Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7185
Peer-review started: June 28, 2017
First decision: July 25, 2017
Revised: August 15, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: October 21, 2017
Gastric submucosal tumors (SMTs) less than 2 cm are generally considered benign neoplasms, and endoscopic observation is recommended, but SMTs over 2 cm, 40% of which are gastrointestinal stromal tumors (GISTs), have malignant potential. Although the Japanese Guidelines for GIST recommend partial surgical resection for GIST over 2 cm with malignant potential as well as en bloc large tissue sample to obtain appropriate and large specimens of SMTs, several reports have been published on tissue sampling of SMTs, such as with endoscopic ultrasound sound fine needle aspiration, submucosal tunneling bloc biopsy, and the combination of bite biopsy and endoscopic mucosal resection. Because a simpler, more accurate method is needed for appropriate treatment, we developed oval mucosal opening bloc biopsy after incision and widening by ring thread traction for submucosal tumor (OMOB) approach. OMOB was simple and enabled us to obtain large samples under direct procedure view as well as allowed us to restore to original mucosa.
Core tip: Gastric submucosal tumors (SMTs) less than 2 cm are generally considered benign neoplasms, and endoscopic observation is recommended, but SMTs over 2 cm, 40% of which are gastrointestinal stromal tumors (GISTs), have malignant potential. Although partial surgical resection for GIST over 2 cm with malignant potential as well as en bloc large tissue sample to obtain appropriate and large specimen of SMTs is recommended, several reports have been published on tissue sampling of SMTs. Because a simpler, more accurate method is needed for appropriate treatment, we developed oval mucosal opening bloc biopsy after incision and widening by ring thread traction approach.
- Citation: Mori H, Kobara H, Guan Y, Goda Y, Kobayashi N, Nishiyama N, Masaki T. Oval mucosal opening bloc biopsy after incision and widening by ring thread traction for submucosal tumor. World J Gastroenterol 2017; 23(39): 7185-7190
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7185
Gastric submucosal tumors (SMTs) less than 2 cm are generally considered benign neoplasms, and endoscopic observation is recommended[1]; however, SMTs over 2 cm, 40% of which are gastrointestinal stromal tumors (GISTs), have malignant potential[2]. The Japanese Guidelines for GIST over 2 cm with malignant potential recommend removal by partial surgical resection as well as en bloc large tissue sample collection to obtain an accurate diagnosis before surgery[3]. To obtain appropriate and large specimens of SMTs and diagnose them accurately, there have been several reports related to tissue sampling of SMTs, such as endoscopic ultrasound sound fine needle aspiration (EUS-FNA)[4,5], submucosal tunneling bloc biopsy (STB)[6], and the combination of bite biopsy and endoscopic mucosal resection (CB-EMR) by which the crown of SMTs was partially resected by EMR[7]. Because a simpler, more accurate method is needed for appropriate treatment, we developed oval mucosal opening bloc biopsy after incision and widening by ring thread traction for submucosal tumor (OMOB) approach.
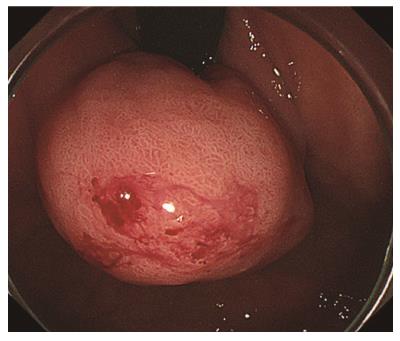
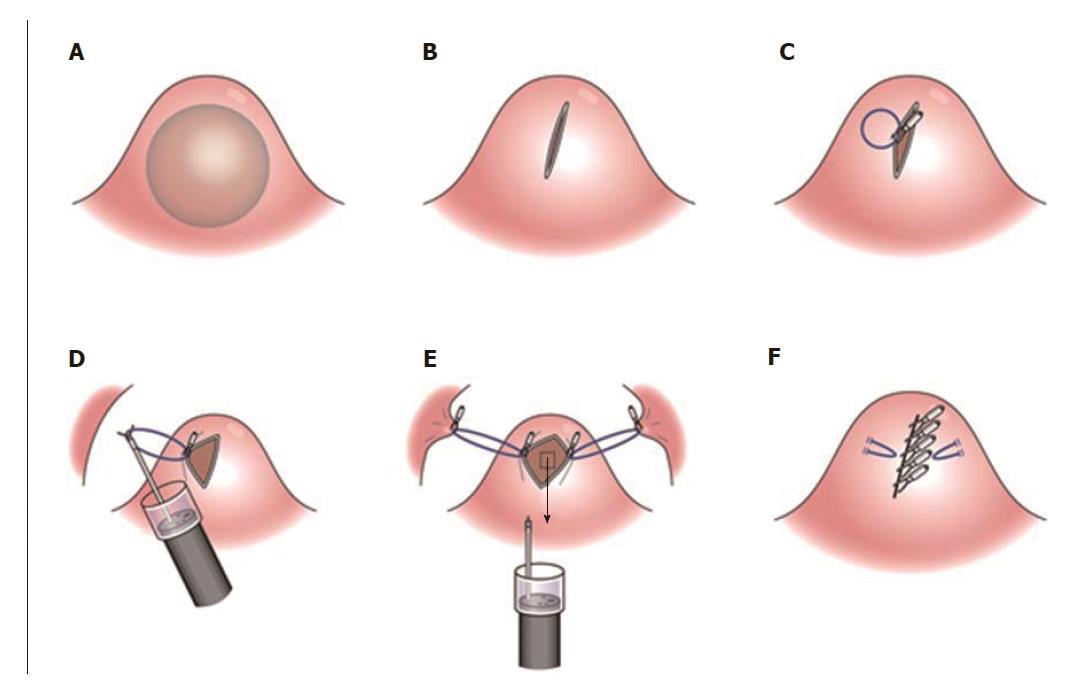
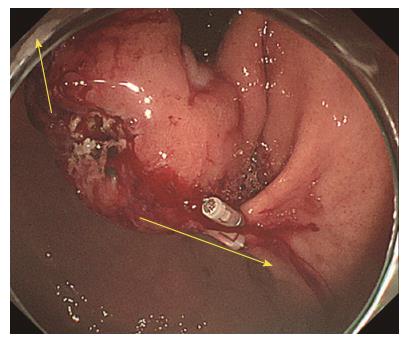
A forty-seven-year-old woman was diagnosed with a gastric SMT that was 30 mm in diameter in the fornix (Figure 1). As the tumor located in the fornix where EUS-FNA was unable to puncture its needle due to maximum bended endoscope position and STB was also difficult to create submucosal tunnel under maximum bended endoscope position, it was difficult to obtain sufficient tissue sample of this tumor (Figures 1 and 2A). A 5-10 mm straight incision was made on the top of the SMT by Dual knife (KD-650L, OLYMPUS Co., Tokyo, Japan) (Figures 2B and 3). After a 5-mm ring-shaped thread was delivered by grasping forceps and clipped on the left side mucosa of the incision edge (Figure 2C), second clip was hooked the ring-shaped thread (Figure 2D) and moved to be tied up the left gastric wall.
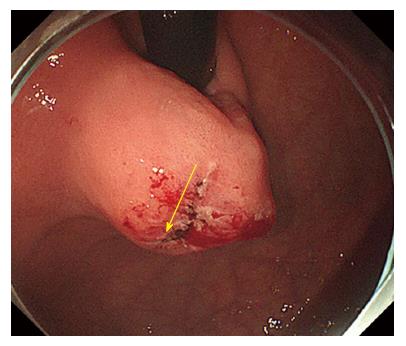
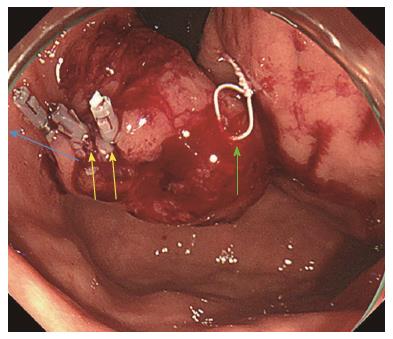
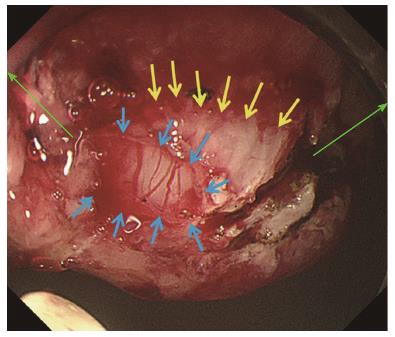
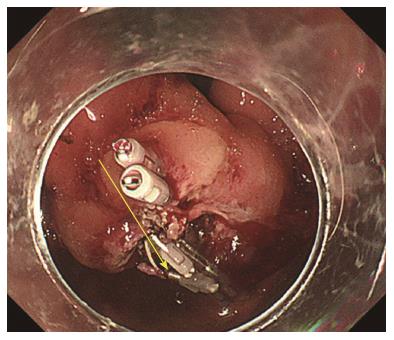
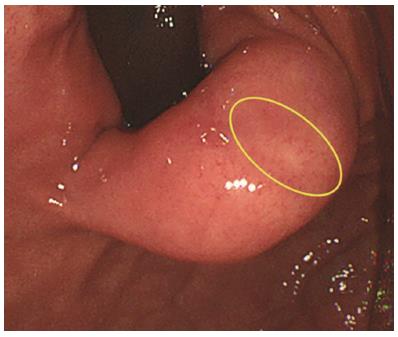
The same procedures were performed on the right side of the incision mucosa (Figure 4) making a straight incision like an oval-shaped incision (Figure 5). With more insufflation, both ring threads expanded the oval incision to a round-shaped incision from which the tumor capsule was clearly recognized (Figure 6). An approximately 5 mm incision of the tumor capsule by Dual knife made it possible to confirm the tumor itself which had abundant tumor vessels (Figures 2E and 6). A 5-mm piece of tumor tissue was obtained by cutting the tumor surface with a Dual knife. After both sides of the ring threads were detached, the opened mucosa was closed by hemoclips to restore it back to the original mucosa (Figures 2F and 7). The total procedure time was only 10 min, and there were no complications, such as bleeding or perforation. The histological result was gastrointestinal stromal tumor. Three weeks after this new bloc biopsy, the incised mucosa was completely recovered with a linear scar. Laparoscopy and endoscopy cooperative surgery (LECS) was successfully performed, and the histological finding of the GIST was low risk in accordance with Fletcher’s classification. An endoscopic image revealed that straight incision on the top of the SMT was completely scarred and closed (yellow ring) (Figure 8) when laparoscopy and endoscopy cooperative surgery (LECS) was performed six week after oval mucosal opening bloc biopsy.
The natural history of 2-5 cm GISTs is unknown. In the Japanese Guidelines of GIST, accurate diagnosis, including the histological grade based on a sufficient tissue sample, is recommended for GIST less than 2 cm, which is growing rapidly, or 2-5 cm GIST rather than endoscopic observation alone[8].
EUS-FNA is very useful for accurate diagnosis for SMTs since it was reported in 1992[9]. Its diagnostic sensitivity for GIST is very high at approximately 70% and the specificity is approximately 85%[10]. On the other hand, EUS-FNA does not always obtain sufficient tissue by needle sample for one of the grading factors of malignancy, such as the mitotic count under a 50 high power microscope field. The diagnostic rate for EUS-FNA was approximately 60% as the obtained samples were too small to pathologically diagnose the mitotic counts[11]. The combination of bite biopsy and endoscopic mucosal resection (CB-EMR) using a snare to cut the top of SMTs enabled us to obtain a large bloc specimen. However, the bleeding rate was very high at approximately 50%-60% from the snare resection site[12]. Bleeding after snare resection occurred due to a large mucosal defect at approximately 15-20 mm in diameter. Compared to CB-EMR, OMOB enable us to perform en bloc large tissue sampling without complications, such as bleeding, for GIST with rich vessels. OMOB consists of a 1-cm linear incision to round shaped excision using ring threads that expand with insufflation. After obtaining large bloc tissue, coagulation of bleeding vessels is performed followed by closure of the opening mucosa. Closure and recovery of mucosal incision is an important point of OMOB. STB using the ESD technique is another way to obtain a large tissue sample of GIST. As STB was safely performed using flexible endoscopic knives, only ESD experts could perform STB. It is difficult for ordinary endoscopists to perform STB[13], because making appropriate size and location of mucosal incision suitable for creating submucosal tunnel was very difficult for ESD beginner. And creating submucosal tunnel to correct direction and adjusting correct depth of submucosal dissection within the submucosal tunnel were more difficult than conventional gastric ESD. Another disadvantage of STB is the creation of a submucosal tunnel that leaves an extra 1-cm tunnel scar outside of the GIST. This extra linear scar makes the surgical margin of LECS larger than that of OMOB.
In conclusion, OMOB was simple and enabled us to obtain a large sample under the direct procedure view; it also allowed us to restore to the original mucosa.
A forty-seven-year-old woman was diagnosed with a gastric submucosal tumor (SMT) that was 30 mm in diameter in the fornix.
The tumor located in the fornix was considered as gastric submucosal tumor.
Gastrointestinal stromal tumor (GIST), leiomyoma, schwannoma, leiomyosarcoma, malignant lymphoma, ectopic pancreas and lipoma.
All labs were within normal limits.
Esophagogastroduodenoscopy showed gastric SMT 30 mm in diameter in the fornix .
The histopathological finding of the SMT was low risk GIST in accordance with Fletcher’s classification.
Complete surgical excision of lesion.
Several reports have been published on tissue sampling of SMTs, such as with endoscopic ultrasound sound fine needle (EUS-FNA) aspiration, submucosal tunneling bloc biopsy, and the combination of bite biopsy and endoscopic mucosal resection.
Oval mucosal opening bloc biopsy by ring thread traction for submucosal tumor is new method for diagnosis of gastric SMT.
Development of oval mucosal opening bloc biopsy after incision and widening by ring thread traction for submucosal tumor (OMOB) approach was useful for simpler, more accurate method for appropriate treatment of gastric SMT.
This case report presented a new biopsy method for GIST of the stomach. The authors demonstrate clearly that “reversible hinged double doors method” is useful to obtain large tissue sample. This method may certainly be of use for tough case even if we use EUS-FNA. This manuscript is well-written in terms of language and seems to be informative to the readers.
We thank Professor Makoto Oryu for providing technical and editorial assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Braden B, Matsuda A, Sinagra E, Syam AFF, Velayos B S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Seo SW, Hong SJ, Han JP, Choi MH, Song JY, Kim HK, Lee TH, Ko BM, Cho JY, Lee JS. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49-vii55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 4. | Niimi K, Goto O, Kawakubo K, Nakai Y, Minatsuki C, Asada-Hirayama I, Mochizuki S, Ono S, Kodashima S, Yamamichi N. Endoscopic ultrasound-guided fine-needle aspiration skill acquisition of gastrointestinal submucosal tumor by trainee endoscopists: A pilot study. Endosc Ultrasound. 2016;5:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Kobara H, Mori H, Rafiq K, Fujihara S, Nishiyama N, Chiyo T, Matsunaga T, Ayaki M, Yachida T, Kato K. Analysis of the amount of tissue sample necessary for mitotic count and Ki-67 index in gastrointestinal stromal tumor sampling. Oncol Rep. 2015;33:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yokoyama T, Nakamura N, Kiyosawa K, Akamatsu T. A biopsy-negative esophageal cancer: diagnosis by combination of bite biopsy and endoscopic mucosal resection using a cap-fitted panendoscope (EMRC). Endoscopy. 2001;33:386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 387] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 10. | Kim GH, Cho YK, Kim EY, Kim HK, Cho JW, Lee TH, Moon JS; Korean EUS Study Group. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014;49:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Kobara H, Mori H, Rafiq K, Fujihara S, Nishiyama N, Ayaki M, Yachida T, Matsunaga T, Tani J, Miyoshi H. Submucosal tunneling techniques: current perspectives. Clin Exp Gastroenterol. 2014;7:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |