Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5969
Peer-review started: May 31, 2017
First decision: June 22, 2017
Revised: July 3, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: August 28, 2017
To assess the efficacy and safety of sofosbuvir and daclatasvir regimens for kidney transplantation (KT) patients with hepatitis C virus (HCV) infection.
This study enrolled a prospective cohort of consecutive Chinese KT patients with HCV infection. They were given sofosbuvir combined with daclatasvir, with or without ribavirin. They were monitored regularly during and after the treatment.
Six patients were recruited in our prospective study cohort. All patients were male and naive to direct-acting antiviral treatment. The treatment duration was 12 wk. Most patients (4/6) were infected with HCV genotype 1b. HCV RNA was undetectable at week 4 after treatment and at the end of treatment in all patients. Sustained virological response rate at 12 wk was 100% (6/6). Two patients had to accept a half dose of sofosbuvir due to serum creatinine elevation during treatment. Kidney function in the remaining patients was stable. No serious adverse events (AEs) were observed. No patient discontinued antiviral therapy due to side effects.
Sofosbuvir and daclatasvir for treatment of KT recipients with HCV infection are highly efficient and safe. Patients tolerated the medications well, and no serious AEs were observed. Larger prospective cohort studies are needed to validate these results.
Core tip: This is a prospective study to assess the efficacy and safety of sofosbuvir and daclatasvir regimens for kidney transplantation (KT) patients with hepatitis C virus (HCV) infection. This study enrolled a prospective cohort of consecutive Chinese KT patients with HCV infection. The recipients were given sofosbuvir combined with daclatasvir with or without ribavirin. Sofosbuvir and daclatasvir treatments are highly efficient and safe. Patients tolerated the regimens and no serious adverse events were observed. Larger prospective cohort studies are needed to validate these results.
- Citation: Xue Y, Zhang LX, Wang L, Li T, Qu YD, Liu F. Efficacy and safety of sofosbuvir and daclatasvir in treatment of kidney transplantation recipients with hepatitis C virus infection. World J Gastroenterol 2017; 23(32): 5969-5976
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5969.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5969
Hepatitis C virus (HCV) infection is common in kidney transplantation (KT) recipients. The worldwide prevalence of HCV infection in patients on hemodialysis is 13.5%, compared with 3% in the general population[1]. The prevalence is significantly higher in patients with KT than in the general population[2]. HCV infection in KT recipients increases the risk of graft loss, liver fibrosis, hepatocellular carcinoma, and death[3,4]. To date, data on the efficacy and safety of direct-acting antivirals (DAAs) in the treatment of KT patients with HCV infection have been limited. New-generation DAAs [e.g., NS5B inhibitor sofosbuvir (SOF) combined with NS5A inhibitor daclatasvir (DCV), with or without ribavirin (RBV)] have been shown to be highly efficient in treating HCV infection in cirrhotic and non-cirrhotic immunocompetent patients[5]. SOF revolutionized the treatment of HCV infection, leading to high rates of sustained virological response (SVR) with few side effects[6]. However, the use of SOF is restricted to patients with an estimated glomerular filtration rate (eGFR) ≥ 30 mL/min per 1.73 m2, as it has not been studied in patients with an eGFR < 30 mL/min per 1.73 m2. In other words, these limitations are not based on current clinical data. GS331007, the active metabolite of SOF, is eliminated by the kidney. Levels of SOF and GS331007 are substantially higher in patients with severe renal impairment (eGFR < 30 mL/min per 1.73 m2)[7]. Premarket animal testing has raised concerns for cardiovascular and hepatobiliary toxicity at higher levels of SOF dosing, but toxicity of the drug and metabolite levels in humans remains unknown[7]. DCV has been recommend for treatment of patients with severe renal disease, as its components are metabolized mainly by the liver. Currently, few data on the treatment of patients post KT are available so far. The aim of this pilot study was to assess the efficacy and safety of SOF combined with DCV for HCV RNA-positive KT patients.
This study enrolled a prospective cohort of consecutive Chinese KT patients with HCV infection from March to September 2016. They were given SOF combined with DCV, with or without RBV therapy at the Department of Infectious Diseases and Hepatology, the Second Hospital of Shandong University, Jinan, China. Written informed consent was obtained from all patients, and the study protocol was approved by the Ethical Committee of the Second Hospital of Shandong University. All patients were non-cirrhotic [diagnosed by either ultrasonography, CT or determination of liver stiffness (FibroScan; cut-off for cirrhosis: 12.5 kPa)]. They were all naive to treatment, and their baseline eGFR was above 30 mL/min per 1.73 m2. All patients received therapy for 12 wk. Patients with coexisting hepatitis B virus infection, human immunodeficiency virus infection, alcoholism, autoimmune hepatitis, or malignancy were excluded. Clinical assessment, conventional liver and kidney biochemistry parameters, serum HCV RNA, as well as the types of immunosuppressive drugs and their doses were assessed routinely as follows: at the beginning of treatment; 2, 4 and 12 wk post treatment; at the end of treatment (EOT); and at 12 wk after therapy was completed. Prothrombin time, alpha-fetoprotein and abdominal ultrasonography were tested when necessary.
Biochemical response was identified as normalization of transaminases. Virological response was identified as rapid virological response (RVR, negative HCV RNA at 4 wk on treatment) and SVR (SVR12, negative HCV RNA at 12 wk after EOT).
Adverse events (AEs) were surveilled during the treatment period.
Liver and kidney biochemical parameters were tested with a Beckman UniCel DXC 800 Chemistry Analyzer (Beckman Coulter, Fullerton, CA, United States). HCV RNA was detected by quantitative real-time PCR assay using the Cobas Taqman HCV test v 2-(LLOQ 15 IU/mL). Serum anti-HCV antibodies were measured by enzyme-linked immunosorbent assay with a diagnostic kit for HCV (Zhuhai Livzon Diagnostics Inc, China). HCV genotypes were determined by direct sequencing of amplicons of the HCV gene using PCR. The eGFR was calculated based on the serum creatinine measurement prior to the initiation of treatment using the Chronic Kidney Disease Epidemiology Collaboration formula[8].
Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, United States). HCV RNA levels were logarithmically transformed for further analysis. Continuous variables are expressed as either mean ± SD or as median and range. Frequencies were used for categorical variables. Quantitative variables were compared using the t test or the Mann-Whitney U test for variables according to different characteristics of distribution when necessary. Categorical data were compared using the Pearson χ2 test or Fisher’s exact test when necessary. P < 0.05 (two-tailed) was considered statistically significant.
Our study cohort included a total of six Chinese KT recipients with HCV infection. One of them (1/6) had received two kidney transplants. All six patients were male and their mean age was 45.3 (40-49) years. None had cirrhosis. They were infected with HCV genotype 1 (4/6 GT1b), genotype 3 (1/6 GT3a) and genotype 6 (1/6 GT6a). Viral load was measured with a range between 0.514 and 29.5 million IU/mL. eGFR was > 30 mL/min per 1.73 m2 at the beginning of treatment. They were all naive to treatment, and all received 12 wk of therapy. Treatment started at 400 mg of SOF and 60 mg of daclatasvir daily in all patients. Dosage of SOF was adjusted to 200 mg daily in two patients due to elevated serum creatinine levels, one on day 2 of treatment, and the other on day 15. One of the patients received RBV (weight-based) in addition to 400 mg of SOF and 60 mg of daclatasvir. The others were treated without RBV. All patients suffered from hypertension. Their antihypertensive medications were switched from calcium channel blockers to angiotensin receptor antagonists or angiotensin converting enzyme inhibitors. The baseline clinical characteristics of the six patients are summarized in Table 1.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 |
| Age/gender | 47/M | 40/M | 40/M | 48/M | 48/M | 49/M |
| Pre-treatment serum HCV viral load (IU/mL) | 3.2E+6 | 8.6E+5 | 1.4E+7 | 5.14E+5 | 2.95E+7 | 8.08E+5 |
| HCV genotype | 1b | 1b | 3a | 1b | 6a | 1b |
| Anti-HCV | Negative | Positive | Positive | Positive | Negative | Positive |
| Cirrhosis | No | No | No | No | No | No |
| Number of kidney transplantations | Two | One | One | One | One | One |
| Prior antiviral therapy | No | No | No | No | No | No |
| Baseline serum Cr (μmol/L) (n = 53-115) | 75.5 | 84.2 | 146.6 | 175.6 | 128 | 89.3 |
| Baseline eGFR (mL/min) | 63.63 | 80.04 | 42.86 | 30.94 | 48.29 | 61.85 |
| Baseline Hgb (g/dL) | 113 | 136 | 121 | 111 | 167 | 137 |
| Baseline ALT (IU/L) | 68 | 67 | 26 | 13 | 26 | 44 |
| Baseline AST (IU/L) | 161 | 143 | 42 | 27 | 42 | 54 |
| Baseline γ-GT (IU/L) | 162 | 621 | 55 | 25 | 60 | 54 |
| Baseline TB (μmol/L) | 30 | 29.2 | 9.6 | 8 | 18.7 | 12.5 |
| Baseline (Hb g/L) | 113 | 136 | 121 | 94 | 167 | 137 |
| Complication | Hypertension | Hypertension | Hypertension | Hypertension | Hypertension/Diabetes | Hypertension |
| Antiviral regimen | Sofosbuvir 400 mg daily + daclatasvir 6 0mg daily | Sofosbuvir 400 mg daily + daclatasvir 60 mg daily | Sofosbuvir 400 mg daily + daclatasvir 60 mg daily + ribavirin 0.6 g daily | Sofosbuvir 400 mg daily + daclatasvir 60 mg daily | Sofosbuvir 400 mg daily + daclatasvir 60 mg daily | Sofosbuvir 400 mg daily + daclatasvir 60 mg daily |
| Treatment duration (wk) | 12 | 12 | 12 | 12 | 12 | 12 |
| Baseline immunosuppressive regimen | Mycophenolatemofetil 500 mg bid | Mycophenolate mofetil 540 mg bid | Mycophenolate mofetil 540 mg bid | Mycophenolate mofetil 750 mg bid | Cyclosporin A 75 mg bid | Mycophenolate mofetil 720 mg bid |
| Tacrolimus (FK506) 0.5 mg bid | Tacrolimus (FK506)1.5 mg bid | Tacrolimus (FK506)2 mg bid | Tacrolimus 2 mg bid | Mycophenolate mofetil 540 mg bid | Tacrolimus (FK506) 0.5 mg bid | |
| Methylprednisolone 4 mg qd | Prednisone 5 mg qd | Prednisone 5mg qd | Prednisone 5 mg qd | Prednisone 5 mg qd | Methylprednisolone 4 mg qd | |
| Baseline anti-hypertension regimen | Metoprolol 12.5 mg qd | Benazepril 10 mg | Benazepril 10 mg bid | Valsartan 80 mg bid | Irbesartan 150 mg bid | None |
| qd | ||||||
| Valsartan 80 mg qd | ||||||
| Other regimens | Benzbromarone tablets 12.5 mg bid | Recombinant human erythropoietin injection 10000 U, IH, biw |
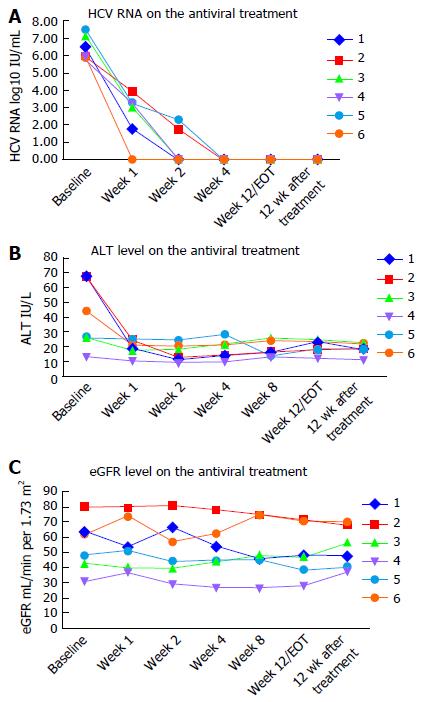
All six patients completed antiviral treatment, and were followed for at least 12 wk post treatment. There were no discontinuations of therapy and none were lost to follow-up. All six patients achieved RVR, of whom four had undetectable viral load by week 2 of treatment. All patients had undetectable HCV viral load at the end of treatment. SVR12 rate was achieved in 100% (6/6) of the recipients. Timelines of virological responses are depicted in Figure 1A.
Serum alanine aminotransferase (ALT), aspartate aminotransferase and γ-glutamyl transferase levels significantly improved with the antiviral treatment, especially during the initial two weeks. Hemoglobin levels were stable during treatment. Timelines of the ALT values are shown in Figure 1B.
No serious AEs were reported during the treatment process. Common AEs included fatigue (1/6), diarrhea (1/6), tinnitus (1/6), abdominal discomfort (1/6), discomfort of transplanted kidney region (1/6), refractory hypertension (1/6), elevation of serum creatinine (2/6), and unstable blood pressure (3/6). Patient 6 suffered from fatigue and diarrhea one week after therapy, but he continued DAA treatment, and his diarrhea gradually ameliorated. Patient 4 suffered from fatigue 40 d after therapy began, along with tinnitus, and discomfort of the abdomen and transplanted kidney regions. However, symptoms spontaneously disappeared during subsequent treatment. Antihypertensive medication was modified in patients 2, 3 and 5 to avoid drug interactions with DCV, causing unstable blood pressure during the first two weeks of treatment. No patient had renal transplant complications related to antiviral treatment, and there were no kidney rejection episodes. Antiviral therapy was not discontinued due to side effects in any patient. Patient 4 had to accept a half-dose SOF on the second day of treatment because his serum creatinine level increased from 175.6 μmol/L to 209 μmol/L, and his eGFR fell to less than 30 mL/min per 1.73 m2. His serum creatinine levels remained stable for the rest of the course. Patient 5 was hospitalized twice for elevated serum creatinine levels in the process of treatment, and the dose of SOF was reduced to 200 mg daily starting in the third week. Kidney function remained stable in the remaining patients, including patient 3, whose baseline serum creatinine level was 146.6 μmol/L. Kidney function remained stable in the remaining patients. Other AEs, such as nausea, headache and myalgia/arthralgia, were not reported. Table 2 displays all AEs reported. eGFR values are shown in Figure 1C.
| Event | Patients | |
| Any adverse event leading to discontinuation | - | 0 |
| Serious adverse events | Gastrointestinal bleeding/Portal vein thrombosis and Streptococcus bacteremia/Sinus bradycardia and first degree A-V block with syncope | 0 |
| Common adverse events | Fatigue | 2 |
| Diarrhea | 1 | |
| Tinnitus | 1 | |
| Elevation in serum creatinine | 2 | |
| Discomfort of abdomen | 1 | |
| Discomfort of transplanted kidney region | 1 | |
| Unstable blood pressure | 3 | |
| Rash | 0 | |
| Insomnia | 0 | |
| Headache | 0 |
All six patients were on immunosuppressive agents. Five patients received tacrolimus and mycophenolate mofetil. The remaining patient received cyclosporine and mycophenolate mofetil (Table 3). Other agents included prednisone or methylprednisolone, and azathioprine. During antiviral treatment, blood concentrations of tacrolimus, mycophenolate mofetil, and cyclosporine varied within goal trough levels according to the risk of immune rejection. Immunosuppressive agents were adjusted in three patients. The other patients’ blood concentrations of immunosuppressive agents remained stable throughout the course of antiviral therapy. All dose adjustments of immunosuppressive agents during treatment are displayed in Table 3.
| KT patient 1 | KT patient 2 | KT patient 3 | KT patient 4 | KT patient 5 | KT patient 6 | |
| Baseline immunosuppressive regimen | Prednisone 5 mg qd | Prednisone 5 mg qd | Prednisone 5 mg qd | Methylprednisolone 4 mg qd | Prednisone 5 mg qd | Methylprednisolone 4 mg qd |
| Mycophenolate mofetil 1.5 g bid | Mycophenolate mofetil 540 mg bid | Mycophenolate mofetil 540 mg bid | Mycophenolate mofetil 750 mg bid | Mycophenolate mofetil 540 mg bid | Mycophenolate mofetil 720 mg bid | |
| Tacrolimus (FK506) 0.5 mg bid | Tacrolimus (FK506) 1.5 mg bid | Tacrolimus (FK506) 2 mg bid | Tacrolimus (FK506) 2 mg bid | Cyclosporine 75 mg bid | Tacrolimus (FK506) 0.5 mg bid | |
| 1st adjustment | FK506 0.5 mg qd + 1 mg qn (3 wk after the treatment for his blood drug concentration of FK506 was 3.7 ng/mL). | No | No | Mycophenolate mofetil 1000 mg bid | Cyclosporine 75 mg qd + 50 mg qn (5 d after the treatment for his blood drug concentration of cyclosporine rose to 277.9 ng/mL). | No |
| FK506 3 mg bid (11 wk after the treatment for his blood drug concentration of FK506 was 5.4 ng/mL). | ||||||
| 2nd adjustment | FK506 4 mg bid (8 wk after the treatment for his blood drug concentration of FK506 was 6.4 ng/mL). | No | No | No | Mycophenolate mofetil 720 mg bid (45 d after the treatment was begun). | No |
| 3rd adjustment | Mycophenolate mofetil 1000 mg bid (one month after the treatment) | No | No | No | No | No |
With a global prevalence rate of approximately 3%, affecting over 170 million individuals, chronic HCV infection is a leading cause of chronic liver disease and hepatocellular carcinoma worldwide[9]. Importantly, for patients with KT, HCV infection is associated with an increased rate of liver fibrosis, graft loss, hepatocellular carcinoma and death[10-12]. To date, treatment recommendations for patients with HCV infection of American Association for the Study of Liver Diseases and European Association for Study of Liver have been modified four times. With these recommendations, new IFN-free DAA therapy may prove to be the treatment of choice.
SOF is an inhibitor of the NS5B polymerase. As a nucleotide analogue, it causes chain termination during the replication of viral genomic RNA. SOF has a pan-genotypic activity and a high resistance barrier. It may only be given to patients with a glomerular filtration rate above 30 mL/min per 1.73 m2 due to its renal elimination.
Daclatasvir is an NS5A inhibitor that has high antiviral activity against genotypes 1 to 4 both in vivo and in vitro, and is also active against genotypes 5 and 6. DCV does not require renal dose adjustment and thus provides a promising option for the “difficult-to-treat” cohort.
The combination of SOF + DCV ± RBV has been investigated in treatment-naive patients with genotype 1, 2 and 3 without cirrhosis in several clinical studies. The results showed high SVR rates between 93%-100% regardless of treatment duration and addition of RBV. The ALLY-1 study investigated SOF + DCV + RBV for 12 wk in patients with cirrhosis (n = 60). For genotype 1, patients with cirrhosis achieved an SVR rate of 82%[13]. Initially, only a 24-wk treatment was evaluated in genotypes 2 and 3 patients. The SVR rates were 92% in genotype 2 and 89% in genotype 3[14]. ALLY-3 study genotype 3 patients were treated with SOF + DCV for 12 wk without RBV. Naive patients without cirrhosis achieved a high SVR rate of 97%, while in case of cirrhosis, the SVR rate was lower (only 58%)[15]. However, the efficacy and safety of DAAs, especially the combination of SOF + DCV ± RBV, in patients post KT are rarely reported[16].
In our study, all KT patients with HCV infection who received SOF and DCV regiments achieved RVR, EOT virological response (HCV RNA were undetectable at the EOT) and SVR12. Liver biochemistry parameters ameliorated significantly during antiviral treatment. The RVR rate and SVR12 rate of this group differed from those in several similar studies. For KT patients with HCV infection, Lin et al[17] reported an overall SVR12 rate of 91% (21/23). Patients in their study were given SOF plus simeprevir, with or without RBV, SOF plus ledipasvir, with or without RBV, or SOF plus RBV. Only two patients who relapsed post treatment had traditionally unfavorable treatment profiles. The two patients were African-American, had genotype 1a infection, high pre-treatment HCV viral load, and underlying advanced liver disease/cirrhosis, were previously treatment-experienced with interferon and RBV, and did not achieve RVR[17]. Kamar et al[18] reported an RVR rate of 88% (22/25) in their study, and their SVR12 rate was 100% (25/25). The three patients who did not achieve RVA had an METAVIR fibrosis score of F2. Two of the three patients were infected with HCV genotype 1b and were given SOF + simeprevir + RBV or SOF + ledipasvir without RBV. The third patient was infected with genotype 4 and treated with SOF + ledipasvir. The combination of SOF + DCV ± RBV in our study showed higher RVR and SVR12 rates than other studies. Possible reasons for this include: (1) our patients are all Asian; (2) they were all given SOF combined with DCV with or without RBV; (3) all patients were non-cirrhotic; (4) they were all naive to treatment; (5) they were younger than those in other studies; and (6) they were all compliant.
To date, data regarding efficacy and safety of DAAs in the treatment of KT patients with HCV infection have been limited. Nazario et al[19] reported few AEs with full dose SOF and simeprevir in patients with end-stage renal diseases, although only 11/17 patients completed the 12-wk post-treatment follow-up. Beinhardt et al[20] reported some serious AEs, such as photosensitivity/sunburn, spontaneous bacterial peritonitis, hemolytic anemia, re-listing for transplantation due to graft failure, as well as common AEs, such as headache and myalgia/arthralgia. We did not observe these in our study.
The majority of patients in our study tolerated SOF and DCV regimens well. Some of the more serious AEs may be related to the disease itself. Hemoglobin levels were stable during treatment, consistent with the results of other reports[18]. Notably, 67% (4/6) of our patients tolerated full-dose SOF well. Only two patients, whose baseline serum creatinine levels were higher than normal, received half-dose SOF. Three patients suffered from unstable blood pressure during the first two weeks, as reported in another study[20]. Common AEs including fatigue, diarrhea, tinnitus, discomfort of abdomen and transplanted kidney region, refractory hypertension, and elevation in serum creatinine levels were reported during treatment, just as reported in another study[20].
Dabbous et al[21] reported one recipient death one week following treatment inception, due to unresolved hepatic encephalopathy. Some AEs, such as gastrointestinal bleeding, portal vein thrombosis and Streptococcus bacteremia, sinus bradycardia and first degree A-V block with syncope, shortness of breath, gout flair, headache, dizziness, pain in the lower extremity, photosensitivity, rash, and insomnia, were reported by Lin et al[17].
Anti-HCV assay by enzyme immunoassay (EIA) technique is the most common screening tool for HCV infection due to its simplicity, availability and low cost. The second generation EIA (EIA-2) assay was frequently associated with false negative results in patients with end stage renal diseases on dialysis, with a reported rate of 2.6%-7%[15,20]. In our cohort, two patients were false negative for anti-HCV. Therefore, we recommend that HCV RNA should be determined in patients following KD.
The main limitation of our study is its small sample size. However, in the current clinical environment of increased need for treatment in the “difficult-to-treat” group, we believe that our study results offer proof of concept and feasibility data for future larger studies.
In conclusion, even though HCV patients with KT are considered “difficult-to-treat”, SOF plus daclatasvir is an attractive therapeutic option. The regimens appear to be safe, well-tolerated and efficacious, resulting in high rates of SVR for up to 12 wk following completion of treatment. The optimal dose of SOF should be adjusted according to the creatinine clearance rate and eGFR. With these adjustments, even patients with elevated baseline serum creatinine levels can achieve satisfactory results.
Hepatitis C virus (HCV) infection is common in kidney transplantation (KT) recipients. HCV infection in KT recipients increases the risk of graft loss, liver fibrosis, hepatocellular carcinoma and death. To date, there are limited data regarding the efficacy and safety of direct-acting antiviral regimens (DAAs) in the treatment of KT patients with HCV infection. New-generation DAAs [i.e., sofosbuvir (SOF) combined with daclatasvir (DCV), with or without ribavirin (RBV)] have been shown to be highly efficient in treating HCV infection in cirrhotic and non-cirrhotic immunocompetent patients. The study was designed to assess the efficacy and safety of SOF combined with DCV for HCV RNA-positive KT patients.
HCV patients post KT are considered “difficult-to-treat”. Outcomes of our study show that SOF plus DCV regimens appear to be safe, well-tolerated and efficacious, resulting in high rates of sustained virological response at 12 wk after treatment completion for these “difficult-to-treat” patients.
Sofosbuvir plus daclatasvir regimens are free of interferon. This study showed that the majority of patients after KT tolerated SOF and DCV regimens well. Thus, it is an attractive option to treat HCV patients after KT.
This study demonstrates a safe, well-tolerated, efficacious and attractive option to treat HCV patients after KT.
The authors have performed a good study, the study is well designed, and the results are interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Faerch K, Mohsen MM S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65:2335-2342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | EBPG (European Expert Group on Renal Transplantation); European Renal Association (ERA-EDTA); European Society for Organ Transplantation (ESOT). European Best Practice Guidelines for Renal Transplantation (part 1). Nephrol Dial Transplant. 2000;15 Suppl 7:1-85. [PubMed] [Cited in This Article: ] |
| 4. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-S99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 889] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 6. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 7. | Gilead. Sofosbuvir for treatment of chronic hepatitis C infection. Antiviral drugs advisory committee meeting briefing document. 2013 Oct 25. Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/antiviraldrugsadvisorycommittee/ucm371877.pdf. [Cited in This Article: ] |
| 8. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] [Cited in This Article: ] |
| 9. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 10. | Fabrizi F, Lunghi G, Ganeshan SV, Martin P, Messa P. Hepatitis C virus infection and the dialysis patient. Semin Dial. 2007;20:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end-stage renal disease. J Gastroenterol Hepatol. 2011;26:228-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Okoh EJ, Bucci JR, Simon JF, Harrison SA. HCV in patients with end-stage renal disease. Am J Gastroenterol. 2008;103:2123-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 14. | Sulkowski MS, Jacobson IM, Nelson DR. Daclatasvir plus sofosbuvir for HCV infection. N Engl J Med. 2014;370:1560-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 500] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 16. | Delabaudière C, Lavayssière L, Dörr G, Muscari F, Danjoux M, Sallusto F, Peron JM, Bureau C, Rostaing L, Izopet J. Successful treatment of fibrosing cholestatic hepatitis with pegylated interferon, ribavirin and sofosbuvir after a combined kidney-liver transplantation. Transpl Int. 2015;28:255-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Lin MV, Sise ME, Pavlakis M, Amundsen BM, Chute D, Rutherford AE, Chung RT, Curry MP, Hanifi JM, Gabardi S. Efficacy and Safety of Direct Acting Antivirals in Kidney Transplant Recipients with Chronic Hepatitis C Virus Infection. PLoS One. 2016;11:e0158431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, Del Bello A, Métivier S, Barange K. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Am J Transplant. 2016;16:1474-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int. 2016;36:798-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Beinhardt S, Al Zoairy R, Ferenci P, Kozbial K, Freissmuth C, Stern R, Stättermayer AF, Stauber R, Strasser M, Zoller H. DAA-based antiviral treatment of patients with chronic hepatitis C in the pre- and postkidney transplantation setting. Transpl Int. 2016;29:999-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Dabbous HM, Montasser IF, Sakr MA, Refai R, Sayam M, Abdelmonem A, Sayed H, F Abdelghafar M, Bahaa M, S Elmeteini M. Safety, Efficacy, and Tolerability of Sofosbuvir and Ribavirin in Management of Recurrent Hepatitis C Virus Genotype 4 After Living Donor Liver Transplant in Egypt: What Have We Learned so far? Hepat Mon. 2016;16:e35339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |