Klein, 2001) for tactile spatial NP and to analyze the locus of spatial NP in the tactile modality (i.e., in order to see whether it is location or response based).
As was outlined in the introduction, a precondition for visuospatial NP is that distractors are processed up to the level of response selection and that, on IR trials, the inhibition of the response would lead to spatial NP (this stands in sharp contrast to identity-based NP, which is clearly not limited to response inhibition; cf. Neill, 2007). Of course, there is ample evidence to suggest that visual distractors are processed up to the level of response selection in a variety of interference paradigms, such as the Eriksen flanker, Stroop, or Simon tasks (see Luck & Vecera, 2002, for a review). In contrast, when it comes to auditory distractors, the evidence is scarce and ambiguous. For example, Wascher, Schatz, Kuder, and Verleger (2001) found no evidence for automatic response activation in response to auditory distractors in a Simon task (but see Leuthold & Schröter, 2006). One reason for this difference in motor activation might be that vision and audition differ with respect to how closely the particular modality and (manual) responding are related; for example, the link between grasping and reaching movements and vision is assumed to be much stronger than the link between these movements and audition. As a result, spatial NP in audition reflects other processes (such as feature mismatch) and not the aftereffects of inhibiting a particular response. Note that this line of argument was recently forwarded by Mayr et al. (2014) in order to explain differences in spatial NP between the visual and auditory modalities. Of course, the line of argument put forward by Mayr and colleagues is only valid as long as one accepts the idea that auditory distractors were not processed up to the level of response selection in the auditory spatial NP task used by Mayr and colleagues.
In a similar vein, one could argue that in touch, action and perception are at least more strongly (if not even more strongly) linked than is the case in vision (for a review, see Dijkerman & de Haan, 2007). In particular, while in vision several studies support the existence of separate “what” (or “how”) and “where” pathways in the visual systems (e.g., DeYoe & Van Essen, 1988; Mishkin, Ungerleider, & Macko, 1983; Treisman, 1996), in touch it has been argued that (although separate what and where pathways do exist; cf. Spence, 2013), these pathways are much more interconnected. For vision, two separate but interacting cortical streams have been proposed for the processing of action and perception. The dorsal stream is involved in the visual guidance of immediate goal-directed movements, whereas the ventral stream is primarily associated with visual perception and recognition (Milner & Goodale, 1995). In contrast, in the tactile modality, action- and perception related pathways are less distinct. The tactile dorsal pathway projects from the anterior parietal cortex (APC) to the posterior parietal cortex (PPC), either directly or via the secondary somatosensory cortex (SII). The tactile ventral pathway includes projections from the APC, via the SII, to the posterior insula and the PPC. Thus, for the somatosensory system, several cortical areas are involved in perception, as well as in action-related processes (Dijkerman & de Haan, 2007). This is not surprising, because typically, somatosensory perception of objects occurs haptically, and therefore, it is a process that inherently involves action. In addition to the findings reported by Dijkerman and de Haan, Drewing and Schneider (2007) have suggested that the somatosensory system should not be divided into two structural streams (e.g., dorsal vs. ventral) but merely into functional streams of “how” and “what” (but see Drewing & Schneider, 2007).
Thus, concerning tactile spatial NP, it could be argued, given the close link between perception and action in touch, that spatial tactile NP is also caused mainly by response inhibition, because distractors are always processed up to the level of response generation. In fact, there is evidence to suggest that tactile distractors are processed up to the level of response selection in flanker tasks (e.g., Craig, 1995; Driver & Grossenbacher, 1996; Evans & Craig, 1992; Frings & Spence, 2010), at least when they are presented to parts of the body that are visible (Wesslein, Spence, & Frings, 2014). Taken together, then, spatial tactile NP might reflect the aftereffects of inhibiting the particular response activated by the distractor. Still, with the data from the present experiment, the locus of inhibition (i.e., whether it is the location of the distractor or the response to this particular location) cannot be pinpointed.
Finally, and more generally, one might want to argue that spatial selection generally differs between audition and vision (and possibly touch as well). Starting with the sensory coding, from the receptors in the retina onward, information is coded spatially in the visual system, as well as in the skin. By contrast, the receptors are arrayed tonotopically in the auditory modality. What is more, functional imaging studies have compared exogenous as well as endogenous cuing variants of the spatial-cuing paradigm in both vision and audition. Stated briefly, the evidence suggests that frontoparietal areas play a dominant role in spatial orienting in both the visual and auditory modalities (e.g., Santangelo & Macaluso, 2012). However, in vision, control functions in the dorsal and ventral frontoparietal regions separately contribute to endogenous and exogenous cuing, respectively (e.g., Arrington, Carr Mayer, & Rao, 2000; Corbetta, Kincade Ollinger, McAvoy, & Shulman, 2000; Corbetta, Miezin, Shulman, & Petersen, 1993; Corbetta & Shulam, 2002). By contrast, when it comes to audition, these two different attentional networks would appear not to be segregated (or to the very least to be segregated less). In particular, in endogenous (e.g., Smith, Jackson, & Rorden, 2009), as well as exogenous (e.g., Mayer, Harrington, Stephen, Adair, & Lee, 2007), cuing studies in audition, both networks have typically been activated (see Spence & Santangelo, 2009, for a review).
In the research on cross-modal cuing, the existence of modality-specific attentional systems has also been discussed (particularly for the case of exogenous attention). Summarizing the experimental evidence at this point, one can say that although some researchers have proposed completely independent modality-specific attentional systems (e.g., Mondor & Amirault, 1998), a majority of researchers argue for a combination of supramodal and modality-specific spatial attention or, at least, closely linked attentional systems (e.g., Buchtel & Butter, 1988; Posner, 1990; Spence & Driver, 2004; Ward, 1994). Thus, even if one accepts that there is a supramodal attentional system and also that spatial sensory input from different modalities is represented in an eye-centered and modality-independent reference frame in the posterior parietal cortex (for an overview, see Y. E. Cohen & Andersen, 2004), there is still room for modality-specific patterns.
In conclusion, we presented the first evidence for spatial NP in the tactile modality. In addition, our data revealed that spatial NP in touch can be observed without feature mismatches between prime and probe stimuli, as has been observed for visual spatial NP. By contrast, tactile and auditory spatial NP differs in that auditory spatial NP completely depends on feature mismatches between prime and probe stimuli. Thus, the spatial NP task might be an interesting tool with which to compare spatial distractor processing between the spatial modalities and to pinpoint modality-specific patterns.
References
Arrington, C. M., Carr, T. H., Mayer, A. R., & Rao, S. M. (2000). Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience, 12, 106–117. doi:10.1162/089892900563975
Buchner, A., Zabal, A., & Mayr, S. (2003). Auditory, visual, and cross-modal negative priming. Psychonomic Bulletin & Review, 10, 917–923. doi:10.3758/BF03196552
Buchtel, H. A., & Butter, C. M. (1988). Spatial attentional shifts: Implications for the role of polysensory mechanisms. Neuropsychologia, 26, 499–509. doi:10.1016/0028-3932(88)90107-8
Buckolz, E., Edgar, C., Kajaste, B., Lok, M., & Khan, M. (2012). Inhibited prime-trial distractor responses solely produce the visual spatial negative priming effect. Attention, Perception, & Psychophysics, 74, 1632–1643. doi:10.3758/s13414-012-0366-0
Buckolz, E., Goldfarb, A., & Khan, M. (2004). The use of a distractor-assigned response slows later responding in a location negative priming task. Perception & Psychophysics, 66, 837–845. doi:10.3758/BF03194977
Chao, H.-F. (2009). Revisiting the role of probe distractors in negative priming: Location negative priming is observed when probe distractors are consistently absent. Attention, Perception, & Psychophysics, 71, 1072–1082. doi:10.3758/APP.71.5.1072
Christie, J., & Klein, R. M. (2001). Negative priming for spatial location? Canadian Journal of Experimental Psychology, 55, 24–38.
Cohen, Y. E., & Andersen, R. A. (2004). Multisensory representations of space in the posterior parietal cortex. In G. A. Calvert, C. Spence, & B. E. Stein (Eds.), The handbook of multisensory processing (pp. 463–479). Cambridge: MIT Press.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Lawrence Erlbaum.
Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P., & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3, 292–297. doi:10.1038/73009
Corbetta, M., Miezin, F. M., Shulman, G. L., & Petersen, S. E. (1993). A PET study of visuospatial attention. Journal of Neuroscience, 13, 1202–1226.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. doi:10.1038/nrn755
Craig, J. C. (1995). Vibrotactile masking: The role of response competition. Perception & Psychophysics, 57, 1190–1200. doi:10.3758/bf03208375
Dalrymple-Alford, E. C., & Budayr, B. (1966). Examination of some aspects of the Stroop color-word test. Perceptual and Motor Skills, 23, 1211–1214. doi:10.2466/pms.1966.23.3f.1211
DeYoe, E. A., & Van Essen, D. C. (1988). Concurrent processing streams in monkey visual cortex. Trends in Neurosciences, 11, 219–226. doi:10.1016/0166-2236(88)90130-0
Dijkerman, H. C., & de Haan, E. H. (2007). Somatosensory processes subserving perception and action. Behavioral and Brain Sciences, 30, 189–201. doi:10.1017/S0140525X07001392
Drewing, K., & Schneider, W. X. (2007). Disentangling functional from structural descriptions, and the coordinating role of attention. Behavioral and Brain Sciences, 30, 205–206. doi:10.1017/S0140525X07001446
Driver, J., & Baylis, G. C. (1993). Cross-modal negative priming and interference in selective attention. Bulletin of the Psychonomic Society, 31, 45–48. doi:10.3758/BF03334137
Driver, J., & Grossenbacher, P. G. (1996). Multimodal spatial constraints on tactile selective attention. In T. Innui & J. I. McClelland (Eds.), Attention and performance XVI: Information integration in perception and communication (pp. 209–235). Cambridge: MIT Press.
Evans, P. M., & Craig, J. C. (1992). Response competition: A major source of interference in a tactile identification task. Perception & Psychophysics, 51, 199–206. doi:10.3758/bf03212244
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). GPower 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191.
Fitzgeorge, L., Buckolz, E., & Khan, M. (2011). Recently inhibited responses are avoided for both masked and nonmasked primes in a spatial negative priming task. Attention, Perception, & Psychophysics, 73, 1435–1452. doi:10.3758/s13414-011-0125-7
Fox, E. (1995). Negative priming from ignored distractors in visual selection: A review. Psychonomic Bulletin & Review, 2, 145–173. doi:10.3758/BF03210958
Frings, C., Amendt, A., & Spence, C. (2011). When seeing doesn't matter: Assessing the after-effects of tactile distractor processing in the blind and the sighted. Journal of Experimental Psychology: Human Perception and Performance, 37, 1174–1181. doi:10.1037/a0022336
Frings, C., Bader, R., & Spence, C. (2008). Selection in touch: Negative priming with tactile stimuli. Attention, Perception, & Psychophysics, 70, 516–523. doi:10.3758/PP.70.3.516
Frings, C., & Spence, C. (2010). Crossmodal congruency effects based on stimulus identity. Brain Research, 1354, 113–122. doi:10.1016/j.brainres.2010.07.058
Frings, C., & Spence, C. (2011). Increased perceptual and conceptual processing difficulty makes the immeasurable measurable: Negative priming in the absence of probe distractors. Journal of Experimental Psychology: Human Perception and Performance, 37, 72–84. doi:10.1037/a0020673
Frings, C., & Spence, C. (2013). Gestalt grouping effects on tactile information processing: When touching hands override spatial proximity. Attention, Perception, & Psychophysics, 75, 468–480. doi:10.3758/s13414-012-0417-6
Frings, C., & Wentura, D. (2006). Strategy effects counteract distractor inhibition: Negative Priming with constantly absent probe distractors. Journal of Experimental Psychology: Human Perception and Performance, 32, 854–864.
Grunwald, M. (2010). Haptic Pad‘s: Eine neue Methode zur Messung und zum Training haptischer Wahrnehmungsleistungen [Hapitc pad’s: A new method to measure and for training haptic perception performance]. Manuelle Medizin, 6, 474–476.
Guy, S., Buckolz, E., & Khan, M. (2006). The locus of location repetition latency effects. Canadian Journal of Experimental Psychology, 60, 307–318. doi:10.1037/cjep2006028
Houghton, G., & Tipper, S. P. (1994). A model of inhibitory mechanisms in selective attention. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory processes in attention, memory, and language (pp. 53–112). San Diego: Academic Press.
Joordens, S., Betancourt, I., & Spalek, T. M. (2006). Selective attention versus selection for action: Negative priming is not the result of distractors being unattended. Perception & Psychophysics, 68, 890–896.
Kane, M. J., May, C. P., Hasher, L., Rahhal, T., & Stoltzfus, E. R. (1997). Dual mechanisms of negative priming. Journal of Experimental Psychology: Human Perception and Performance, 23, 632–650.
Leboe, J. P., Mondor, T. A., & Leboe, L. C. (2006). Feature mismatch effects in auditory negative priming. Perception & Psychophysics, 68, 897–910. doi:10.3758/BF03193353
Leuthold, H., & Schröter, H. (2006). Electrophysiological evidence for response priming and conflict regulation in the auditory Simon task. Brain Research, 1097, 167–180. doi:10.1016/j.brainres.2006.04.055
Luck, S. J., & Vecera, S. P. (2002). Attention. In S. Yantis (Ed.), Stevens’ handbook of experimental psychology: Vol. 1: Sensation and perception (pp. 235–286). New York: Wiley.
Mayer, A. R., Harrington, D. L., Stephen, J., Adair, J. C., & Lee, R. R. (2007). An event-related fMRI study of exogenous facilitation and inhibition of return in the auditory modality. Journal of Cognitive Neuroscience, 19, 455–467.
Mayr, S., & Buchner, A. (2007). Negative priming as a memory phenomenon: A review of 20 years of negative priming research. Journal of Psychology, 215, 35–51. doi:10.1027/0044-3409.215.1.35
Mayr, S., Buchner, A., Möller, M., & Hauke, R. (2011). Spatial and identity negative priming in audition: Evidence of feature binding in auditory spatial memory. Attention, Perception, & Psychophysics, 73, 1710–1732. doi:10.3758/s13414-011-0138-2
Mayr, S., Hauke, R., & Buchner, A. (2009). Auditory location negative priming: A case of feature mismatch. Psychonomic Bulletin & Review, 16, 845–849. doi:10.3758/PBR.16.5.845
Mayr, S., Möller, M., & Buchner, A. (2014). Auditory spatial negative priming: What is remembered of irrelevant sounds and their locations? Psychological Research. doi:10.1007/s00426-013-0515-7
Milliken, B., Joordens, S., Merikle, P. M., & Seiffert, A. E. (1998). Selective attention: A reevaluation of the implications of negative priming. Psychological Review, 105, 203–229. doi:10.1037/0033-295X.105.2.203
Milliken, B., Tipper, S. P., Houghton, G., & Lupiáñez, J. (2000). Attending, ignoring, and repetition: On the relation between negative priming and inhibition of return. Perception & Psychophysics, 62, 1280–1296. doi:10.3758/BF03212130
Milliken, B., Tipper, S. P., & Weaver, B. (1994). Negative priming in a spatial localization task: Feature mismatching and distractor inhibition. Journal of Experimental Psychology: Human Perception and Performance, 20, 624–646. doi:10.1037/0096-1523.20.3.624
Milner, A., & Goodale, M. (1995). The visual brain in action. Oxford: Oxford University. Press.
Mishkin, M., Ungerleider, L. G., & Macko, K. A. (1983). Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences, 6, 414–417. doi:10.1016/0166-2236(83)90190-X
Möller, M., Mayr, S., & Buchner, A. (2013). Target localization among concurrent sound sources: No evidence for the inhibition of previous distractor responses. Attention, Perception, & Psychophysics, 75, 132–144. doi:10.3758/s13414-012-0380-2
Mondor, T. A., & Amirault, K. J. (1998). Effect of same-and different-modality spatial cues on auditory and visual target identification. Journal of Experimental Psychology: Human Perception and Performance, 24, 745–755. doi:10.1037/0096-1523.24.3.745
Neill, W. T. (1997). Episodic retrieval in negative priming and repetition priming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23, 1291–1305. doi:10.1037/0278-7393.23.6.1291
Neill, W. T. (2007). Mechanisms of transfer-inappropriate processing. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 63–78). Washington: American Psychological Association.
Neill, W. T., & Valdes, L. A. (1992). Persistence of negative priming: Steady state or decay? Journal of Experimental Psychology: Learning, Memory, and Cognition, 18, 565–576. doi:10.1037/0278-7393.18.3.565
Olsson, M. J. (1999). Implicit testing of odor memory: Instances of positive and negative repetition priming. Chemical Senses, 24, 347–350. doi:10.1093/chemse/24.3.347
Park, J., & Kanwisher, N. (1994). Negative priming for spatial locations: Identity mismatching, not distractor inhibition. Journal of Experimental Psychology: Human Perception and Performance, 20, 613–623. doi:10.1037/0096-1523.20.3.613
Posner, M. I. (1990). Hierarchical distributed networks in the neuropsychology of selective attention. In A. Caramazza (Ed.), Cognitive neuropsychology and neurolinguistics: Advances in model of cognitive function and impairment (pp. 187–210). Hillsdale: Erlbaum.
Rothermund, K., Wentura, D., & De Houwer, J. (2005). Retrieval of incidental stimulus–response associations as a source of negative priming. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 482–495. doi:10.1037/0278-7393.31. 3.482
Santangelo, V., & Macaluso, E. (2012). Spatial attention and audiovisual processing. In B. E. Stein (Ed.), The new handbook of multisensory processing (pp. 359–370). Cambridge: The MIT Press.
Smith, D. T., Jackson, S. R., & Rorden, C. (2009). Repetitive transcranial magnetic stimulation over frontal eye fields disrupts visually cued auditory attention. Brain Stimulation, 2, 81–87. doi:10.1016/j.brs.2008.07.005
Spence, C. (2013). Just how important is spatial coincidence to multisensory integration? Evaluating the spatial rule. Annals of the New York Academy of Sciences, 1296, 31–49. doi:10.1111/nyas.12121
Spence, C., & Driver, J. (Eds.). (2004). Crossmodal space and crossmodal attention. Oxford: Oxford University Press.
Spence, C., & Santangelo, V. (2009). Auditory attention. In C. Plack (Ed.), Auditory perception (pp. 249–270). Oxford: Oxford University Press.
Tipper, S. P. (1985). The negative priming effect: Inhibitory priming by ignored objects. Quarterly Journal of Experimental Psychology Section A, 37, 571–590. doi:10.1080/14640748508400920
Tipper, S. P. (2001). Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. Quarterly Journal of Experimental Psychology: Section A, 54, 321–343. doi:10.1080/713755969
Tipper, S. P., Brehaut, J. C., & Driver, J. (1990). Selection of moving and static objects for the control of spatially directed action. Journal of Experimental Psychology: Human Perception and Performance, 16, 492–504. doi:10.1037//0096-1523.16.3.492
Tipper, S. P., & Cranston, M. (1985). Selective attention and priming: Inhibitory and facilitatory effects of ignored primes. Quarterly Journal of Experimental Psychology, 37, 591–611. doi:10.1080/14640748508400921
Tipper, S. P., Weaver, B., & Milliken, B. (1995). Spatial negative priming without mismatching: Comment on Park and Kanwisher (1994). Journal of Experimental Psychology: Human Perception and Performance, 21, 1220–1229. doi:10.1037/0096-1523.21.5.1220
Treisman, A. (1996). The binding problem. Current Opinion in Neurobiology, 6, 171–178.
Tukey, J. W. (1977). Exploratory data analysis. Reading: Addison-Wesley.
Ward, L. M. (1994). Supramodal and modality-specific mechanisms for stimulus-driven shifts of auditory and visual attention. Canadian Journal of Experimental Psychology, 48, 242–259. doi:10.1037/1196-1961.48.2.242
Wascher, E., Schatz, U., Kuder, T., & Verleger, R. (2001). Validity and boundary conditions of automatic response activation in the Simon task. Journal of Experimental Psychology: Human Perception and Performance, 27, 731–751. doi:10.1037/0096-1523.27.3.731
Wesslein, A.-K., Spence, C., & Frings, C. (2014). When vision influences the invisible distractor. Tactile response compatibility effects require vision. Journal of Experimental Psychology: Human Perception and Performance, 40, 763–774. doi:10.1037/a0035047
Wühr, P., & Frings, C. (2008). A case for inhibition: Visual attention suppresses the processing of irrelevant objects. Journal of Experimental Psychology: General, 137, 116–130. doi:10.1037/0096-3445.137.1.116
Acknowledgments
The research reported in this article was supported by a grant of the Deutsche Forschungsgemeinschaft to Christian Frings and Charles Spence (FR 2133/5-1). We would like to thank Caroline Bermes for drawing Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frings, C., Mast, F. & Spence, C. Tactile spatial negative priming occurs without feature mismatch. Atten Percept Psychophys 76, 2305–2314 (2014). https://doi.org/10.3758/s13414-014-0721-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-014-0721-4