Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.298
Revised: May 16, 1998
Accepted: June 9, 1998
Published online: August 15, 1998
AIM: To detect the presence of HPV DNA and study the alteration of p53 expression in anal cancers in Chinese.
METHODS: HPV DNA was amplified by PCR. The amplified HPV DNA was classified by DBH. HPV antigen and p53 expression were respectively detected by immunohistochemistry.
RESULTS: HPV DNA was amplified only in one case of squamous cell carcinoma of the 72 Chinese anal cancers and further classified as HPV type 16. Others were all HPV negative. HPV antigen and p53 expression were also detected in this case. Positive stainings with anti-p53 antibody were seen in 61.2% anal cancers. There were no statistically significant differences between anal squamous cell carcinomas and adenocarcinomas and between anal adenocarcinomas and rectal adenocarcinomas. p53 protein expression was observed in the basal cells of squamous epithelium of condyloma acuminatum and morphologically normal squamous epithelium in 2 cases invaded by anal adenocarcinoma.
CONCLUSION: HPV infection was not associated with these cases of anal cancer. p53 alteration was a common event. Positive p53 immunostaining can not be regarded as a marker for differentiating benign from malignant lesions.
- Citation: Lai MD, Luo MJ, Yao JE, Chen PH. Anal cancer in Chinese: human papillomavirus infection and altered expression of p53. World J Gastroenterol 1998; 4(4): 298-302
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.298
Anal cancer is an uncommon tumor accounting for 2%-3% of all anorectal tumors. The association between human papillomavirus (HPVs) and lesions of anus has been reported, but the reported incidence of HPV infection in anal lesions varies in different studies. HPVs are infectious agents with tropism for cutaneous or mucosal epithelium. Infection with high-risk HPV types 16, 18 and 33 has been detected in a high percentage of patients with several types of cancers including anal cancers, suggesting that it may be a risk factor for some carcinomas[1].
The oncogene products of several DNA tumor viruses appear to target the retinoblastoma gene (Rb) product and p53 protein. The E6 and E7 oncoproteins encoded by HPV respectively bind to p53 protein and to Rb product, resulting in inactivation of them[2]. The binding of E6 protein encoded by HPV16 and 18 rapidly degrades p53 via the ubiquitin-directed pathway[3]. Therefore, it is obvious that the role of the E6 oncoprotein is to eliminate or inactivate p53 as a tumor suppressor, although the functional consequences of p53 bound and degraded by E6 in HPV-induced cancers remain unclear. It is often found that there is an inverse relationship between p53 mutation and HPV infection in HPV-related tumors, but the actual correlation between HPV infection and p53 mutation needs to be clarified.
In this study, anal cancers in Chinese were screened for the presence of HPV DNA and for the alteration of p53 expression.
Tumor samples. Archival, formaldehyde-fixed, paraffin-embedded tissue blocks of anal carcinomas were obtained from the First Affiliated Hospital of Zhejiang Medical University from 1979 to 1984. The term “anal cancer” is defined according to the WHO 1989 standard as the cancer occurring in the anal canal, “which extends from the upper to the lower border of the internal anal sphincter (from pelvic floor to anal verge)”. The anal cancer in this series include 19 squamous cell carcinomas, 8 cloacogenic carcinomas, 23 adenocarcinomas, 6 adenosquamous cell carcinomas and 16 other tumors of the anus. In addition, 23 rectal adenocarcinomas were also tested. In each case, all the available hematoxylin and eosin-stained sections were reviewed, and a representative block was chosen for further studies. Altered expression of p53 was immunohistochemically detected in most of the above samples because there were not enough tissues in some cases, although all the above cases were detected for HPV infection.
Polymerase chain reaction (PCR) amplification was used with confirmation of the products by dot blot hybridization (DBH). DNA was extracted from paraffin-embedded tissues as standard procedures using phenol and chloroform. HPV target sequences were amplified for 40 cycles using HPV L1 consensus sequence primers (MY11 and MY09) (Shanghai Institute of Cell Biology). Reaction volume of 100 μL included 50 mmol/L KCl, 4 mmol/L MgCl2, 10 mmol/L Tris-HCL pH 8.5, 0.2 mmol/L each of dNTPs, 2.5 unit Taq DNA polymerase (Perkin-Elmer/Cetus), 0.5mmol/L each of the primer sequences and 10 μL purified DNA sample. Positive control sample (Hela cell DNA) and negative control sample (distilled water) were run simultaneously with the test specimens. Each cycle consisted of 94 °C for 1 min, annealed at 52 °C for 1 min and extended at 72 °C for 1 min. After the last cycle of amplification,10 μL of product was analyzed by agarose gel electrophoresis, ethidium bromide staining and visualization under UV light. The amplification product was confirmed using dot blot hybridization by five biotinylated type specific probes as follows:
HPVL1 MY11(+): GCMCAGGGWCATAAYAATGG
MY09(-): CGTCCMARRGGAWACTGATC
M = A + C, R = A + C, W = A + T, Y = C + T
MY12HPV6: CATAAGTAACTACATCTTCCA
MY13HPV11: TCTGTGTCTAAATCTGCTACA
MY14HPV16: CATACACCTCCAGCACCTAA
MY74HPV18:GGATGCTGCACCGGCTGA
MY16HPV33: CACACAAGTAACTAGTGACAG
DBH procedure. In brief, 5 μL amplified product was first heat-denatured, then directly spotted to the nitrocellulose membrane and air dried. The membrane was put into denatured solution for 10 min, then neutralizing solution for 1 min, air-dried and baked for 60 min in an 80 °C vacuum oven . The membrane was put into prehybridization solution at 65 °C for 2 h. The bionylated probe was added to the above solution at the last concentration of 2 ng/L. Hybridization was performed at 65 °C overnight . The membranes were washed at high stringency and incubated at 63 °C for 60 min with 3% (W/V) bovine serum albumin (BSA). Treated membranes were incubated straptavidin (4 ng/L) at 37 °C for 60 min and washed and finally incubated with bionylated alkaline phosphatase (2 ng/L) at 37 °C for 60 min. Substrate was naphol As-MX, stained with fast blue . Hela cell DNA was used as positive control and distilled water at negative control.
Immunohistochemistry. HPV antigen was assessed by immunohistochemical examination with rabbit anti-bovine papillomavirus (Dako, dilution 1:100), detected by goat antibody against rabbit (Dako, dilution 1:50), then by rabbit PAP (Dako, dilution 1:200), and stained by AEC. P53 protein expression was examined with a monoclonal antibody (D0-M7001), detected by SABC(streptavidin-biotin complex peroxidase kit, 1:100) and stained by DAB. Antibodies were checked by positive and negative controls.
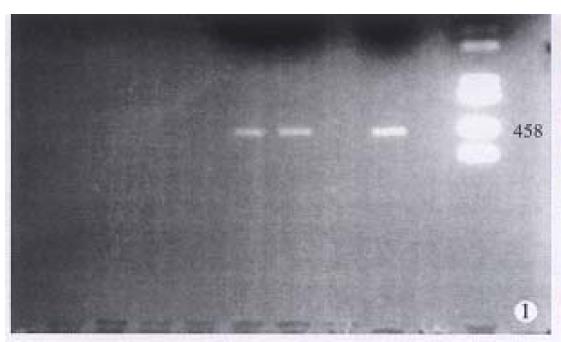
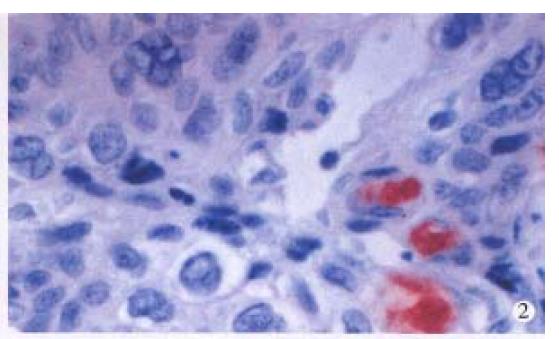
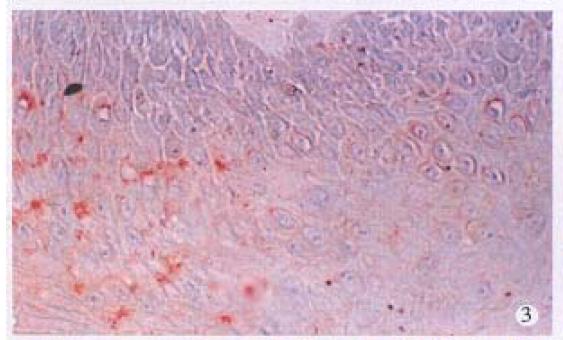
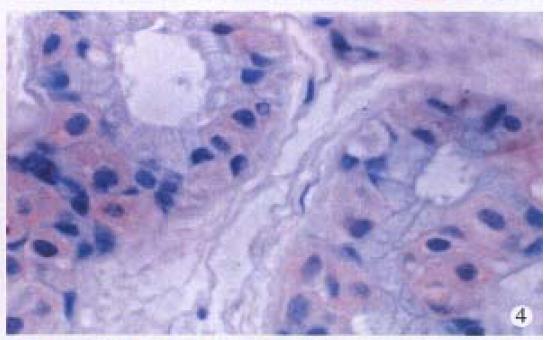
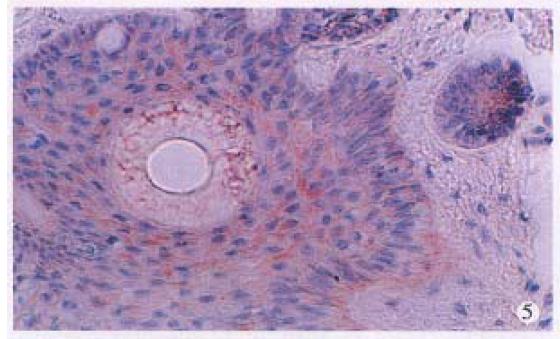
HPV DNA was amplified only in one squamous cell carcinoma (SCC) (Figure 1), representing 5% of anal SCC (1/19). The amplified HPV DNA was further classified as HPV16 by DBH. Others were all HPV negative. HPV antigen was also immunohistochemically detected in this case. HPV antigen positive cells were not only distributed in cancer cells (Figure 2), but also found in nonneoplastic superficial epithelium (Figure 3), sebaceous glands, sweat glands (Figure 4) and hair follicles (Figure 5) adjacent to the tumor positive for HPV DNA.
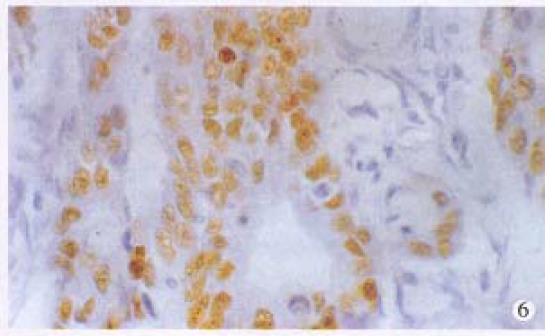
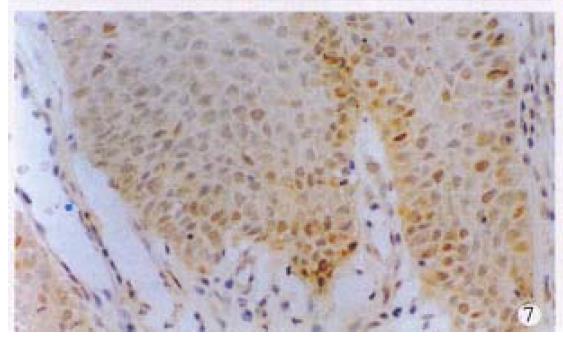
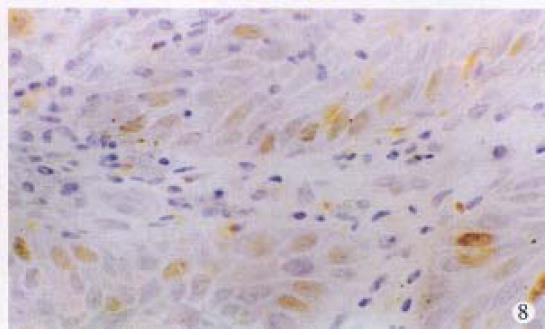
Positive stainings with the anti-p53 antibody were detected in 61.2% anal cancers (30/49) (Figure 6). The positive rates in different histological types of anal cancers were 71.4% (10/14) in SCC, 57.1% (16/28) in adenocarcinomas, 50% (3/6) in cloacogenic carcinomas, 100% (1/1) in malignant melanoma and 60% (9/15) in rectal adenocarcinomas. There were no statistically significant differences between anal SCC and adenocarcinomas and between anal adenocarcinomas and rectal adenocarcinomas. P53 protein expression was also found in the SCC case with positive HPV DNA and antigen and the basal cells of squamous epithelium of two cases of condyloma acuminatum (Figure 7) and morphologically normal squamous epithelium invaded by anal adenocarcinoma (Figure 8).
More and more evidence have shown that HPV plays a causal role in benign and malignant lesions of the urogenital tract and the head and neck region. Carcinoma of the cervix, aerodigestive tract, lung and esophagus have been proved to be related to HPV. HPV DNA has been found in up to 90% of cases of cervical dysplasia and 100% of SCC of the cervix[4]. Sixteen HPV types are involved in genital tract lesions . HPV was epidemiologically implicated in the pathogenesis of anal cancers. HPV antigen has been immunohistochemically found in both biopsy specimens of condyloma acuminatum and at least half of the biopsy specimens of anal squamous cell carcinomas[5]. HPV16 or 18 DNA were detected in more than half of the cases (Palmer, et al 1989) by southern blot or dot blot hybridization, and HPV 16 was found positive in 49 of 207 cases of anal SCC (Scholefield, et al 1990) by alkaline hydrolysis. Histochemical localization of the virus by in situ hybridization was demonstrated in 22%-67% of anal SCC. HPV types 6/11, 16/18, 31, or 33 were identified in up to 100% of cases of invasive anal SCC by PCR[6]. Using immunohistochemical staining and PCR techniques, HPV DNA and antigen were found in only one of 19 SCC cases in our series (5%) and HPV type 16 was further confirmed by DBH. It is considered that the pathogenesis of this group of anal carcinoma is not associated with HPV infection.
It has been suggested that multiple factors may be operative in the pathogenesis of anal carcinoma. Risk factors for anal cancer include cigarette smoking, anorectal chronic inflammation, radiation of anorectal area, virus infections such as herpes simplex virus-2, increasing number of sexual partners and poor sexual hygiene, immunosuppression, etc[1]. Scholefield et al[7] have shown that positive rate of HPV DNA in SCC was much higher in Sweden and Brazil than in South African and India, that there may exist demographic differences in the prevalence of HPV infection. An increasing prevalence of HPV infection in homosexual men is associated with an increasing incidence of anal cancer. Therefore, prevalences of HPV infection vary in different ethnic population and areas. It was reported that 25% of American women had experienced heterosexual anal intercourse and it occurred frequently in 8%. The low HPV infection in our series may be related to different sexual habit from that in western countries. Anal intercourse may contribute to both anal condyloma and carcinoma. It has been suggested that anal cancer in older group, especially older women, is not associated with HPV infection[4]. The average age of our patients with SCC is 61 years (ranging from 50-81) and 60% were women.
It was suggested that there were HPV-positive and HPV-negative subgroups of HPV-related tumors . In HPV-negative subgroup, mutation of p53 often occurred, while in HPV-positive tumor it did not. E6 protein of HPV bound and degraded wild p53 protein by ubiquitin-directed pathway[3]. The wild-type p53 protein, a product of the p53 gene located at 17p13, is a normal growth controll protein. Mutation of the p53 gene produced a mutant p53 protein which promoted tumor formation through loss of growth suppression. The deletion, mutation and inactivation of the p53 gene were present in the majority of solid tumors. In our study 61.2% of total anal carcinomas and 71.4% of SCC were immunohistochemically p53 positive. This result indicated that the alteration of the p53 gene is a common phenomenon. Auvinen et al[8] reported that 69% of 144 cases of colorectal carcinomas were p53 positive. Jakate et al[9] demonstrated that mutant p53 protein was present in 58.6% of anal cancers, in 85.7% of anal adenocarcinoma and 42.1% of SCC, and coexpression of both mutant p53 and E6 protein was seen in three cases (10.3%). p53 overexpression also existed in the SCC with HPV infection in our group. The above results indicated that HPV infection and p53 mutation are two independent genetic events in anal cancers.
It has been generally accepted that the p53 protein detected immunohistochemically is a mutant p53 protein and is seen only in malignant cells[10], but accumulating evidences deny this concept . Overexpression of wild p53 gene may also be detected by immunohistochemistry and identified in benign lesions[11-13]. Two cases of normal squamous epithelium adjacent to anal carcinomas and 2 cases of anal condyloma acuminatum had p53 overexpression and immunohistochemical positivity of p53 in our series. Weaker staining signals were seen in the basal cells of epithelium. Combined with the fact that almost 40% of anal cancers were p53 negative, immunohistochemical staining of p53 protein can not be regarded as a marker for differentiating benign from malignant, although p53 protein detected in benign lesions can not be exclusively ruled out to be a mutant protein.
Project supported by the National Natural Science Foundation of China, No.39300050.
| 1. | Noffsinger A, Witte D, Fenoglio-Preiser CM. The relationship of human papillomaviruses to anorectal neoplasia. Cancer. 1992;70:1276-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 2. | Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1772] [Cited by in F6Publishing: 1886] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 3. | Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1751] [Cited by in F6Publishing: 1733] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 4. | Richart RM. Causes and management of cervical intraepithelial neoplasia. Cancer. 1987;60:1951-1959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Gal AA, Meyer PR, Taylor CR. Papillomavirus antigens in anorectal condyloma and carcinoma in homosexual men. JAMA. 1987;257:337-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Zaki SR, Judd R, Coffield LM, Greer P, Rolston F, Evatt BL. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1992;140:1345-1355. [PubMed] [Cited in This Article: ] |
| 7. | Scholefield JH, Kerr IB, Shepherd NA, Miller KJ, Bloomfield R, Northover JM. Human papillomavirus type 16 DNA in anal cancers from six different countries. Gut. 1991;32:674-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Auvinen A, Isola J, Visakorpi T, Koivula T, Virtanen S, Hakama M. Overexpression of p53 and long-term survival in colon carcinoma. Br J Cancer. 1994;70:293-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Jakate SM, Saclarides TJ. Immunohistochemical detection of mutant P53 protein and human papillomavirus-related E6 protein in anal cancers. Dis Colon Rectum. 1993;36:1026-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Eriksson ET, Schimmelpenning H, Aspenblad U, Zetterberg A, Auer GU. Immunohistochemical expression of the mutant p53 protein and nuclear DNA content during the transition from benign to malignant breast disease. Hum Pathol. 1994;25:1228-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kennedy SM, Macgeogh C, Jaffe R, Spurr NK. Overexpression of the oncoprotein p53 in primary hepatic tumors of childhood does not correlate with gene mutations. Hum Pathol. 1994;25:438-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Busby-Earle RM, Steel CM, Williams AR, Cohen B, Bird CC. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994;69:732-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Cooper K, Herrington CS, Evans MF, Gatter KC, McGee JO. p53 antigen in cervical condylomata, intraepithelial neoplasia, and carcinoma: relationship to HPV infection and integration. J Pathol. 1993;171:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |