Abstract
Tactile expertise, resulting from extensive use of hands, has previously been shown to improve tactile perception in blind people and musicians and to be associated with changes in the central processing of tactile information. This study investigated whether expertise, due to precise and deliberate use of the fingers at work, relates to improved tactile perception and whether this expertise interacts with age. A tactile pattern and a frequency discrimination task were conducted while ERPs were measured in experts and nonexperts of two age groups within middle adulthood. Independently of age, accuracy was better in experts than in nonexperts in both tasks. Somatosensory N70 amplitudes were larger with increasing age and for experts than for nonexperts. P100 amplitudes were smaller in experts than in nonexperts in the frequency discrimination task. In the pattern discrimination task, P300 difference wave amplitude was reduced in experts and late middle-aged adults. In the frequency discrimination task, P300 was more equally distributed in late middle-aged adults. We conclude that extensive, dexterous manual work leads to acquisition of tactile expertise and that this expertise might delay, but not counteract, age effects on tactile perception. Comparable neurophysiological changes induced by age and expertise presumably have different underlying mechanisms. Enlarged somatosensory N70 amplitudes might result from reduced inhibition in older adults but from enhanced, specific excitability of the somatosensory cortex in experts. Regarding P300, smaller amplitudes might indicate fewer available resources in older adults and, by contrast, a reduced need to engage as much cognitive effort to the task in experts.
Similar content being viewed by others
Blind Braille readers have superior tactile abilities, as compared with people with normal vision (Frings, Amendt, & Spence, 2011; Goldreich & Kanics, 2003, 2006; Pascual-Leone & Torres, 1993; Van Boven, Hamilton, Kauffman, Keenan, & Pascual-Leone, 2000). Musicians, including string instrumentalists and pianists, have been shown to have different cortical representations of tactile stimuli (Elbert, Pantev, Wienbruch, Rockstroh, & Taub, 1995) and to perform better in tactile tasks than other individuals (Ragert, Schmidt, Altenmüller, & Dinse, 2004; Wong, Gnanakumaran, & Goldreich, 2011).This superior performance in tactile perception is likely due to the extensive use-dependent stimulation of the fingers (Ragert et al., 2004; Wong et al., 2011).
Whether other work-related tactile expertise acquired during many years of on-the-job training of manual dexterity is beneficial for touch perception is not known. Existing findings are inconsistent and even include reports of reduced tactile perception as a consequence of extensive hand use (Hilsenrat & Reiner, 2010; Shahbazian, Bertrand, Abarca, & Jacobs, 2009; Tremblay, Mireault, Létourneau, Pierrat, & Bourrassa, 2002). In our own previous study on age- and expertise-related differences in touch perception, we compared experts in finger dexterity (e.g., precession mechanics) with nonexperts (e.g., service employees) with regard to tactile and haptic performance (Reuter, Voelcker-Rehage, Vieluf, & Godde, 2012). We did not find significant support for the assumption that frequent use of the hands in the workplace improves touch perception in the right hand (Reuter et al., 2012). However, in right-handed people, the right hand is extensively used in everyday manual tasks, too. Thus, beneficial effects of work-related expertise might have been masked.
Expertise and aging
Age-related changes, such as increased tactile thresholds (Bowden & McNulty, 2013; Deshpande, Metter, Ling, Conwit, & Ferrucci, 2008; Dinse, 2006; Reuter et al., 2012; Tremblay, Wong, Sanderson, & Cote, 2003) and reduced performance in tactile discrimination tasks (Manning & Tremblay, 2006; Master, Larue, & Tremblay, 2010; Reuter et al., 2012), become visible already in late middle-adulthood and increase with older age (Kaneko, Asai, & Kanda, 2005; Reuter et al., 2012; Wickremaratchi & Llewelyn, 2005). Results from studies in the cognitive (e.g., Kennedy, Taylor, Reade, & Yesavage, 2010) and motor (e.g., Vieluf, Mahmoodi, Godde, Reuter, & Voelcker-Rehage, 2012) domains suggest that expertise can postpone age-related functional decline (Horton, Baker, & Schorer, 2008; Krampe & Charness, 2006). In the tactile domain, evidence for stability of tactile acuity on the fingertip in older blind Braille readers has been reported (Legge, Madison, Vaughn, Cheong, & Miller, 2008). Others report decline also in blind people, but with superior tactile acuity as compared with sighted age-matched individuals across the lifespan (Goldreich & Kanics, 2003, 2006; Stevens, Foulke, & Patterson, 1996).
Event-related potentials evoked by tactile stimuli
Mechanically applied tactile stimuli evoke event-related potentials (ERPs) to investigate early, stimulus-driven, somatosensory processing markers, as well as later, endogenously driven, cognitive processes (Bolton & Staines, 2012). The early somatosensory ERP components P50 and N70 are generated in the contralateral primary somatosensory cortex (Allison, McCarthy, Wood, & Jones, 1991; Schubert et al., 2008) and represent processing of the stimuli’s physical properties (Schubert, Blankenburg, Lemm, Villringer, & Curio, 2006). The N70 is followed by the P100, which is sensitive to attention and is thought to represent bilateral secondary somatosensory cortical processing (Bolton & Staines, 2012; Hämäläinen, Kekoni, Sams, Reinikainen, & Näätänen, 1990; Tanaka et al., 2008).
The P300 is a modality-independent cognitive ERP component that is most prominent at parietal electrode sites (Kida, Kaneda, & Nishihira, 2012). Its amplitude is related to attentional resource allocation to a given task when memory or context updating is involved (Daffner et al., 2011; Kida et al., 2012; Kok, 2001; Polich, 2007). The P300 is larger at a smaller deviant-to-standard ratio and for easier, relative to more difficult, tasks (Polich, 1987, 2007). P300 latencies reflect the timing of stimulus evaluation processes (Kutas, McCarthy, & Donchin, 1977; McCarthy & Donchin, 1981).
Influence of expertise and age on somatosensory cortical processing
Enhanced perceptual abilities in musicians have been argued to result from larger cortical representations of the stimulated fingers and increased cortical excitability in the somatosensory cortex (Ragert et al., 2004). Increased excitability of the somatosensory system, as a result of extensive tactile stimulation, was shown to lead to larger amplitudes of early somatosensory ERPs (Giriyappa, Subrahmanyam, Rangashetty, & Sharma, 2009; Höffken et al., 2007; Ragert, Franzkowiak, Schwenkreis, Tegenthoff, & Dinse, 2008). Using fMRI, increased activity in the somatosensory cortex has been shown in violinists while processing tactile stimuli (Elbert et al., 1995). Similar results are available for early ERPs of other modalities (auditory, Baumann, Meyer, & Jäncke, 2008; visual, Curran, Gibson, Horne, Young, & Bozell, 2009).
The amplitudes of early somatosensory ERPs also increase with age (Adler & Nacimiento, 1988; Desmedt & Cheron, 1980; Drechsler, 1978; Stephen et al., 2010; Stephen et al., 2006), likely due to general disinhibition and more unspecific activation associated with aging (Drechsler, 1978; Lenz et al., 2012; Pellicciari, Miniussi, Rossini, & De Gennaro, 2009). Until now, interaction effects of age and expertise on somatosensory ERPs have not been investigated together in a single study.
Influence of expertise and age effects on the P300
Data on expertise-related modulation of cognitive processes in the somatosensory domain are rare, especially for tactile perception. Using an active oddball task, Iwadate, Mori, Ashizuka, Takayose, and Ozawa (2005) found soccer players to have larger P300 amplitudes than had nonsoccer players for median nerve stimuli applied to a lower limb. This might point to facilitated cognitive processing of somatosensory stimuli applied to the foot, as a result of the frequent use and, hence, stimulation of the foot in soccer players. Similarly, studies on auditory expertise revealed that musicians had larger P300 amplitudes than nonmusicians in a pitch discrimination task (Tervaniemi, Just, Koelsch, Widmann, & Schröger, 2005) and in a deviant cadences detection task (James, Michel, Britz, Vuilleumier, & Hauert, 2011). However, Radlo, Janelle, Barba, and Frehlich (2001) found that expert baseball players had reduced P300 amplitudes, as compared with intermediate baseball players, during a visual discrimination task. Given that experts outperformed nonexperts behaviorally, the results were interpreted as indicative of more efficient perceptual decision making in experts.
Age-related changes in the P300 are well described (Friedman, 2012; Polich, 1996; Pontifex, Hillman, & Polich, 2009). Prolongation of P300 latency in older adults indicates slower information processing (Cona, Arcara, Amodio, Schiff, & Bisiacchi, 2013; Gaál, Csuhaj, & Molnár, 2007; Riis et al., 2009). Age-related decrease of P300 amplitude is most prominent at parietal electrodes (Fjell & Walhovd, 2001; Polich, 1996; Walhovd, Rosquist, & Fjell, 2008). It might reflect reduced availability of attentional resources to be allocated to the task and to suppress irrelevant neuronal operations (Pontifex et al., 2009; Walhovd et al., 2008). With increasing age, the P300 scalp topography becomes more uniform, in contrast to a pronounced frontal to parietal amplitude increase in young adults (Friedman, Kazmerski, & Fabiani, 1997; Friedman, Simpson, & Hamberger, 1993; Polich, 2012). Age-related differences in tactile discrimination performance have been shown to be related to differences in the topographical distribution of the P300 (Reuter, Voelcker-Rehage, Vieluf, Winneke, & Godde, 2013).
Overall study aims and hypotheses
The present study had two main research aims. First, we investigated the effect of work-related expertise on tactile perception. We asked whether extensive stimulation of the fingers in experts is related to improved tactile discrimination, as well as to neurophysiological changes on two levels: the somatosensory processing level and a higher order cognitive processing level. We assessed tactile discrimination performance and electrophysiological data in early and late middle-aged experts and nonexperts by use of a tactile pattern and a tactile frequency discrimination task.
On the basis of the assumption that frequent stimulation of the finger tips induces perceptual learning (Ragert et al., 2004; Wong et al., 2011), we hypothesized that experts should perform better than nonexperts, as has been shown for blind people (Goldreich & Kanics, 2006; Legge et al., 2008) and musicians (Ragert et al., 2004). We further expected larger amplitudes for the early ERP components P50 and N70 in experts, indicative of greater excitably of the somatosensory system (Giriyappa et al., 2009; Höffken et al., 2007). We assumed reduced P100 amplitudes in experts as a possible indicator of reduced attentional effort (Bolton & Staines, 2012). Moreover, we hypothesized larger P300 amplitudes in experts, as compared with nonexperts, indicating more efficient use of preattentively encoded neural information for stimulus categorization (James et al., 2011; Tervaniemi et al., 2005).
Second, we investigated the interaction of age and expertise to reveal whether extensive use of hands is able to counteract age-related decline in tactile perception. On the behavioral level, expertise might influence age trajectories in two different ways. On the one hand, it might reduce the slope of age-related decline in experts (i.e., age × expertise interaction). On the other hand, expertise might result in better performance at any age, but the slope of age-related decline should be similar in experts and nonexperts (i.e., main effects of age and expertise but no interaction). With respect to the somatosensory processing level, we expected larger somatosensory ERP amplitudes for both older adults and experts (Lenz et al., 2012). Consequently, if both age and expertise have the same facilitating effect on somatosensory ERP components, regardless of underlying mechanisms, highest amplitudes should be found in older experts. Regarding cognitive ERP components, we assumed that expertise might influence age-related differences in P300 distribution (Reuter et al., 2013). A more “youth-like” P300 topography in older experts, as compared with older nonexperts, was expected to parallel the benefit in behavioral performance.
Finally, we expected longer peak latencies for somatosensory ERPs and the P300 in older adults, indicating general slowing of processing with age (Bolton & Staines, 2012; Peters, 2002; Salthouse, 1996). Since comparable data for expertise is missing, we did not have firm hypotheses regarding expertise effects or expertise × age interaction effects on peak latency, but we postulated that age-related slowing would be less pronounced in older experts than in older nonexperts.
Method
Participants
Forty-seven healthy, right-handed participants took part in the experiment in the framework of the Bremen-Hand-Study@Jacobs, Bremen, Germany. Data for the two groups of nonexperts on the pattern discrimination task have been published previously in an article comparing tactile performance in middle-aged and young nonexperts (Reuter et al., 2013).
Participants were recruited through information flyers and articles in local newspapers. All of them took part voluntarily and gave their informed consent to the procedure, which was approved by the ethics commission of the German Psychological Society. They received 8 Euros per hour as monetary compensation.
Participants were assigned to four subsamples depending on age and level of expertise: “early middle-aged nonexperts” (EMN), 36–47 years of age; “early middle-aged experts” (EME), 37–48 years of age; “late middle-aged nonexperts” (LMN), 56–66 years of age; and “late middle-aged experts” (LME), 55–66 years of age.
To be considered as expert, at least 10 years of work experience in an occupation with high demands on fine motor control were required (Ericsson & Smith, 1991). We considered precision mechanics (opticians, dentists, goldsmiths, watchmakers, dental technicians, and hearing care professionals) as experts, since they frequently manipulate small objects and, thus, stimulate their fingertips. By contrast, nonexperts did not perform any tasks with a high demand of fine motor control in their job (e.g,, consultants, insurance agents, office clerks) (Reuter et al., 2012; Vieluf, Godde, Reuter, & Voelcker-Rehage, 2013; Vieluf et al., 2012).
The inclusion criteria of at least 10 years of work experience and being part of the active workforce determined our age range. While younger participants would not have had the chance to acquire expertise yet, older participants would have been likely to be retired and, thus, to differentially use their hands in everyday life. All participants did not engage in hobbies involving extensive finger dexterity.
Participants were screened for demographic information that included their educational background, hand usage during work and leisure time, hand dominance, and health by use of a questionnaire. Additionally, the Purdue Pegboard test (Purdue Pegboard model 32020, Lafayette Instruments, Lafayette, IN) was used to measure clinical dexterity. The test confirmed that, independently of the group, participants placed more pegs with the right than with the left hand (Vieluf et al., 2012). Screenings further confirmed that the two expert groups used their hands more frequently at work than did the two nonexpert groups. Table 1 shows screening results for each group. The four groups did not differ in their levels of education, weekly working hours, weekly hours of handwriting, hand dominance, or weekly hours of typing but did with respect to their health status.
Experimental tasks and behavioral data analysis
Participants performed two tactile, two-choice discrimination tasks with unequal probabilities of the two stimulus types, similar to active oddball tasks. Spatial and temporal discrimination tasks were conducted in order to assess both domains of tactile perception. In both tasks, participants had to actively discriminate tactile stimuli produced by a piezoelectric wafer (piezo: TeleSensory, MountainView, Ca; casing and controller: metec AG, Stuttgart, Germany) to the tip of participants’ left index finger. We tested the left hand because we assumed that expertise-related differences might be more prominent in the left than in the right hand, since experts use their left hand more often than do nonexperts (Jäncke, Schlaug, & Steinmetz, 1997).
Figure 1 illustrates the wafer and the stimulation patterns and frequencies. The wafer consisted of eight plastic pins in a 2 × 4 orientation that could be individually controlled. In the spatial task, participants had to discriminate between two tactile patterns that were presented in a stable, nonoscillating manner, with a maximal amplitude of 1.8 mm. Either a straight line or a zigzag line, both formed by four pins, was presented. This task will be referred to as pattern discrimination task (PDT) henceforth. In the temporal task, the stimuli consisted of all eight pins and were presented with different frequencies. A frequency of 120 Hz and a frequency of 180 Hz had to be distinguished. The rationale for choosing frequencies of 120 and 180 Hz, which are much higher as compared with previous studies (e.g., Hodzic, Veit, Karim, Erb, & Godde, 2004; Reuter et al., 2012; Voelcker-Rehage & Godde, 2010), was to avoid artifacts in the EEG signal of interest (~0.1–30 Hz) caused by the on- and offset of the pins in each frequency cycle. The stimuli in this task were matched for total power (squared product of amplitude and frequency) (Harris, Arabzadeh, Fairhall, Benito, & Diamond, 2006; Voelcker-Rehage & Godde, 2010). In the following, this task is referred to as frequency discrimination task (FDT). A custom-made amplifier (QueroSys, Schotten, Germany) drove the stimulation and was controlled by the software Presentation (Neurobehavioral Systems, Albany, CA).
Experimental stimuli. a Piezoelectric wafer with finger held close to the pins. b Tactile stimuli with straight line (left) and zigzag line (right). c Schematic drawing of fast (180 Hz) and slow (120 Hz) frequencies; deviant and standard stimuli were chosen in a randomized fashion across participants. Stimuli were delivered to left index fingers, and the response buttons were pressed with the right index and middle fingers
Following the oddball design (Polich, 1996), in both tasks, for each participant, one of the two stimuli was randomly selected as standard stimulus and was presented on approximately 80 % of the trials (number of trials in the PDT, M = 315. 43, SE= 3.85; in the FDT, M = 322.93, SE = 1.18). On the remaining approximately 20 % of the trials (PDT, M = 80.19, SE = 1.34 trials; FDT, M = 77.51, SE = 1.01 trials), the other, deviant stimulus was presented.
Stimuli were presented for 600 ms each. The interstimulus interval was varied randomly between 800 and 1,200 ms, with a mean of 1,000 ms. A total of 400 trials, separated into eight blocks, were conducted for each tasks. A pause interval of 10 s was given between the blocks. Participants were asked to fixate a cross on a screen and to respond to each stimulus as quickly and as accurately as possible by pressing the left or right button of a custom-made two-button response box with their right index and right middle fingers, respectively. The fixation cross was used to avoid eye or head movements that might disturb the EEG and to ensure that participants kept their eyes open.
For each task, the definition of the two stimuli as either standard or deviant and the assignment of the buttons was randomized and counterbalanced for each participant. The participants wore both ear plugs and headphones, to prevent using the sound of the stimulation to identify the stimuli. All individuals first performed the PDT, followed by the FDT. This order was chosen to avoid potential effects of tactile high-frequency stimulation on the performance in the PDT (Voelcker-Rehage & Godde, 2010). Before each task, participants were given test trials with feedback to make sure that the tasks were well understood.
Performance was measured by means of d-prime (d′) as an indicator of accuracy. We calculated d′ as the difference between the standardized probability of correct responses and false alarms for the deviant stimuli. This method is an unbiased measure of stimulus discriminability that takes both sensitivity and response tendency into account (Stanislaw & Todorov, 1999).
To account for perfect performance, we followed a procedure described by Stanislaw and Todorov (1999) and added 0.5 to both the number of hits and the number of false alarms and added 1 to both the number of signal trials and the number of noise trials, before calculating the hit rates and false alarm rates.
EEG data recording and ERP analysis
EEG data were recorded using a 32-channel active electrode system (ActiveTwo, BioSemi, Amsterdam, Netherlands). Electrodes were placed according to the 10–20 system (Jasper, 1958). Vertical and horizontal eye movements, as well as mastoid potentials, were recorded with six facial electrodes designed for body-surface applications.
The signal was digitized with a sampling rate of 2048 Hz and online band-pass filtered between 0.16 and 100 Hz. The EEG was offline analyzed and processed with Brain Vision Analyzer Software 2.0 (Brain Products, Munich, Germany). For ERP analyses, the signal was offline down-sampled to 512 Hz, and linked mastoids were used as references. A low-pass filter of 30 Hz and a notch filter of 50 Hz, were applied, and direct current (DC) shifts were corrected by DC detrend correction. Eye movements were identified and corrected by use of individual independent component analysis implemented in the analysis software. EEG activity with a gradient steeper than 5 μV/ms or voltages exceeding −75 or 75 μV were automatically detected and rejected as artifacts. Trials with artifacts were excluded channel-wise from further analysis. Only correct trials were analyzed. It was ensured that the participants included in the analysis had a minimum of 26 accepted trials in both conditions (M = 53.31, SE = 2.91, deviant condition of PDT; M = 272.88, SE = 9.76, standard condition of PDT; M = 71.55, SE = 1.49, deviant condition of FDT; M = 321.13, SE = 3.29, standard condition of FDT; and see Supplementary Table 1 for mean number of trials per group) (Cohen & Polich, 1997). As a result of this criterion, some participants had to be excluded from the ERP analysis due to insufficient numbers of correct trials. ERP data were obtained by averaged segments of −100 until 900 ms from the stimulus onset. Peaks were automatically identified in given time windows (see below). These peaks were visually inspected and adjusted, if necessary. Amplitudes were measured as baseline-to-peak values. Latencies refer to the time from stimulus onset until the peak amplitude was reached. Early somatosensory components, P50 (40–60 ms) and N70 (60–100 ms), known to arise largely from the primary somatosensory cortex (Allison et al., 1991; Hämäläinen et al., 1990), were analyzed at the electrode position C4, situated above the somatosensory cortex contralateral to the stimulated left hand (Reuter et al., 2013) and surrounding electrodes: Cz, Cp2, Cp6, Fc2, Fc6.
We focused on the standard condition only, since more reliable peaks were revealed, due to the higher number of available trials. The most pronounced and consistent peaks were detected at C4; therefore, we restricted the statistical analysis to peaks measured at this site. The P100 (90–150 ms) component was analyzed for the standard condition only at electrodes C3 and C4, ipsi- and contralateral to the stimulated hand, respectively, since its source is likely located in secondary somatosensory cortices (Bolton & Staines, 2012; Hämäläinen et al., 1990).The P300 (~250–800 ms) is known to be maximal over the midline electrodes Fz, Cz, and Pz (Fabiani, Gratton, & Coles, 2000) and was analyzed at these electrodes. For the P300 analysis, we calculated difference waves by subtracting the ERP in the standard condition from the ERP of the deviant condition. Difference waves reflect the relationship between responses to deviant and to standard stimuli. In the following, the term P300 refers to P300 difference wave. We conducted an automatic P300 peak detection on the difference wave in the time window 250–700 ms. Again, all peaks were visually inspected and adjusted, if necessary.
Statistics
Statistical analyses were done with SPSS for Windows version 20.0 (IBM Corp., Armonk, NY) and Statistica (StatSoft Europe, Hamburg, Germany). In order to examine differences among the age and expertise groups in their tactile discrimination performance, a 2 (age: early middle-aged, late middle-aged) × 2 (expertise: nonexperts, experts) analysis of variance (ANOVA) on d′ to deviant trials were computed separately for both tasks. Similarly, to analyze differences in early somatosensory components (i.e., P50 and N70), this ANOVA model was applied to peak amplitudes and latencies of the ERPs in the standard condition. For the analysis of P100 amplitude and latency, a 2 (age) × 2 (expertise) × 2 (electrode: C3, C4) repeated measures ANOVA was conducted. For the analysis of P300, we calculated a 2 (age) × 2 (expertise) × 3 (electrode: Fz, Cz, Pz) repeated measures ANOVA.
We found group differences in health (see Table 1) and assumed that health might influence performance or neurophysiological parameters. Therefore, we included health as a covariate in all statistical models. In cases in which we found at least marginally significant covariate effects, we report the ANCOVA model; otherwise, the ANOVA model is reported. For within-subjects factors with more than one degree of freedom (P300 analysis), the Greenhouse–Geisser nonsphericity correction was used. Effect sizes are given as partial eta squares (η p 2). In order to correct for unequal sample size per group, we used the complete linear model analysis method introduced by Overall and Spiegel (1969). Scheffé’s test was used for post hoc comparisons. Correlation analyses were used to assess the association between d′ and P300 amplitudes at electrodes Fz and Pz (Reuter et al., 2013), as well as between d′ and N70 peak amplitudes. In the following, only the statistical results reaching at least a level of marginal significance of p = .10 are reported. An overview of all statistical outcomes is given in the Supplementary Table 2.
Results
Tactile discrimination performance
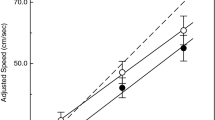
Figure 2 illustrates the behavioral performance of all groups in both tasks. Descriptive results are given in Table 2. For the analysis of d′ in the PDT the 2 (age) × 2 (expertise) ANOVA revealed a significant main effect for expertise, with experts performing better than nonexperts, F(1, 43) = 5.423, p = .025, η p 2 = .11, and a main effect of age, F(1, 43) = 5.490, p = .024, η p 2 = .11, with early middle-aged adults performing better than late middle-aged adults.
In the FDT, a significant covariate effect for health was found, F(1, 42) = 4.773, p = .035, η p 2 = .10, and thus health was included as a covariate. We found a main effect of expertise, F(1, 42) = 6.032, p = .018, η p 2 = .13, again with experts performing better than nonexperts. The significant covariate effect indicated that a better health condition positively influenced accuracy in the FDT.
Electrophysiological correlates of tactile discrimination
Figure 3 shows the ERP waveform of the standard stimuli at the electrode C4 for each group in both tasks. Figures 4 and 5 depict ERPs for both the deviant and the standard conditions and the respective difference waves at the midline electrodes for all groups in both tasks. Descriptive results of ERP parameters are given in Table 3, while a detailed overview on the statistical results is provided in Supplementary Table 2.
Pattern discrimination task
Amplitudes of somatosensory ERP components
The age × expertise ANOVA did not reveal any significant effect for P50 amplitudes. For N70 amplitudes, an ANCOVA model (2 age × 2 expertise with the covariate health) was used. We found main effects for the factors expertise, F(1, 32) = 4.226, p = .048, η p 2 = .12, and age, F(1, 32) = 4.334, p = .045, η p 2 = .12, with larger N70 amplitudes for experts, in comparison with nonexperts, and for late middle-aged, as compared with early middle-aged, adults (see Fig. 3). The covariate effect of health was marginally significant, F(1, 32) = 3.002, p = .093, η p 2 = .09, suggesting that a better health condition might be associated with larger N70 peak amplitudes. The age × expertise × electrode did not reveal any significant effects for P100 amplitudes.
Latencies of somatosensory ERP components
For P50 and P100 latencies, no significant effects were revealed. With respect to N70, we found a significant effect of age, F(1, 33) = 12.363, p = .001, η p 2 = .27, with late middle-aged participants having longer latencies than early middle-aged participants.
Amplitude of P300 difference wave
A repeated measures 2 (age) × 2 (expertise) × 3 (electrodes) ANOVA revealed significant between-groups effects of the factors expertise, F(1, 28) = 5.232, p = .030, η p 2 = .16, with experts having smaller P300 peak amplitudes than nonexperts, and age, F(1, 28) = 5.074, p = .032, η p 2 = .15, with late middle-aged adults showing smaller peak amplitudes than early middle-aged adults (see Fig. 4). We further found a main effect of electrode, F(2, 56) = 18.548, p < .001, η p 2 = .40. A post hoc analysis indicated significantly larger amplitudes at Pz than at Cz (p =.002) and Fz (p < .001). Amplitudes at Fz and Cz did not differ from each other. Moreover, the electrode × age × expertise interaction was significant, F(2, 56) = 4.339, p = .036, η p 2 = .13. A post hoc analysis revealed that only in EMN was the P300 amplitude at Pz significantly larger than at Fz. In the other groups, activity was rather equally distributed.
Latency of P300 difference wave
The same ANOVA model was applied to P300 peak latencies for pattern discrimination. An effect of electrode, F(2, 56) = 12.518, p < .001, η p 2 = .31, with longer latencies at Pz than at Cz (p = .009) and Fz (p < .001), and a marginally significant interaction of electrode and expertise, F(2, 56) = 2.843, p = .068, η p 2 = .09, were found. Post hoc analysis indicated that this interaction was driven by latency differences between electrodes in experts (Pz > Cz with p = .002, and Pz > Fz with p = .001), while no such difference was revealed in nonexperts.
Association between ERP amplitudes and PDT performance
Only for EMN was there a significant correlation between P300 amplitude at Fz and d′ (r = .728, p = .041). In the other groups, this association was not significant, and neither was the relation between performance and P300 amplitudes at Pz. Also, N70 peak amplitudes were not related to performance.
Frequency discrimination task
Amplitudes of somatosensory components
Conducting a 2 (age) × 2 (expertise) ANOVA, we did not find any effects for P50 amplitudes in the FDT. To analyze N70 amplitudes in the FDT, health was included as covariate. We found significant main effects of expertise, F(1, 31) = 4.309, p = .046, η p 2 = .12, and age, F(1, 31) = 6.405, p = .017, η p 2 = .17, with larger amplitudes for experts, as compared with nonexperts, and for late middle-aged adults, as compared with early middle-aged adults (see Fig. 3). Health revealed a significant covariate effect, F(1, 31) = 6.738, p = .014, η p 2 = .18, indicating that, as in the PDT, better health was associated with larger N70 amplitudes. For P100 amplitudes, the 2 (age) × 2 (expertise) × 2 (electrode) repeated measures ANCOVA revealed a main effect of expertise, F(1, 31) = 4.559, p = .041, η p 2 = .13, with smaller amplitudes for experts, as compared with nonexperts. Age and interaction effects were not significant. The covariate effect of health reached marginal significance, F(1, 31) = 3.853, p = .059, η p 2 = .11.
Latencies of somatosensory components
The analysis of P50 latency did not reveal any significant effects. With respect to N70, the 2 (age) × 2 (expertise) ANOVA revealed a significant effect of age, F(1, 32) = 6.903, p = .013, η p 2 = .18, with late middle-aged participants having longer latencies than early middle-aged participants. In the analysis of P100 latencies, the 2 (age) × 2 (expertise) × 2 (electrode) ANOVA revealed only a marginally significant interaction effect of age and expertise, F(1, 32) = 3.181, p = .084, η p 2 = .09.
Amplitudes of P300 difference wave
The 2 (age) × 2 (expertise) × 3 (electrodes) ANOVA revealed a main effect of electrode, F(2, 70) = 14.339, p < .001, η p 2 = .29, with larger peak amplitudes at Pz than at Cz (p = .001) and at Fz (p < .01). The analysis further showed an interaction effect of electrode and age, F(2, 70) = 3.964, p = .034, η p 2 = .10 (see Fig. 5). Post hoc analysis revealed that in early middle-aged adults, the P300 amplitude was larger at Pz than at Fz (p < .001). On the contrary, in late middle-aged participants, P300 amplitudes were not significantly different between electrode positions. Unlike for the PDT, no expertise effects were found.
Latency of P300 difference wave
The same ANOVA model was used for peak latencies analysis in the FDT. As for the PDT, we found a main effect of electrode, F(2, 70) = 26.897, p < .001, η p 2 = .435, with latencies longer at Pz, as compared with Cz (p < .001) and with Fz (p < .001).
A main effect of age, F(2, 35) = 7.385, p = .010, η p 2 = .17, was revealed, with late middle-aged participants showing longer peak latencies than did early middle-aged participants. Expertise did not influence these effects. However, a trend toward an age × electrode interaction, F(2, 70) = 2.820, p = .081, η p 2 = .075, was found, indicating that only the peak latencies measured at the electrode site Pz were longer in late middle-aged, in comparison with early middle-aged, adults (p = .033), while there were no differences between age groups at the other electrodes.
Association between ERP amplitudes and FDT performance
A positive correlation between d′ and P300 amplitude at Fz was found for EMN, r = .725, p = .018, and LMN, r = .734, p = .016. This association was not shown for the groups of experts. As for the PDT, we did not find any correlations between N70 amplitudes and performance.
Discussion
We examined the influence of work-related expertise and age on tactile pattern and frequency discrimination performance. Response accuracy (d′) was positively related to expertise in both tasks and was negatively related to age in the PDT. No significant interactions of age and expertise were found.
In both tasks, N70 amplitudes were larger for late middle-aged adults and experts, relative to early middle-aged adults and nonexperts, respectively. Later middle-aged adults also showed prolonged N70 latencies, whereas no expertise-related differences in latencies were found. P100 amplitudes were found to be smaller for experts than for nonexperts in the FDT. Regarding the P300, both older age and expertise were associated with reduced amplitudes in the PDT. In the FDT, only age revealed this effect. Late middle-aged adults had prolonged P300 peak latencies in the FDT. Unlike in nonexperts (Reuter et al., 2013), experts’ behavioral tactile discrimination performance was not shown to be associated with P300 amplitudes.
Effects of expertise on tactile discrimination and neurophysiological correlates
Experts outperformed nonexperts of the same age in tactile discrimination tasks
In line with our hypothesis and with findings in blind persons and musicians (Ragert et al., 2004; Wong et al., 2011), experts performed better than nonexperts in tactile discrimination tasks with their left hand. In light of previous opposing results for the right hand (Hilsenrat & Reiner, 2010; Reuter et al., 2012), this supports our assumption that expertise effects are more prominent for the left than for the right hand. We argue that differences between experts and nonexperts in the right hand are diminished due to the more frequent use of the right hand in regular activities of daily living. Having investigated only the left hand here, this, however, has to remain speculative, and further investigation involving both hands with different tasks is required.
Since this was a cross-sectional study, the causal relations between expertise resulting from the frequent stimulation of hands in the work place and tactile perception cannot be inferred. Especially with respect to work-related expertise, one might argue that the improved tactile abilities do not result from the frequent use of hands at work but that participants have chosen their occupation because of their inborn superior manual dexterity. However, since we found neither expertise-related benefits in tactile perception of the right hand (Reuter et al., 2012) nor improved performance of experts in a clinical dexterity test that demanded fast manual actions in precision grip (Vieluf et al., 2012), it does not seem likely that experts are naturally more dexterous with their left hand and, therefore, became precision mechanics. By contrast, we argue that their improved tactile perception in fact results from the long term and extensive use of hands in the work place.
Expertise resulted in increased excitability of the primary somatosensory cortex
We expected larger amplitudes of early somatosensory ERP components in experts, as compared with nonexperts. This was true for N70 in both tasks, but not for P50. According to Höffken et al. (2007), changes in cortical excitability, which are likely to lead to enlarged amplitudes, are one key mechanism underlying plastic somatosensory cortical reorganization. This change in excitability has previously been shown after short-term tactile learning (Godde, Ehrhardt, & Braun, 2003; Hodzic et al., 2004; Höffken et al., 2007; Ragert et al., 2008). Our results now indicate that excitability also increases with long-term tactile learning—that is, expertise.
With respect to long-term use, enhanced amplitudes of a negative somatosensory ERP were previously shown only for blind persons (Giriyappa et al., 2009). Furthermore, increased activity in the somatosensory cortex of experts has been shown with fMRI for violinists (Elbert et al., 1995). The present study is the first to show increased ERP amplitudes associated with occupation-related long-term tactile stimulation (expertise).
Latencies of somatosensory ERP components did not differ between experts and nonexperts, suggesting that expertise does not influence the timing of afferent somatosensory information processing.
Expertise effects on the cognitive processing level were task dependent
While comparable expertise effects on early somatosensory ERP components were revealed in both tasks, the effects of expertise on P300 were different for the PDT and FDT. Only in the PDT did experts have a smaller P300 than nonexperts. This finding contradicts our hypothesis that P300 amplitudes, as neural correlate of stimulus categorization, should be enhanced in experts, as it has been shown for musical expertise (James et al., 2011; Tervaniemi et al., 2005).
Perceptual learning, which has been associated with P300 reduction, might explain this finding (Kok, 2001; Sailer, Fischmeister, & Bauer, 2010). Once a task is learned, participants require fewer attentional resources while performing it. They habituate to the tasks and execute it on a more automated level (Seppänen, Pesonen, & Tervaniemi, 2012). Sailer et al. showed differences in P300 habituation between fast and slow learners and associated the reduced P300 in fast learners with changes in the subjective outcome probability and a reduction in attentional effort devoted to the task. We assume that in our study, experts habituated more quickly to the deviant stimuli, most likely already during the practice trials. The behavioral results show that the task was more difficult for nonexperts, indicating that they, in contrast to the experts, were required to allocate more attentional resources to do the PDT task.
With regard to the FDT, the significantly reduced P100 amplitudes in experts also indicate that experts direct less attention to the stimuli. This supports the notion of more automated processing also for the FDT. It remains puzzling, however, why we did not find expertise-related differences in P300 amplitude in the FDT. The PDT and FDT differed in terms of difficulty, since d′ values were much higher in the FDT than in the PDT. It is possible that this is a reason why no expertise effects emerged in the P300 in the FDT. Moreover, with regard to the FDT, although equated for total power, we cannot exclude the possibility that participants used intensity cues, and not purely temporal cues, to discriminate between the two stimuli. Moreover, tactile spatial information and temporal information are encoded and processed differently and constitute two domains of tactile perception (Hollins, 2002; Li Hegner, Lee, Grodd, & Braun, 2010). This might have contributed to the different expertise effects in both tasks.
Interactive effects of age and expertise
No interaction effects on the behavioral level
We did not find interaction effects of age and expertise on the behavioral level, indicating similar age trajectories for experts and nonexperts. Thus, acquired expertise might result in higher performance levels but might not prevent age-related decline.
In the PDT, early middle-aged participants performed better than late middle-aged participants. No effect of age was found in the FDT. One might assume that age differentially affects the two domains of tactile perception. However, tactile perception has been shown to deteriorate in both domains with increasing age (Reuter et al., 2012; Wickremaratchi & Llewelyn, 2005). We thus suppose that the FDT was too easy to reveal age effects, especially since age groups differed only by 17 years, on average. Increasing the age difference between the groups and/or task difficulty is likely to lead to stronger age effects.
Increased amplitudes of early somatosensory ERP components with age and expertise might be attributable to different causes
With respect to N70, both age and expertise were associated with an amplitude increase in PDT and FDT. Interaction effects were not revealed, but on a descriptive level, it seems that older experts had the largest amplitudes in both tasks. We could not find a significant expertise effect for P50 amplitude, although, again on the descriptive level, amplitudes seemed to be larger in late than in early middle-aged adults. Probably, the age difference between the groups was too small to reveal significant age effects for P50.
In younger adults, cortical map expansion and increased excitability of the somatosensory cortex have previously been associated with improved performance after tactile training interventions (Hodzic et al., 2004; Höffken et al., 2007; Ragert et al., 2008). By contrast, impaired performance in older adults has been shown to be associated with increased somatosensory excitability caused by reduced inhibition (Lenz et al., 2012; Pellicciari et al., 2009). In this context, we suggest that in experts, increase of somatosensory cortical N70 amplitudes reflects stronger but more specific activation, whereas in nonexperts, the age-related increase reflects reduced inhibition and resulting unspecific broader activation, accompanied by lower performance (Drechsler, 1978; Lenz et al., 2012; Pellicciari et al., 2009). In our study, tactile discrimination performance did not correlate with N70 amplitudes. It remains speculative whether these similar effects on early ERP components have different underlying mechanisms and effects on behavioral outcomes.
In respect to somatosensory processing speed, prolonged N70 latencies in older adults confirmed age-related slowing (Adler & Nacimiento, 1988; Desmedt & Cheron, 1980; Drechsler, 1978; Stephen et al., 2010; Stephen et al., 2006).
Reduced P300 amplitudes with older age and expertise might represent different effects on cognitive processing
Regarding the P300, age and expertise were both associated with reduced amplitudes in the PDT. We assume that this reduction is due to different plastic changes induced by age and expertise. Reduced P300 amplitudes in older adults, as were found for both tasks, are a common finding. It might reflect reduced availability of attentional resources for stimulus categorization and suppression of irrelevant neuronal operations (Pontifex et al., 2009; Walhovd et al., 2008). In the FDT, an interaction of age and electrode was found, due to more equal distribution of P300 activity in late than in early middle-aged adults. The latter showed a topographical distribution of P300 amplitudes with a clear parietal focus. This complements the existing literature on a parietal-to-frontal shift with aging (e.g., Adrover-Roig & Barceló, 2010; Reuter et al., 2013; West, Schwarb, & Johnson, 2010). Amplitude reduction in experts, by contrast, is assumed to be the result of less need to engage as much cognitive effort in the task (Kok, 2001; Sailer et al., 2010), as also is indicated by the reduced P100 amplitude in the FDT. This assumption is further supported by the finding that in nonexperts, P300 positively correlated with tactile discrimination performance (Reuter et al., 2013), while this association could not be confirmed for experts.
P300 latency was found to depend on ag but not expertise
Age-related prolongation of P300 latency was revealed only in the FDT. We assume that the expected latency prolongation and electrode × age interaction did not become apparent in the PDT, because mean age difference between groups was relatively small. Prolongation with age has previously been shown for PDT when also including young adults (Reuter et al., 2013). In addition, the fact that the FDT was less difficult than the PDT might have led to the relatively stronger age effect on P300 latencies in the FDT (Gaál et al., 2007).
Summary and future directions
Our results confirmed expertise-related differences in tactile discrimination accuracy and, thus, complement findings regarding expertise-related benefits in tactile perception for musicians and blind persons (Ragert et al., 2004; Wong et al., 2011). Electrophysiological findings revealed that expertise is associated with increased N70 amplitudes, indicating enhanced activation of the somatosensory system. Herewith, we have now confirmed theoretical considerations regarding the effect of frequent stimulation of the fingers also for the frequent use of hands at work. With respect to cognitive processing of tactile information, we, for the first time, have shown that extensive use of hands not only affects somatosensory processing, but also might influence the cognitive processing of tactile stimuli. Reduced P300 amplitudes in the PDT and smaller P100 amplitudes in the FDT indicated less attentional effort in experts, as compared with nonexperts.
As was expected, age-related differences were found for most measures, but neither behavioral performance nor electrophysiological data revealed expertise × age interactions. We found larger N70 amplitudes in older adults and experts but assume different underlying mechanisms, such as stronger, focal, and highly specific excitation with increasing expertise but much broader and unspecific activation as a result of general disinhibition with increasing age. Together with our behavioral findings that experts outperform nonexperts and older adults perform worse than young adults, this interpretation is well in line with the model described by Lenz et al. (2012). Also, similar age and expertise effects on P300 amplitudes are thought to be caused by different underlying processes. Smaller amplitudes might indicate fewer available resources to be allocated to the task in older adults and, by contrast, a reduced need to engage as much cognitive effort to the task in experts. In sum, our results indicate that the frequent, precise, and deliberate use of the hands is beneficial for tactile perception; however, the slope of the age-related decline seems to be unaffected by expertise. Thus, expertise might buffer age-related functional deterioration and, thereby, contribute to the maintenance of finger dexterity in older age.
This was the first study to investigate the effects of work-related expertise on tactile discrimination using electrophysiological data. Future studies are necessary to replicate our results. The proposed neural mechanisms involved remain speculative. Future research using other imaging techniques with higher spatial resolution (e.g., fMRI) or methods enabling the investigation of the balance between excitation and inhibition (e.g.,TMS/tDCS) might complement our findings. Ideally, longitudinal data should be obtained to learn more about the development of tactile expertise and about changes in tactile information processing throughout the working life span.
References
Adler, G., & Nacimiento, A. (1988). Age-dependent changes of short-latency somatosensory evoked potentials in healthy adults. Stereotactic and Functional Neurosurgery, 51(1), 55–59. doi:10.1159/000099383
Adrover-Roig, D., & Barceló, F. (2010). Individual differences in aging and cognitive control modulate the neural indexes of context updating and maintenance during task switching. Cortex, 46(4), 434–450. doi:10.1016/j.cortex.2009.09.012
Allison, T., McCarthy, G., Wood, C. C., & Jones, S. J. (1991). Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. Brain, 114(6), 2465–2503. doi:10.1093/brain/114.6.2465
Baumann, S., Meyer, M., & Jäncke, L. (2008). Enhancement of auditory-evoked potentials in musicians reflects an influence of expertise but not selective attention. Journal of Cognitive Neuroscience, 20(12), 2238–2249. doi:10.1162/jocn.2008.20157
Bolton, D. A. E., & Staines, W. R. (2012). Age-related loss in attention-based modulation of tactile stimuli at early stages of somatosensory processing. Neuropsychologia, 50(7), 1502–1513. doi:10.1016/j.neuropsychologia.2012.03.002
Bowden, J. L., & McNulty, P. A. (2013). The magnitude and rate of reduction in strength, dexterity and sensation in the human hand vary with ageing. Experimental Gerontology. doi:10.1016/j.exger.2013.03.011
Cohen, J., & Polich, J. (1997). On the number of trials needed for P300. International Journal of Psychophysiology, 25(3), 249–255. doi:10.1016/S0167-8760(96)00743-X
Cona, G., Arcara, G., Amodio, P., Schiff, S., & Bisiacchi, P. S. (2013). Does executive control really play a crucial role in explaining age-related cognitive and neural differences? Neuropsychology, 27(3), 378.
Curran, T., Gibson, L., Horne, J. H., Young, B., & Bozell, A. P. (2009). Expert image analysts show enhanced visual processing in change detection. Psychonomic Bulletin & Review, 16(2), 390–397. doi:10.3758/PBR.16.2.390
Daffner, K. R., Chong, H., Sun, X., Tarbi, E. C., Riis, J. L., McGinnis, S. M., & Holcomb, P. J. (2011). Mechanisms underlying age-and performance-related differences in working memory. Journal of Cognitive Neuroscience, 23(6), 1298–1314. doi:10.1162/jocn.2010.21540
Deshpande, N., Metter, E. J., Ling, S., Conwit, R., & Ferrucci, L. (2008). Physiological correlates of age-related decline in vibrotactile sensitivity. Neurobiology of Aging, 29(5), 765–773. doi:10.1016/j.neurobiolaging.2006.12.002
Desmedt, J. E., & Cheron, G. (1980). Somatosensory evoked potentials to finger stimulation in healthy octogenarians and in young adults: Wave forms, scalp topography and transit times of pariental and frontal components. Electroencephalography and Clinical Neurophysiology, 50(5–6), 404–425. doi:10.1016/0013-4694(80)90007-3
Dinse, H. R. (2006). Cortical reorganization in the aging brain. Progress in Brain Research, 157, 57–80. doi:10.1016/S0079-6123(06)57005-0
Drechsler, F. (1978). Quantitative analysis of neurophysiological processes of the aging CNS. Journal of Neurology, 218(3), 197–213. doi:10.1007/BF00313013
Elbert, T., Pantev, C., Wienbruch, C., Rockstroh, B., & Taub, E. (1995). Increased cortical representation of the fingers of the left hand in string players. Science, 270(5234), 305–307. doi:10.1126/science.270.5234.305
Ericsson, K. A., & Smith, J. (1991). Toward a general theory of expertise: Prospects and limits. Cambridge: Cambridge University Press.
Fabiani, M., Gratton, G., & Coles, M. G. H. (2000). Event-related brain potentials. In J. T. Cacioppo, L. G. Tassinary & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 53–84). New York: Cambridge University Press.
Fjell, A. M., & Walhovd, K. B. (2001). P300 and neuropsychological tests as measures of aging: Scalp topography and cognitive changes. Brain Topography, 14(1), 25–40. doi:10.1023/A:1012563605837
Friedman, D. (2012). The components of aging. In S. J. Luck & E. S. Kappenman (Eds.), Oxford handbook of event-related potential components (pp. 513–536). New York, NY: Oxford University Press.
Friedman, D., Kazmerski, V., & Fabiani, M. (1997). An overview of age-related changes in the scalp distribution of P3b. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 104(6), 498–513. doi:10.1016/S0168-5597(97)00036-1
Friedman, D., Simpson, G., & Hamberger, M. (1993). Age‐related changes in scalp topography to novel and target stimuli. Psychophysiology, 30(4), 383–396. doi:10.1111/j.1469-8986.1993.tb02060.x
Frings, C., Amendt, A., & Spence, C. (2011). When seeing doesn’t matter: Assessing the after-effects of tactile distractor processing in the blind and the sighted. Journal of Experimental Psychology: Human Perception and Performance, 37(4), 1174. doi:10.1037/a0022336
Gaál, Z. A., Csuhaj, R., & Molnár, M. (2007). Age-dependent changes of auditory evoked potentials–effect of task difficulty. Biological Psychology, 76(3), 196–208. doi:10.1016/j.biopsycho.2007.07.009
Giriyappa, D., Subrahmanyam, R. M., Rangashetty, S., & Sharma, R. (2009). Index finger somatosensory evoked potentials in blind braille readers. Neurologia i Neurochirurgia Polska, 43(5), 439–445.
Godde, B., Ehrhardt, J., & Braun, C. (2003). Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport, 14(4), 543–546. doi:10.1097/01.wnr.0000060833.03650.f7
Goldreich, D., & Kanics, I. M. (2003). Tactile acuity is enhanced in blindness. The Journal of Neuroscience, 23(8), 3439–3445.
Goldreich, D., & Kanics, I. M. (2006). Performance of blind and sighted humans on a tactile grating detection task. Attention, Perception, & Psychophysics, 68(8), 1363–1371. doi:10.3758/BF03193735
Hämäläinen, H., Kekoni, J., Sams, M., Reinikainen, K., & Näätänen, R. (1990). Human somatosensory evoked potentials to mechanical pulses and vibration: Contributions of SI and SII somatosensory cortices to P50 and P100 components. Electroencephalography and Clinical Neurophysiology, 75(1–2), 13–21. doi:10.1016/0013-4694(90)90148-D
Harris, J. A., Arabzadeh, E., Fairhall, A. L., Benito, C., & Diamond, M. E. (2006). Factors affecting frequency discrimination of vibrotactile stimuli: Implications for cortical encoding. PLoS One, 1, e100.
Hilsenrat, M., & Reiner, M. (2010). Discrimination capabilities of professionals in manual skills in a haptic task not related to their expertise. In A. M. L. Kappers, J. B. F. van Erp, W. M. Bergmann Tiest & Van Der Helm, Frans CT. (Eds.), Haptics: Generating and Perceiving Tangible Sensations, International conference, EuroHaptics 2010, Amsterdam July 2010, Proceedings Part II (pp. 31–36). Amsterdam: Springer.
Hodzic, A., Veit, R., Karim, A. A., Erb, M., & Godde, B. (2004). Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. The Journal of Neuroscience, 24(2), 442–446. doi:10.1523/JNEUROSCI.3731-03.2004
Höffken, O., Veit, M., Knossalla, F., Lissek, S., Bliem, B., Ragert, P., & Tegenthoff, M. (2007). Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. The Journal of Physiology, 584(2), 463–471. doi:10.1113/jphysiol.2007.140079
Hollins, M. (2002). Touch and haptics. In H. E. Pashler (Ed.), Stevens’ handbook of experimental psychology (3rd ed.). New York: Wiley.
Horton, S., Baker, J., & Schorer, J. (2008). Expertise and aging: Maintaining skills through the lifespan. European Review of Aging and Physical Activity, 5(2), 89–96. doi:10.1007/s11556-008-0034-5
Iwadate, M., Mori, A., Ashizuka, T., Takayose, M., & Ozawa, T. (2005). Long-term physical exercise and somatosensory event-related potentials. Experimental Brain Research, 160(4), 528–532.
James, C. E., Michel, C. M., Britz, J., Vuilleumier, P., & Hauert, C. A. (2011). Rhythm evokes action: Early processing of metric deviances in expressive music by experts and laymen revealed by ERP source imaging. Human Brain Mapping, 33(12), 2751–2767. doi:10.1002/hbm.21397
Jäncke, L., Schlaug, G., & Steinmetz, H. (1997). Hand skill asymmetry in professional musicians. Brain and Cognition, 34(3), 424–432. doi:10.1006/brcg.1997.0922
Jasper, H. H. (1958). The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10(2), 371–375.
Kaneko, A., Asai, N., & Kanda, T. (2005). The influence of age on pressure perception of static and moving two-point discrimination in normal subjects. Journal of Hand Therapy, 18(4), 421–424. doi:10.1197/j.jht.2005.09.010. quiz 425.
Kennedy, Q., Taylor, J. L., Reade, G., & Yesavage, J. A. (2010). Age and expertise effects in aviation decision making and flight control in a flight simulator. Aviation, Space, and Environmental Medicine, 81(5), 489.
Kida, T., Kaneda, T., & Nishihira, Y. (2012). Dual-task repetition alters event-related brain potentials and task performance. Clinical Neurophysiology, 123, 1123–1130. doi:10.1016/j.clinph.2011.10.001
Kok, A. (2001). On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology, 38(3), 557–577. doi:10.1017/S0048577201990559
Krampe, R. T., & Charness, N. (2006). Aging and expertise. In K. A. Ericsson, N. Charness, P. J. Feltovich & R. R. Hoffmann (Eds.), The Cambridge handbook of expertise and expert performance (pp. 723-742; 40). Cambridge; New York: Cambridge University Press.
Kutas, M., McCarthy, G., & Donchin, E. (1977). Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science, 197(4305), 792–795. doi:10.1126/science.887923
Legge, G. E., Madison, C., Vaughn, B. N., Cheong, A. M., & Miller, J. C. (2008). Retention of high tactile acuity throughout the life span in blindness. Attention, Perception, & Psychophysics, 70(8), 1471–1488. doi:10.3758/PP.70.8.1471
Lenz, M., Tegenthoff, M., Kohlhaas, K., Stude, P., Höffken, O., Gatica Tossi, M. A., & Dinse, H. R. (2012). Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. The Journal of Neuroscience, 32(5), 1811–1816. doi:10.1523/JNEUROSCI.2722-11.2012
Li Hegner, Y., Lee, Y., Grodd, W., & Braun, C. (2010). Comparing tactile pattern and vibrotactile frequency discrimination: A human FMRI study. Journal of Neurophysiology, 103(6), 3115–3122. doi:10.1152/jn.00940.2009
Manning, H., & Tremblay, F. (2006). Age differences in tactile pattern recognition at the fingertip. Somatosensory & Motor Research, 23(3–4), 147–155. doi:10.1080/08990220601093460
Master, S., Larue, M., & Tremblay, F. (2010). Characterization of human tactile pattern recognition performance at different ages. Somatosensory & Motor Research, 27(2), 60–67. doi:10.3109/08990220.2010.485959
McCarthy, G., & Donchin, E. (1981). A metric for thought: A comparison of P300 latency and reaction time. Science, 211(4477), 77–80. doi:10.1126/science.7444452
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. doi:10.1016/0028-3932(71)90067-4
Overall, J. E., & Spiegel, D. K. (1969). Concerning least squares analysis of experimental data. Psychological Bulletin, 72(5), 311–322.
Pascual-Leone, A., & Torres, F. (1993). Plasticity of the sensorimotor cortex representation of the reading finger in braille readers. Brain, 116(1), 39–52. doi:10.1093/brain/116.1.39
Pellicciari, M., Miniussi, C., Rossini, P., & De Gennaro, L. (2009). Increased cortical plasticity in the elderly: Changes in the somatosensory cortex after paired associative stimulation. Neuroscience, 163(1), 266–276. doi:10.1016/j.neuroscience.2009.06.013
Peters, A. (2002). The effects of normal aging on myelin and nerve fibers: A review. Journal of Neurocytology, 31(8–9), 581–593.
Polich, J. (1996). Meta-analysis of P300 normative aging studies. Psychophysiology, 33(4), 334–353. doi:10.1111/j.1469-8986.1996.tb01058.x
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. doi:10.1016/j.clinph.2007.04.019
Polich, J. (1987). Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 68(4), 311–320.
Polich, J. (2012). In S. J. Luck & E. S. Kappenman (Eds.), Neuropsychology of P300 (pp. 159–188). New York, NY: Oxford University Press.
Pontifex, M. B., Hillman, C. H., & Polich, J. (2009). Age, physical fitness, and attention: P3a and P3b. Psychophysiology, 46(2), 379–387. doi:10.1111/j.1469-8986.2008.00782.x
Radlo, S. J., Janelle, C. M., Barba, D. A., & Frehlich, S. G. (2001). Perceptual decision making for baseball pitch recognition: Using P300 latency and amplitude to index attentional processing. Research Quarterly for Exercise and Sport, 72(1), 22–31.
Ragert, P., Franzkowiak, S., Schwenkreis, P., Tegenthoff, M., & Dinse, H. R. (2008). Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Experimental Brain Research, 184(1), 1–11. doi:10.1007/s00221-007-1122-x
Ragert, P., Schmidt, A., Altenmüller, E., & Dinse, H. R. (2004). Superior tactile performance and learning in professional pianists: Evidence for meta-plasticity in musicians. European Journal of Neuroscience, 19(2), 473–478. doi:10.1111/j.0953-816X.2003.03142.x
Reuter, E., Voelcker-Rehage, C., Vieluf, S., & Godde, B. (2012). Touch perception throughout working life: Effects of age and expertise. Experimental Brain Research, 216(2), 287–297. doi:10.1007/s00221-011-2931-5
Reuter, E., Voelcker-Rehage, C., Vieluf, S., Winneke, A. H., & Godde, B. (2013). A parietal-to-frontal shift in the P300 is associated with compensation of tactile discrimination deficits in late middle-aged adults. Psychophysiology, 50(6), 583–593. doi:10.1111/psyp.12037
Riis, J. L., Chong, H., McGinnnis, S., Tarbi, E., Sun, X., Holcomb, P. J., & Daffner, K. R. (2009). Age-related changes in early novelty processing as measured by ERPs. Biological Psychology, 82(1), 33–44. doi:10.1016/j.biopsycho.2009.05.003
Sailer, U., Fischmeister, F. P. S., & Bauer, H. (2010). Effects of learning on feedback-related brain potentials in a decision-making task. Brain Research, 1342, 85–93. doi:10.1016/j.brainres.2010.04.051
Salthouse, T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403. doi:10.1037/0033-295X.103.3.403
Schubert, R., Blankenburg, F., Lemm, S., Villringer, A., & Curio, G. (2006). Now you feel it—now you don’t: ERP correlates of somatosensory awareness. Psychophysiology, 43(1), 31–40. doi:10.1111/j.1469-8986.2006.00379.x
Schubert, R., Ritter, P., Wüstenberg, T., Preuschhof, C., Curio, G., Sommer, W., & Villringer, A. (2008). Spatial attention related SEP amplitude modulations covary with BOLD signal in S1—a simultaneous EEG—fMRI study. Cerebral Cortex, 18(11), 2686–2700. doi:10.1093/cercor/bhn029
Seppänen, M., Pesonen, A. K., & Tervaniemi, M. (2012). Music training enhances the rapid plasticity of P3a/P3b event-related brain potentials for unattended and attended target sounds. Attention, Perception, & Psychophysics, 74(3), 600–612. doi:10.3758/s13414-011-0257-9
Shahbazian, M., Bertrand, P., Abarca, M., & Jacobs, R. (2009). Occupational changes in manual tactile sensibility of the dentist. Journal of Oral Rehabilitation, 36(12), 880–886. doi:10.1111/j.1365-2842.2009.02014
Stanislaw, H., & Todorov, N. (1999). Calculation of signal detection theory measures. Behavior Research Methods, 31(1), 137–149. doi:10.3758/BF03207704
Stephen, J. M., Montaño, R., Donahue, C. H., Adair, J. C., Knoefel, J., Qualls, C., & Aine, C. J. (2010). Somatosensory responses in normal aging, mild cognitive impairment, and alzheimer’s disease. Journal of Neural Transmission, 117(2), 217–225. doi:10.1007/s00702-009-0343-5
Stephen, J. M., Ranken, D., Best, E., Adair, J., Knoefel, J., Kovacevic, S., & Aine, C. J. (2006). Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clinical Neurophysiology, 117(1), 131–143. doi:10.1016/j.clinph.2005.09.003
Stevens, J. C., Foulke, E., & Patterson, M. (1996). Tactile acuity, aging, and braille reading in long-term blindness. Journal of Experimental Psychology: Applied, 2, 91–106. doi:10.1037/1076-898X.2.2.91
Tanaka, E., Inui, K., Kida, T., Miyazaki, T., Takeshima, Y., & Kakigi, R. (2008). A transition from unimodal to multimodal activations in four sensory modalities in humans: An electrophysiological study. BMC Neuroscience, 9(1), 116. doi:10.1186/1471-2202-9-116
Tervaniemi, M., Just, V., Koelsch, S., Widmann, A., & Schröger, E. (2005). Pitch discrimination accuracy in musicians vs nonmusicians: An event-related potential and behavioral study. Experimental Brain Research, 161(1), 1–10. doi:10.1007/s00221-004-2044-5
Tremblay, F., Mireault, A. C., Létourneau, J., Pierrat, A., & Bourrassa, S. (2002). Tactile perception and manual dexterity in computer users. Somatosensory & Motor Research, 19(2), 101–108. doi:10.1080/08990220120113066
Tremblay, F., Wong, K., Sanderson, R., & Cote, L. (2003). Tactile spatial acuity in elderly persons: Assessment with grating domes and relationship with manual dexterity. Somatosensory & Motor Research, 20(2), 127–132. doi:10.1080/0899022031000105154
Van Boven, R. W., Hamilton, R. H., Kauffman, T., Keenan, J. P., & Pascual-Leone, A. (2000). Tactile spatial resolution in blind braille readers. Neurology, 54(12), 2230–2236. doi:10.1212/WNL.54.12.2230
Vieluf, S., Godde, B., Reuter, E., & Voelcker-Rehage, C. (2013). Effects of age and fine motor expertise on the bilateral deficit in force initiation. Experimental Brain Research, 231(1), 107–116. doi:10.1007/s00221-013-3673-3
Vieluf, S., Mahmoodi, J., Godde, B., Reuter, E., & Voelcker-Rehage, C. (2012). The influence of age and work-related expertise on fine motor control. The Journal of Gerontopsychology and Geriatric Psychiatry, 25(4), 199–206. doi:10.1024/1662-9647/a000071
Voelcker-Rehage, C., & Godde, B. (2010). High frequency sensory stimulation improves tactile but not motor performance in older adults. Motor Control, 14(4), 460–477.
Walhovd, K. B., Rosquist, H., & Fjell, A. M. (2008). P300 amplitude age reductions are not caused by latency jitter. Psychophysiology, 45(4), 545–553. doi:10.1111/j.1469-8986.2008.00661.x
West, R., Schwarb, H., & Johnson, B. N. (2010). The influence of age and individual differences in executive function on stimulus processing in the oddball task. Cortex, 46(4), 550–563. doi:10.1016/j.cortex.2009.08.001
Wickremaratchi, M. M., & Llewelyn, J. G. (2005). Effects of ageing on touch. Postgraduate Medical Journal, 2006(82), 301–304. doi:10.1136/pgmj.2005.039651
Wong, M., Gnanakumaran, V., & Goldreich, D. (2011). Tactile spatial acuity enhancement in blindness: Evidence for experience-dependent mechanisms. The Journal of Neuroscience, 31(19), 7028–7037. doi:10.1523/JNEUROSCI.6461-10.2011
Author note
This research was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, VO 1432/7-1 / SPP 1184 and GO 802-7-1). We thank Sandra Fellehner and Janine Ohmann for their help with data collection and Samuel Fynes-Clinton and Christopher Adams for carefully proofreading. Solveig Vieluf’s current affiliation is the Institute of Sport Science, Saarland University, Saarbrücken, Germany. Axel H. Winneke’s current affiliation is the Fraunhofer Institute for Digital Media Technology, Project Group Hearing, Speech and Audio Technology, Oldenburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 17 kb)
Supplementary Table 2
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Reuter, EM., Voelcker-Rehage, C., Vieluf, S. et al. Extensive occupational finger use delays age effects in tactileperception—an ERP study. Atten Percept Psychophys 76, 1160–1175 (2014). https://doi.org/10.3758/s13414-014-0634-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-014-0634-2