Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.992
Peer-review started: November 25, 2019
First decision: December 23, 2019
Revised: January 14, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 7, 2020
Proton pump inhibitors use increases hepatic encephalopathy risk in patients with liver disease.
Core tip: Proton pump inhibitors (PPIs) have been widely used in patients with liver disease. In general, PPIs are considered safe. However, accumulating evidence indicates that long-term and excessive use of PPIs without clear indication can lead to serious adverse reactions. Some epidemiological studies have investigated the association of PPI use with the risk of hepatic encephalopathy. However, the results are controversial. The study reveals that PPI use increases hepatic encephalopathy risk in patients with liver disease. This reminds clinicians to be more cautious when using PPIs in patients with liver disease.
- Citation: Liu RQ, Shao Y. Results of meta-analysis should be treated critically. World J Gastroenterol 2020; 26(9): 992-994
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.992
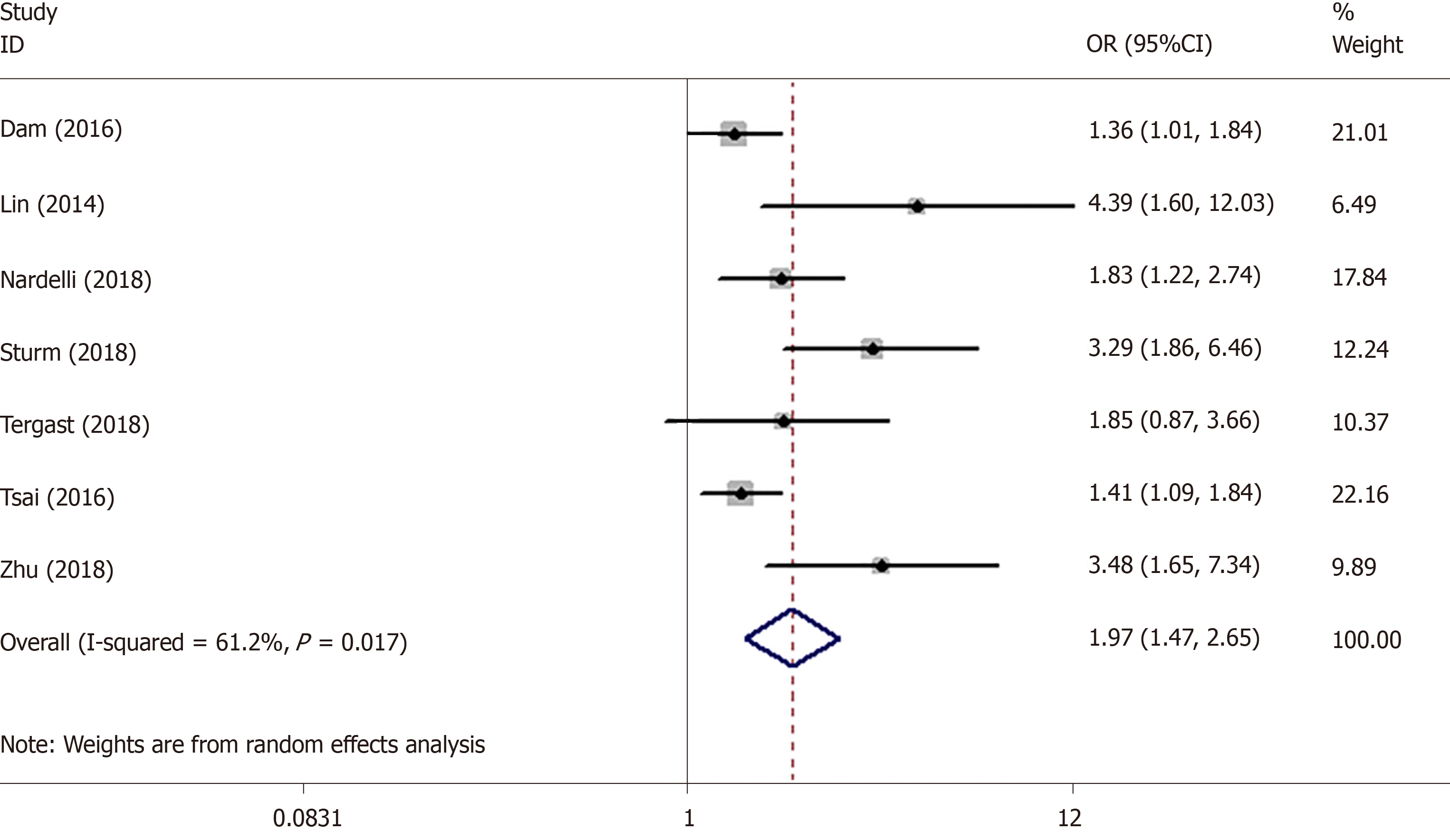
We read the recently published meta-analysis on the association between proton pump inhibitor (PPI) and hepatic encephalopathy (HE)[1]. The authors found that there was a significant association between PPI use and HE risk with low heterogeneity (I2 = 14.2%). In fact, a correct analysis showed an even stronger association. First, the author made an error in extracting the data. The study by Tsai et al[2] provided three dose-related odds ratios (ORs) of 1.41 (1.09-1.84), 1.51 (1.11-2.06) and 3.01 (1.78-5.10), and the meta-analysis by Ma et al[1] incorrectly used only the first OR. According to the study of Hamling et al[3], we recalculated that the correct OR was 1.59 (1.30-1.95). The results of the meta-analysis revealed that PPI use was significantly associated with the risk of HE with a pooled OR of 1.97 (95% confidence interval [CI]: 1.51–2.58) by using a random effects model (I2 = 57.1%). The forest plot is shown in Figure 1. In addition, we used the data which were given by Ma et al[1] to perform comprehensive analysis. The pooled OR was 1.97 (1.47, 2.65) instead of 1.50 (1.25, 1.75) and the I2 was 61.2% instead of 14.2%. The results are shown in Figure 2. The incorrect operations of Ma et al[1] from using software may be the cause of inconsistencies. Due to the obvious heterogeneity, subgroup analysis and meta-regression should be performed to explore the sources of heterogeneity.
The fixed effects model assumes that all the included studies have the same true effect size, while the true effect size in the random effects model varies with different studies. When the observed effect size of each study approaches or equals its true effect size, the heterogeneity is not obvious and the fixed effect model should be used. Otherwise, the random effects model is used. The Cochrane Handbook has stated that when I2 is less than 40%, a fixed effects model should be used for meta-analysis. Otherwise, a random effect model should be used. In general, the conclusions obtained by random effects models tend to be conservative, and thus can be used in any case. However, when the heterogeneity is significant, only the random effect model can be used, and further analysis is needed to find the sources of heterogeneity.
In conclusion, our results demonstrated that PPI use increased HE risk, consistent with the study of Ma et al[1]. However, the meta-analysis by Ma et al[1] is flawed. More accurate statistical analysis is necessary to improve the quality of this meta-analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Barreto SG, Chiu CC, Triantafyllou K S-Editor: Zhang L L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Ma YJ, Cao ZX, Li Y, Feng SY. Proton pump inhibitor use increases hepatic encephalopathy risk: A systematic review and meta-analysis. World J Gastroenterol. 2019;25:2675-2682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 495] [Article Influence: 30.9] [Reference Citation Analysis (0)] |