Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.30
Revised: September 30, 1997
Accepted: October 20, 1997
Published online: February 15, 1998
AIM: To determine whether antisense insulin-like growth factor-I (IGF-I) gene can modulate CEA and AFP expression in human hepatoma cells (HepG2).
METHODS: Transfection of HepG2 cells was accomplished using Lipofectin reagent. Northern blot analysis confirmed the antisense IGF-I RNA of the transfected cells. CEA and AFP levels were measured using radioimmunoassay.
RESULTS: Human hepatoma cell lines (HepG2) were transfected with antisense IGF-I gene. Northern blot analysis confirmed that antisense IGF-I RNA was expressed in the transfected cells. The effect of antisense IGF-I gene on CEA and AFP expression was demonstrated by the fact that the CEA and AFP levels in the supernatant of transfected cell culture were significantly lower as compared with the parent cells, [CEA 7.0 μg/L ± 0.76 μg/L and 3.29 μg/L ± 1.80 μg/L (P < 0.05) and AFP 53.63 μg/L ± 6.02 μg/L and 9.0 μg/L ± 5.26 μg/L (P < 0.01), respectively].
CONCLUSION: The malignant potentiality of the transfected cells was partially suppressed.Antisense IGF-I gene can modulate the expression of CEA and AFP in human hepatoma cell lines (HepG2)
- Citation: Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol 1998; 4(1): 30-32
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/30.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.30
Insulin-like growth factor-I (IGF-I) is a cytokine with multiple biological functions, which can stimulate cell differentiation and DNA synthesis and transcription[1]. In recent years, it has been found that many kinds of tumors expressed abnormal IGF-I, e.g., hepatoma, astrocytomas, etc. IGF-I might be important mitogens in the growth of many tumors[2]. Tumor cells may be able to enhance their own growth by synthesis of endogenous IGF-I. This process of autocrine secretion contributes to the partialautonomy and rapid growth in tumor cells[2-4]. We have applied the antisense strategy to block the function (e.g., by binding to a splice junction) of IGF-I in tumor cells so as to promote its degradation, and alter the structure of the target sequence. In a recent report, the stable transfectants of rat glioblastoma cells transfected with antisense IGF-I gene lost its tumorigenicity, and the inhibition of IGF-I expression elicited a highly immunogenic phenotype in glioma cells[3,4]. On the other hand, tumor markers related with the malignant hepatocarcinoma are CEA and AFP. IGF-I expression was increased significantly in the cell proliferation and high malignancy of human hepatoma. It can be used in the subsidiary diagnosis of the relapse and the metastasis of cancer, and in the research of neoplastic transformation and primary tumor diagnoses. To observe the effect of antisense IGF-I gene on the tumor markers (CEA and AFP), antisense IGF-I gene was transfected into human hepatoma cells (HepG2) to observe whether antisense IGF-I gene can regulate the changes of CEA and AFP, and the effect of neoplastic transformation.
Human hepatoma cells were purchased from the Institute of Shanghai Cell Biology. Antisense IGF-I expression plasmid was obtained from the University of Case Western Researve. Hygromycin was purchased from Sigma. Lipofectin and RPMI-1640 were purchased from GIBCO. The DIG system for hybridization was purchased from Boehringer Mannheim Biochemica. The CEA and AFP kit were the products of Institute of Shanghai Biologic-Products.
Detailed methodology followed Kliieg Ler M[5] and Trojan et al[3]’s.
Transfection of HepG2 cells was accomplished using Lipofectin reagent ( GIBCO ) according to the instructions. Solution A: 100 μL RPMI-1640 containing 10 μg/L antisense IGF-I gene. Solution B: 90 μL RPMI-1640 containing 10 μL Lipofectin. Both solutions were mixed gently, and placed at room temperature for 15 min. Two serum-free RPMI-1640 was added to each tube containing the Lipofectin reagent-DNA complexes, and mixed gently and overlay out cells. The cells were incubated for 5 h-12 h at 37 °C in a CO2 incubator. Two mL of RPMI-1640 was supplemented with 10% FCS and the cells were incubated at 37 °C, 5% CO2 incubator for another 48 h. The cells grow in the presence of hygromycin until positive clones were selected.
Total RNA in cells was extracted according to Kliieg Ler M[5]. Transfer blot hybridization was carried out as described in manual of DIG system kit. A dilution series of the total RNA was transferred to Nylon membrane and baked for 30 min at 120 °C. The membranes were put in a sealed plastic bag and used for Northern hybridization
CEA kit contained CEA marker, 125I-CEA, CEA antibody and immune separation reagent. The operation and analysis of the results were carried out according to the manual.
AFP kit contained marker,125I-AFP, first antibody of AFP, analysis reagent of AFP and second antibody of AFP. The operation and analysis of the results were carried out according to the manual.
Data were analyzed using the Student’s t test.
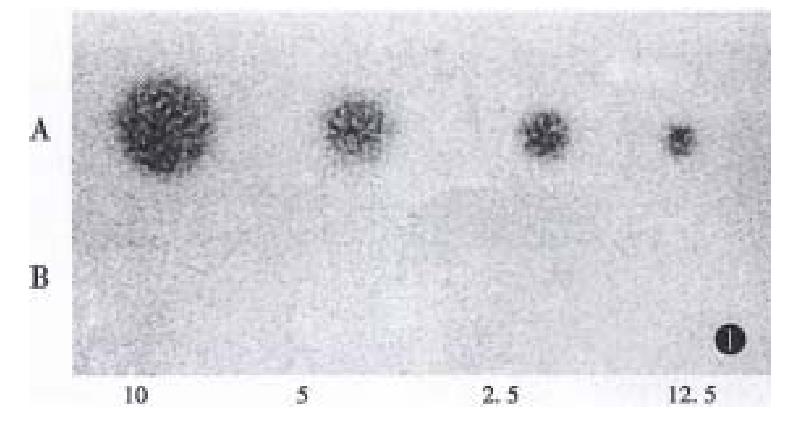
The antisense IGF-I plasmid was purified by abstraction and electrophoresis. It was wrapped up by Lipofectin and conducted into human hepatoma cells (HepG2). IGF-I antisense transcripts were selected in the presence of hygromycin. The total RNA in positive clone was extracted and its RNA transcription levels were analyzed by Northern Blot hybridization (Figure 1). The results showed that strong expression of the antisense transcript and the IGF-I transcripts of parent HepG2 cells was not apparent.
The transfectant cells were kept in the presence of hygromycin for 8-12 days. After the transfectants and parent cells were cultured for 24 h, the supernatants were collected and CEA levels were determined (Figure 2). The CEA levels of positive clones were markedly lower than that of the parent cells ( 7.0 μg/L ± 0.76 μg/L and 3.29 μg/L ± 1.80 μg/L, respectively) (P < 0.05).
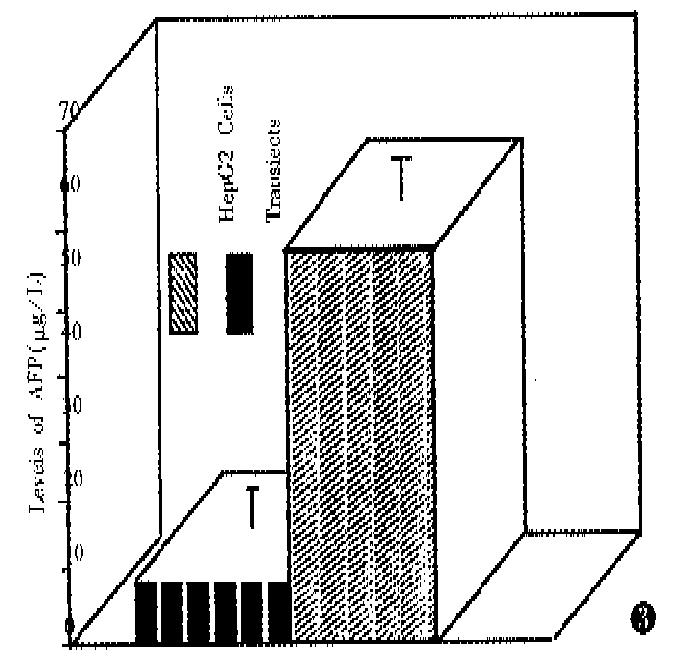
The supernatants were collected by the same method for CEA. AFP levels are shown in Figure 3. The AFP levels of positive clones were lowered markedly as compared with parent cells (53.63 μg/L ± 6.02 μg/L and 9.0 μg/L ± 5.26 μg/L, respectively) (P < 0.01).
It has been known for a long time, abnormal materials related with the diseases existin the sera of cancer patients. The tumor markers are used for the diagnosis, prognosis and treatment of tumor. Although, the tumor markers were expressed nonspecifically by tumors and produced differently, they are still important indexes to predict carcinogenesis. CEA and AFP are carcinoembryonic protein. They appear in the embryonic term and disappear in adult. When they appear in the serum of adult, it shows that the growth of tumor was active so that these proteins were secreted into the blood. The half-life of CEA and AFP in plasma were 6-8 and 6.5 days. Recent reports indicated that CEA was a member of immunoglobulin supergene family[6]. Both were important tumor markers in the course of the carcinogenesis and development. As both were regarded as the index of the tumor biological changes and of the malignant degree in vivo, in this study, CEA and AFP were used as the index of positive clone biological changes after tumor was transfected by antisense IGF-I gene. It showed that antisense IGF-I gene affect the changes of tumor markers related with malignancy of the tumors. Because many gastrointestinal tumors were produced by CEA and AFP, human hepatoma cells (HepG2) were selected as the target tumors. Antisense IGF-I gene was wrapped up and transfected into the HepG2 cells, and cells grew in the presence of hygromycin for the positive clone. Northern Blots proved that transfectants of HepG2 cells showed detectable antisense IGF-I transcripts. The positive clone grew in the presence of hygromycin for 8-12 days. After the transfectants and parent cells were adjusted to 2 × 106/well for 24 h the supernatants were collected. Figure 1, Figure 2 show the results of radioactive immune technique. The CEA and AFP levels of positive clones were markedly lower than that of the parent cells [CEA, 7.0 μg/L ± 0.76 μg/L and 3.29 μg/L ± 1.80 μg/L (P < 0.05) and AFP, 53.63 μg/L ± 6.02 μg/L and 9.0 μg/L ± 5.26 μg/L (P < 0.01), respectively]. The results indicated that transfectants could affect the levels of CEA and AFP. When the secretive levels of both decreased, it suggested that malignancy of positive clone was lower than that of the parent cells and original biological courses were changed. At present, a lot of papers show that many kinds of tumors expressed abnormal IGF-I and suggest the involvement of the continuous proliferation and tumorigenic phenotype[2]. After antisense IGF-I gene was transfected into target cells, a specially nucleotide sequence was expressed in cells that was complementary to a portion of IGF-I gene in target cells. The process and transcript of IGF-I mRNA were inhibited. As the secretion of IGF-I in target cells were changed, it could affect the role of biology in cells, the event could inhibit the growth and the tumorigenicity of rat glioblastoma cells and murine teratocarcinoma in vivo[3,4]. That antisense IGF-I gene blocks the corresponding IGF sequences may be the reason for the decrease of CEA and AFP in target cells. The exact machanisms await further researches.
Project supported by the Natural Science Foundation of Guangdong Province, No. 930305.
| 1. | Froesch ER, Schmid C, Schwander J, Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 759] [Cited by in F6Publishing: 826] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 2. | Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 371] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 245] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Trojan J, Johnson TR, Rudin SD, Blossey BK, Kelley KM, Shevelev A, Abdul-Karim FW, Anthony DD, Tykocinski ML, Ilan J. Gene therapy of murine teratocarcinoma: separate functions for insulin-like growth factors I and II in immunogenicity and differentiation. Proc Natl Acad Sci USA. 1994;91:6088-6092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kliieg Ler M. Gene transfer and expression. New York: M Stockton Press. 1990;47-81. [Cited in This Article: ] |
| 6. | Zimmermann W, Thompson J. Recent developments concerning the carcinoembryonic antigen gene family and their clinical implications. Report on the XVIIth meeting of the International Society for Oncodevelopmental Biology and Medicine, Freiburg, FRG (September 18-22, 1989). Tumour Biol. 1990;11:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |