INTRODUCTION
Angiogenesis is indispensable for various physiological processes including reproduction, development, wound repair, and tissue regeneration. However, abnormal neovascularization is also involved in the development and progression of pathogenic processes in a variety of disorders, including diabetic retinopathy, psoriasis, chronic inflammation, lepra alphos, rheumatoid arthritis and cardiovascular diseases. Since Folkman put forward the hypothesis that malignant tumor is angiogenesis dependent, direct and indirect evidences have shown that tumor growth and metastasis are accompanied by the growth of new blood vessels, which proves the rationality and feasibility of anti-angiogenic therapy in treatment of cancer[1,2]. Shifting the balance with in the tumor microenvironment from overproduction of angiogenic stimulators toward an overproduction of angiogenic inhibitors represents one potential anti-angiogenic strategy[3,4]. To date, a number of angiogenic inhibitors have been demonstrated to inhibit experimental tumor growth in animal models, and some of them have already entered clinical trials[5].
Gastric cancer is a malignant neoplasm, for which the common treatment is suboptimal and the prognosis of patients remains dismal. It is suggests that tumor-mediated angiogenesis due to high levels of angiogenic stimulators and down-regulation of endogenous angiogenic inhibitors play a fundamental role in the pathogenesis of malignant gastric cancer[6,7]. Molecular mechanisms of angiogenesis in gastric cancer have indicated an attractive therapeutic strategy by targeting those angiogenic regulators[8].
Angiostatin, a potent specific inhibitor of proliferating endothelial cells, shows significant antiangiogenic and anticarcinogenic efficacies on various tumors in vivo. It specifically inhibits the proliferation, migration, and formation of capillary tubes in vitro or in vivo, and induces apoptosis in endothelial cells[9-11]. Although no directed inhibition on tumor cell growth in vitro is observed, evidence shows that angiostatin inhibits the occurrence of the primary as well as metastatic tumor, and remains dormant. Systemic delivery of purified angiostatin protein, however, raises a number of difficult practical problems which make the large-scale implementation inefficient and/or inefficacious. And these problems also make it unavailable logically and pharmaceutically, including difficulties in producing large quantities of biologically active protein, the short half-life of such proteins in vivo, and the requirement for long-term intermittent or continuous treatment. The transfer of angiostatin gene, therefore, may represent a useful alternative delivery strategy. Transducing tissues at risk for tumor progression in vivo with genes encoding antiangiogenic proteins offers the potential of creating a local antiangiogenic microenvironment that can be stably maintained through continuous expression of the transduced transgene from surrounding tumor cells.
We have constructed and administered eukoryotic vector encoding mouse angiostatin into human gastric cancer cells to further characterize the antitumor function of angiostatin and explore the potential activity in gene therapy for gastric cancer. Our data demonstrated statistically significant inhibition of tumor formation and growth in nude mice accompanied by decreased microvascular density and upregulation of angiostatin, meanwhile no cytostatic effect on gastric cancer cells was observed in vitro. These results suggested that the delivery of angiostatin gene may represent a potentially new treatment modality for malignant gastric cancer, accounting for antiangiogenic activity in a paracrine way on surrounding endothelial cells.
MATERIALS AND METHODS
Cell culture and reagents
The human gastric cancer cell line SGC7901 was obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). Cells were cultured in RPMI1640 (Gibco) supplemented with 10% fetal bovine serum (FBS) (Sijiqing, Hangzhou, China), penicillin (100 units/ml) and streptomycin (100 mg/ml) in a humidified atmosphere of 5% CO2 at 37 °C. Liposome (TfxTM Reagent) was purchased from Promega Company; total RNA Isolation System (Sino-American Biotechnology Company) G418 from Gibco company; and bicinchoninic acid (BCA) protein assay kit was obtained from Pierce Chemicals (Rockford, Illinois).
Recombinant eukaryotic expression vector pcDNA3.1(+)-angio construction and transfection
Mouse angiostatin cDNA fragment encoding for the NH2-terminal secretory signal sequence(SS) and kringle1-4 (K1-4) regions of mouse plasminogen, fused with an antigenic epitope tag HA(HA tag) to the COOH terminus of kringle 4 was inserted into enkaryotic expression vector pcDNA3.1(+). The structure of the recombinant vector pcDNA3.1(+)-angio was confirmed by the restriction enzyme digest and DNA sequencing.
Gastric cancer cells SGC7901 in logarithmic growth phase were planted in 6-well plates at 5 × 105 cells/well, and reached approximately 80% confluent after overnight incubation on the day of the transfection. DNA/liposome (TfxTM Reagent) complexes were prepared and transfected according to the protocol provided by the manufacturer. The experimental group was transfected with pcDNA3.1(+)-angio 2 μg/liposome 10 μL, the vector control with pcDNA3.1(+) 2 μg/liposome 10 μL and the mock control with liposome 10 μL. After 48 h of transfection, the selective medium containing G418 (400 mg/L) was used to culture cells for 30 d. Then the isolated resistant cell clones were then selected and amplified.
RNA dot blot analysis of angiostatin transcription
Total RNA was extracted with total RNA isolation system, resuspended in DEPC-treated water, quantitated with OD260 and OD280, and dotted onto nitrocellulose filters. The linear mouse angiostatin cDNA fragment was released from the vector pcDNA3.1(+)-angio and labeled with [α-32P] in nick translation reaction as the probe. The specific activity of the probe was examined by TCA method. Hybrid was performed sequentially: prehybrided at 42 °C for 3 h, the probe denatured in water-bath at 100 °C for 10 min and cooled on ice for 5 min, hybrided at 42 °C overnight, washed in 2 × SSC and 0.2 × SSC at RT, and then hybrid signals were detected by antoradiogragh.
Western blot analysis of HA-tagged protein
The cells were cultured in conditioned media for 5 d. Cell supernatants was mixed with Lysine-sepharose and incubated at 4 °C overnight. The resin was washed with 50 mM Tris-HCl (pH8.0), and protein was eluted and stored at -20 °C. Protein concentration was determined by bicinchoninic acid assay (BCA) with bovine serum albumin as standard. Equal aliquots (40 μg) of protein from cell supernatants were subjected to electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, followed by transfer to PVDF membranes (mMillipore) using the transfer buffer for 2 hours and detection with the rabbit polyclonal anti-HA antibody (diluted 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C and a secondary peroxidase-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Final detection was performed by the enhanced chemiluminescence (ECL) Western blotting analysis system.
Viable cell number counting and FACS analysis of cell cycle
Cell viability and cell growth in vitro were determined by cell number counting. Briefly, the three groups of cells were plated on 24-well plates at 1 × 104 cells/well and cultured for 7 d. Everyday viable cell numbers of the three groups were counted under microscope.
Three groups of cells 3 × 105 in the logarithmic growth phase were collected and fixed in 70% ethanol overnight. After being dyed with PI at 4 °C avoid of light for 30 minutes, the cells through screening were checked in FACS to estimate the changes in cell cycle.
Animal studies
In vitro tumorigenesis assay. BalBc nuke mice were randomly divided into three groups, 5 in each group and injected subcutaneously with gastric cancer cells SGC7901 at 1.2 × 107 cells/mouse, the experimental group with pcDNA3.1(+)-angio/SGC7901, the vector control with pcDNA3.1(+)/SGC7901 and the mock control with SGC7901 untransfected. After 30 d, the mice were sacrificed to measure the size of tumor formed and calculate the percentage of inhibition on tumorigenesis in vivo.
Quantitative analyses for microvessel densities (MVD)
The tumor tissues were fixed and embedded in paraffin and MVD were detected by SABC method. Briefly, the sections were incubated with 0.3% H2O2 and methanol for 30 min, blocked with normal goat serum at RT for 2 h, stained with a primary anti-mouse vWF monoclonal antibody at 4 °C overnight and then biotin-conjugated anti-mouse IgG antibody at RT for 1 h. After being incubated with ABC compounds for 40 min, DAB was used to develop color reaction. Under light microscope, MVD was counted in 4 fields (400 ×) selected randomly.
Statistical analysis
The results were verified by variance analysis and χ2 analysis with SPLM statistical software offered by the Department of Statistics, the Fourth Military Medical University.
RESULTS
Indentification of the recombinant eukaryotic expression vector encoding angiostatin cDNA
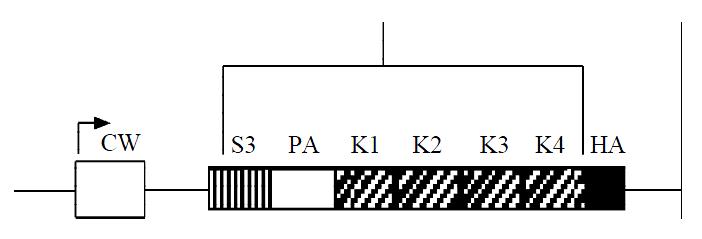
Angiostatin cDNA was cloned into an eukaryotic expression vector pcDNA3.1(+), under the cytomegalovirus promoter, encoding for the NH-terminal secretory signal sequence (SS), the preactivation peptide (PA), and kringle1-4 (K1-4) regions of mouse plasminogen, and an antigenic epitope tag derived from the influenza HA fused to the COOH terminus of kringle4 (Figure 1). As shown in electrophoresis, the linear recombinant plasmid was about 7.0 kb, and a fragment 1.4 kb was released by restrictive digest with HindIII and XbaI, which confirmed that the targeted gene was cloned into pcDNA3.1(+)-angio successfully (Figure 2). Gene sequencing showed that it had the same sequence as supposed.
Figure 1 Schematic diagram of angiostatin cDNA fragment.
Figure 2 Identification of angiostatin cDNA insertion in pcDNA3.
1(+)-angio. M: λdsDNA/HindIII marker; 1: pcDNA3.1(+)-angio/HindIII; 2: pcDNA3.1(+)-angio; 3: pcDNA3.1(+)-angio/HindIII+XbaI; 4: pcDNa3.1(+)-angio/BamHI.
Vector-mediated expression and secretion of angiostatin in vitro
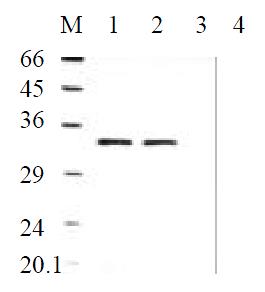
To determine the level of transgene-encoded angiostatin transcription and protein expression and secretion, gastric cancer cells SGC7901 transfected with the corresponding vectors were selected by G418 for 30 d and formed macroscopic cell clones in the experimental and vector control groups (Figure 3). The mock group of cells, however, were dead completely after 7 d of selection. Strong RNA hybrid signals of angiostatin mRNA were detected in the experimental group of cell clones genetically engineered but not in the controls (Figure 4). Western blot analysis of cell supernatants (Figure 5) revealed the detected protein of the size consistent with angiostatin in the experiment group, whereas no specific band was observed in the controls.
Figure 3 Cell clones transfected with pcDNA3.
1(+)-angio and pcDNA3.1(+) respectively. A: Cell clone pcDNA3.1(+)-angio transfected; B: Cell clone pcDNA3.1(+) transfected.
Figure 4 Angiostatin mRNA expression by Dot Blot analysis.
1: SGC7901; 2: SGC7901(+); 3:SGC7901-pcDNA3.1(+)-angio.
Figure 5 Angiostatin protein expression by Western Blot analysis.
M: Marker; 1: SGC7901pcDNA3.1(+)-angio; 2: SGC7901pcDNA3.1(+)-angio; 3: SGC7901pcDNA3.1(+); 4: SGC7901.
Biological activity of angiostatin proteins expression in vitro
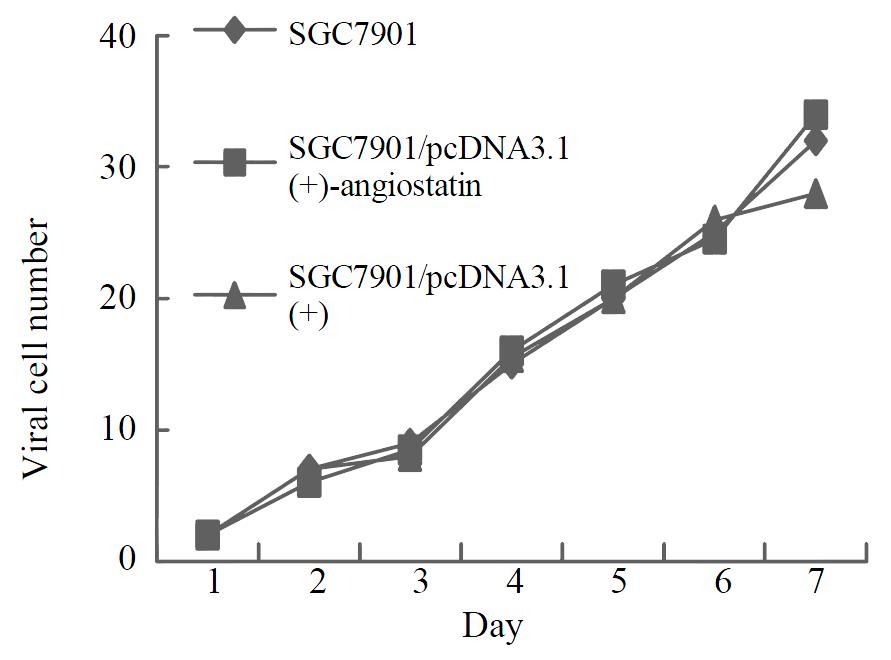
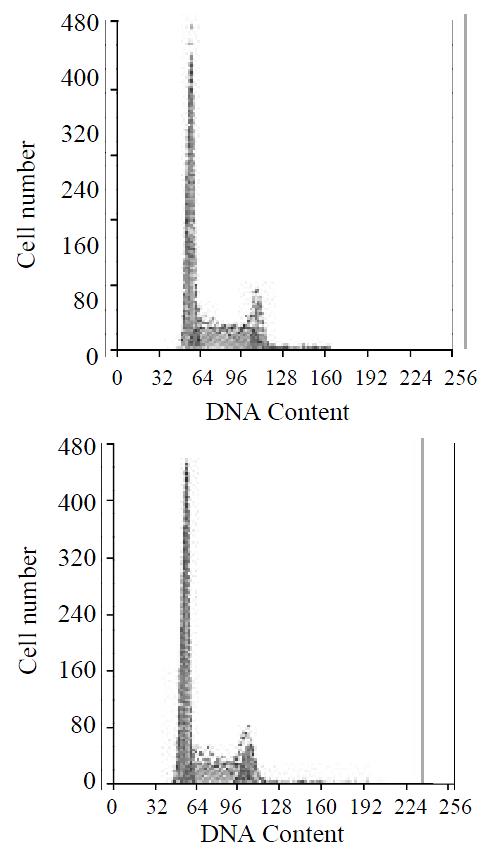
To assay for biological activity of the encoded angiostatin in vitro, tumor cells transduced with and without the corresponding vectors were cultured for 7 d to make cell growth curve (Figure 6). Under microscope, no obvious difference was observed in the cell morphology among the three groups of cells. And cell growth curves indicated no change in cell growth speed and doubling time among the three groups. Comparing the cell cycle of the three groups, no significant differences were found in the distribution of G0/G1, S and G2/M (Figure 7). These results indicated that up-regulated angiostatin expression neither directly inhibits cell growth and proliferation nor affects cell cycle in vitro.
Figure 6 Cell growth curves.
Figure 7 Cell cycle distributions by FACS analysis.
A: SGC7901 transfected with pcDNA3.1(+)-angio; B: SGC7901.
Biological activity of angiostatin proteins expression in vivo
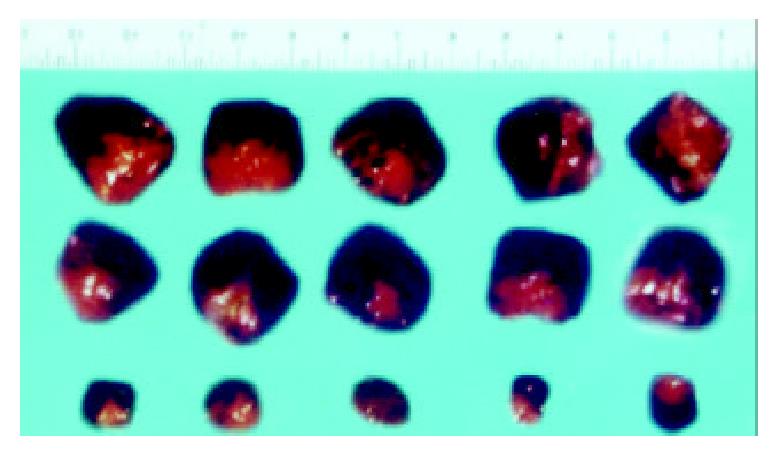
The macroscopic tumors were observed on day 7 after injection and expanded fast in the vector and mock control groups of nude mice. However, no tumors were observable until day 10 after injection in the experimental group of nude mice, and tumors expanded slowly. After 30 d of injection, no mice died in the three groups and the tumors were resected and measured. Small, pale tumor nodules were observed in the angiostatin-tranfected tumors, whereas large, red and hypervascularized tumors were present in the vector-transfected control and mock control tumor cells. The average size of tumors in the experimental group was 2.11 × 0.53 cm3, much less than the average size of the vector control 4.32 × 1.00 cm3 and the mock control (Figure 8). The inhibition by angiostatin overexpression reached 72%.
Figure 8 Tumors formed in nude mice by SGC7901/pcDNA-angio, SGC7901/pcDNA, and SGC7901 cells (n = 5).
A:SGC7901; B: SGC7901/pcDNA3.1(+); C: SGC7901/pcDNA3.1(+)-angio.
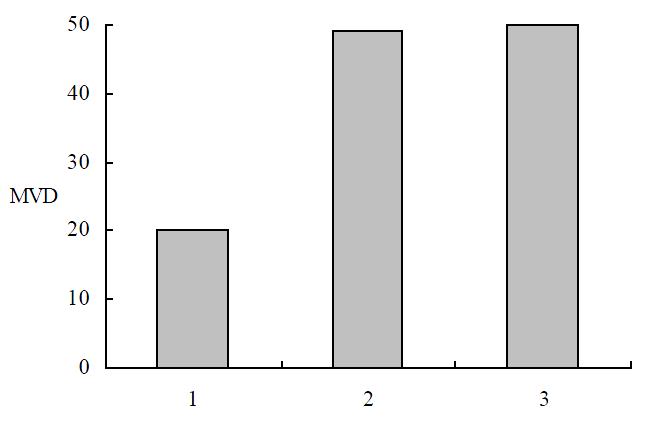
The tumor tissues in the experimental group showed more typical symptoms of poor vascularization than the control groups. More markedly diminished microvascular densities were detected by immunohistochemical staining in the tumors in the experimental group than in the vector or mock control groups respectively (P < 0.01) (Figure 9), which indicated that overex pression of angiostatin probably decreased tumorigenesis by inhibiting neovascularization in tumors in vivo. And angiostatin exerted its inhibitory actions in some paracrine ways on ECs surrounding tumor tissues and indirectly inhibited tumorigenesis.
Figure 9 MVD in immunochemistry staining.
1: SGC7901/pcDNA-angio; 2: SGC7901/pcDNA; 3: SGC7901.
DISCUSSION
Since Dr. Folkman raised the hypothesis of tumor angiogenic dependence, experimental evidences have validated that tumor growth requires the company of new blood vessel growth. At the prevascular stage, the tumor is unable to grow to a size beyond 2-3 mm3 and remains in its dormant state. However, once the angiogenic phenotype of the tumor is switched on, tumor growth rate changes from linear to exponential[12-14]. Metastases are also dependent on angiogenesis in at least two steps of the metastatic events[15]. First, metastatic tumor cells must exit from a primary tumor which has been vascularized. Second, upon arrival at their target organ, metastatic tumor cells must undergo neovascularization in order to grow to a clinically detectable size. It is speculated that complete inhibition of tumor angiogenesis may result in a loss of survival factors essential for tumor cells from the endothelial cell as paracrine factors[16]. The regulation of tumor angiogenesis is a complex process involving enzymatic and signal-transduction cascades that function in fibrinolysis, matrix remodeling, inflammation, hemodynamic control of oxygenation, and growth regulation[17-19]. The local balance between positive and negative factors determines the net tendency toward angiogenesis or angiostasis[20].
Angiogenesis is closely related to gastric cancer as a pertinent predictive factor in addition to having prognostic value[6]. The average number of blood vessels is significantly higher in gastric cancer specimens than in normal gastric specimens, higher in advanced disease than in early-stage disease, higher in specimens with metastases or blood vessel invasion than in those without such metastasis or invasion[21,22]. VEGF positive immunostaining is observed in the gastric tissues with different severities of lesions, the positive rates increased with the lesion progressing from CAG to IM to DYS[23]. And its expression is associated with hematogenous invasion, metastasis, and clinical prognosis of gastric cancer[24]. As a malignant disease with the highest mortality rates in China, gastric cancer is refractory to the routine chemotherapy and radiotherapy, which are mainly adopted as the adjunctive therapy during and/or post operation to prolong the survival rates[25,26]. Moreover, chemotherapy has obvious toxicity and is subject to drug resistance in the treatment of gastric cancer[27]. Until now the only method possible to cure gastric cancer is surgical resection, despite its importance for the late-stage cancer and various types of high-rate recurrences. Meanwhile the chromosomal instability in DNA of gastric cancer cells unfavorably obstructs the curative effects of the methods directly attacking tumor cells.
Thus antiangiogenic treatment may be necessary and potential for gastric cancer, which has been validated in many experiments. In in vivo experiments, antiangiogenic agents with cytotoxic anticancer drugs formed a highly effective modulator combination for the treatment against primary and metastatic carcinoma. The combination of anti-VEGF antibody with mitomycin C markedly enhanced anti-tumor and anti-metastasis effects in nude mice transplanted with human gastric cancer SGC-7901[25]. The VEGF receptor KDR/Flk-1 antisense strategy significantly increases the number of gasric cancer cells undergoing apoptosis, and decreases tumor dissemination[28]. Angiogenesis inhibitor endostatin inhibits both tumor growth and metastasis of human gastric cancer in nude mice[29]. And octreotide inhibits the migration and invasion of SGC-7901 gastric cancer cells in vitro and the metastasis of cancer in vivo through down-regulation of MMP-2 expression and tumor angiogenesis[30]. And the clinical applications of antivascular, anti-angiogenic and angiostatic agents for the treatment of gastric carcinoma may be valuable for long-term administration to maintain tumor dormancy because drug resistance does not develop, and these agents have a sustained effect with less side effects than the traditional methods.
Angiostatin was firstly isolated as a circulating angiogenesis inhibitor, whose sequence has greater than 98% identity with an internal fragment of plasminogen, containing the first four of five triple loop disulfide-linked kringle structures. Purified angiostatin specifically and reversibly inhibits proliferation of endothelial lineages in a dose-dependent manner, but not proliferation of normal and neoplastic nonendothelial cell lines[9-11]. Systemic administration of human angiostatin potently inhibits the growth of transplanted human breast carcinoma by 95%, colon carcinoma by 97% and prostate carcinoma by almost 100% in mice, without obvious weight loss or other toxicity observed[31,32]. It causes human primary carcinomas to regress to a dormant state by a net balance of tumor cell proliferation and apoptosis. And in the presence of angiostatin, metastatic tumor cells form microscopic perivascular cuffs around the pre-existing microvessel, rarely expanding beyond 0.3 mm in diameter[33-35]. Although angiostatin is a potent inhibitor of angiogenesis and tumor growth, the need of high dosages, repeated injections and long-term administration of this protein into the body have made it less attractive for clinical trials.
In order to develop alternative strategies for therapy, the potential of angiostatin in gene therapy has been investigated. In our study, human gastric cancer cells SGC7901 are transfected with mouse angiostatin cDNA and stable cell lines expressing the secreted form of angiostatin are established. Despite the high levels of its expression in cell clones, angiostatin has no direct influence on tumor-cell growth in vitro. Implantation of the stable cell clones in nude mice produces inhibition of primary tumor growth by an average of 72%. Inhibition of tumor growth is correlated with reduced vascularization, suggesting that angiostatin exerts the antitumor effects through antiangiogenesis. These results are similar to the previously report that angiostatin cDNA transfection into the murine T241 fibrosarcoma cells inhibits primary tumor growth by an average of 77% in C57Bl6/J mice[36]. Thus angiostatin gene therapy is possibly available for gastric cancer, especially for its simple manipulation, highly specificity, wide-spectrum inhibitory effects on various tumors. It has been observed that retroviral transduction of angiostatin in rat glioma cells inhibits tumor growth by 70% in vivo, however, a relatively ineffective process[37]. Stable gene transfer of the angiostatin cDNA by retroviral vectors in Kaposi’s sarcoma KS-IMM cells resulted in delayed tumor growth in nude mice, which was associated with reduced vascularization[38]. Direct injections of replication-deficient angiostatin-expressing adenoviral vector inhibit tumor growth, and represent a potentially new treatment modality for malignant ascites, which are efficient and capable of transducing dividing cells as well as non-dividing cells in vivo[37,39]. The specific targeting of tumors to inhibit angiogenesis using an adenovirus expressing angiostatin, may deliver localized concentrations of protein having a greater impact on inhibition of tumor growth[40]. AAV-mediated antiangiogenesis gene therapy offers efficient and sustained systemic delivery of the therapeutic product, which in turn effectively suppresses glioma growth in the brain[41]. Considering the possibility of viral immunoreaction and toxicity, non-viral angiostatin-delivery system has also been explored. Intravenous injection of cationic liposome-angiostatin cDNA complex produced a significant antimetastatic effect on murine B16 melanoma compared to either reporter gene-treated and untreated controls[42]. These results support that angiostatin-gene therapy is a potential strategy in the clinical treatment of gasatric cancer.
In order to modify the specificity, we are exploiting gastric cancer specific single-chained antibodies to guide the angiostatin-gene therapy. Another potential pathway is delivering angiostatin gene directly into gastric cancer tissues under the endoscope. Because neovascularization in tumors is a multi-step process, the key molecules involved in various steps are potentially combined to improve anti-cancer efficacy of angiogenic gene therapy, including inhibition of endothelial cell proliferation, migration, invasion and matrix degradation. More effective inhibition has been observed in the combined gene therapy of angiostatin and endostatin than the angiostatin-or endostatin-therapy respectively[43]. Meanwhile, angiogenic genes are possibly combined with other curative molecules, such as immunoregulators, suppressors of oncogene, enzymatic precursor drugs. When angiostatin-mediated antiangiogenic therapy is used in combination with intratumor delivery of the IL-12 gene, this produces a synergistic therapeutic effect[44]. It has been validated the potential of combining a destructive strategy directed against the tumor cells with an anti-angiogenic approach to fight cancer. The combination of radiotherapy and angiostatin intratumoral injection reveals a significant inhibition of tumor growth as compared with either treatment[45]. These combined therapies will open new possibilities of being less toxic and more effective than the traditional therapies in the clinical treatment of gastric cancer.
In conclusion, it is the first time that approves the potential of angiostatin anti-cancer effect has been proved on gastric cancer and its functional mechanism of antiangiogenesis in tumor has been revealed. And these data offers a new way to comeover the disadavantages in traditional therapies in clinc.