Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9394
Peer-review started: June 30, 2016
First decision: July 29, 2016
Revised: August 24, 2016
Accepted: September 12, 2016
Article in press: September 12, 2016
Published online: November 14, 2016
To evaluate the morphology of the colon in patients with irritable bowel syndrome (IBS) by using computed tomography colonography (CTC).
Twelve patients with diarrhea type IBS (IBS-D), 13 patients with constipation type IBS (IBS-C), 12 patients with functional constipation (FC) and 14 control patients underwent colonoscopy following CTC. The lengths of the rectosigmoid colon, transverse colon and the total colon were measured. The diameters of the rectum, sigmoid colon, descending colon, transverse colon, and ascending colon were measured.
The mean length of the total colon was 156.5 cm in group C, 158.9 cm in group IBS-D, 172.0 cm in group IBS-C, and 188.8 cm in group FC. The total colon in group FC was significantly longer than that in group C (P < 0.05). The mean length of the rectosigmoid colon was 56.2 cm, 55.9 cm, 63.6cm, and 77.4 cm (NS). The mean length of the transverse colon was 49.9 cm, 43.1 cm, 57.0 cm, and 55.0 cm. The transverse colon in group IBS-D was significantly shorter than that in group IBS-C (P < 0.01) and that in group FC (P = 0.02). The mean diameter of the sigmoid colon was 4.0 cm, 3.3 cm, 4.2 cm, and 4.3 cm (NS). The mean diameter of the descending colon was 3.6 cm, 3.1 cm, 3.8 cm, and 4.3 cm. The descending colon diameter in group IBS-D was significantly less than that in group IBS-C (P = 0.03) and that in group FC (P < 0.001). The descending colon diameter in group FC was significantly greater than that in group C (P = 0.04). The mean diameter of the transverse colon was 4.4 cm, 3.3 cm, 4.2 cm, and 5.0 cm (NS).
CT colonography might contribute the clarification of subtypes of IBS.
Core tip: We report the morphology difference between diarrhea type IBS (IBS-D) and constipation type IBS (IBS-C). 12 patients with IBS-D, 13 patients with IBS-C, 12 patients with functional constipation (FC) and 14 control patients underwent colonoscopy following computed tomography colonography (CTC). The lengths and the diameters of the colon were measured. The rectosigmoid colon and transverse colon in IBS-D are shorter than that in IBS-C and FC. The sigmoid colon and descending colon in IBS-D has a diameter smaller than that in IBS-C and FC. The colonic morphology in IBS-D might be different from that in IBS-C and FC. CTC might contribute the clarification of IBS.
- Citation: Ohgo H, Imaeda H, Yamaoka M, Yoneno K, Hosoe N, Mizukami T, Nakamoto H. Irritable bowel syndrome evaluation using computed tomography colonography. World J Gastroenterol 2016; 22(42): 9394-9399
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9394.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9394
Irritable bowel syndrome (IBS), one of the most common gastrointestinal (GI) disorders in the world, is characterized by abdominal pain, discomfort, bloating, and disturbed defecation[1,2]. It has four types: IBS with diarrhea (IBS-D), IBS with pain or discomfort and predominant constipation (IBS-C), mixed IBS (IBS-M), and unsubtyped IBS (IBS-U). IBS is very common, with a prevalence of 10%-20% in the world[3]. In Japan, there are approximately 6%-17% of patients with IBS[4].
Because IBS lacks characteristic imaging features and has no diagnostic biomarkers, symptom-based criteria (Rome III) are recommended for its diagnosis[1,2]. IBS is a prototypic functional GI disorder generally accompanied by visceral hypersensitivity, increased gut reactivity, and altered central processing in response to various stressors[5,6]. Its features are affected by psychosocial stress, infection, gut microbiota and by the patient’s genetics, gender, age, society, culture and perspective[7-11].
Computed tomographic colonography (CTC), similar to colonoscopy but less invasive, is an examination procedure developed for detecting colonic adenomatous lesions[12-14]. Both colonoscopy and CTC examine the full length of the colon, and their use in screening would be expected to result in colorectal cancer (CRC)-related mortality rates lower than those obtained using sigmoidoscopy or stool-based tests. For CRC and large precursor adenomas (≥ 10 mm), the sensitivity of CTC is comparable with that of colonoscopy. Not only colonic adenomatous lesions but also colonic morphology is able to be examined by using CTC. However, there is a paucity of information regarding the appropriate use of abdominal imaging in patients with IBS, and few studies have investigated typical diagnostic yields in them[15]. Moreover, the diagnostic threshold of each IBS subtype has not been reported before.
The aim of this study was to retrospectively evaluate the GI tract morphology revealed using CTC in patients with IBS.
Twelve patients with IBS-D (group IBS-D), 13 patients with IBS-C (group IBS-C), 13 patients with functional constipation (group FC), and 14 patients with colonic polyps and without abnormal defecation (control group C) were enrolled in this study at Saitama Medical University from May 2012 to February 2016. Patients underwent CTC soon after colonoscopy.
Preexamination preparation included a clear-liquid diet the day prior to examination and 75 mg of Laxoberon after dinner of the day prior to examination. In addition to fasting (12 h) before colonoscopy, patients underwent a standardized bowel preparation protocol using 244 g of Moviprep or 137 g of Niflec (EA Paharma Co., Japan). During the examination the patients first underwent colonoscopy with CO2 insufflation (PCFQ260, PCFQ260AZ, or PCFH290, Olympus Medical Science Corp., Japan) using intramuscular injection of 20 mg butylscopolamine or 1 mg of glucagon. Colonoscopy was performed by three endoscopists, and as much intracolonic fluid as possible was suctioned during withdrawal of the colonoscope. Next, soon after colonoscopy, patients underwent CTC. The CTC examination entailed insertion of a small flexible rectal catheter with colonic distension produced by an automated CO 2 insufflator (20 mmHg, PROTOCO2L, Eidia Co., Japan) immediately before the scan. Single-breath-hold multidetector supine and prone CT images were obtained using a 128-channel scanner (Siemens SOMATOM Definition Flash; Siemens Healthineers, Japan). Each image was acquired using 0.75-mm slice collimation, 1-mm reconstruction slice thickness and reconstruction increment, 120 kVp and 80 mAs after the subject had received an intramuscular injection of 20 mg butylscopolamine.
The lengths of the entire colon, rectosigmoid colon and transverse colon were measured, as the diameters of the rectum, sigmoid colon, descending colon, transverse colon and ascending colon were measured.
The study protocol was in accordance with the tenets of the revised Declaration of Helsinki (1989) and was approved by the institutional review board at our institutions. Written informed consent was obtained from all the patients.
The statistical significance of length and diameter differences was evaluated, by analysis of variance with Scheffe’s method of multiple comparison, using SPSS software, version 17 (SPSS Inc., Chicago, IL). All probability values calculated in this analysis were one-sided sided, and P < 0.05 was considered significant.
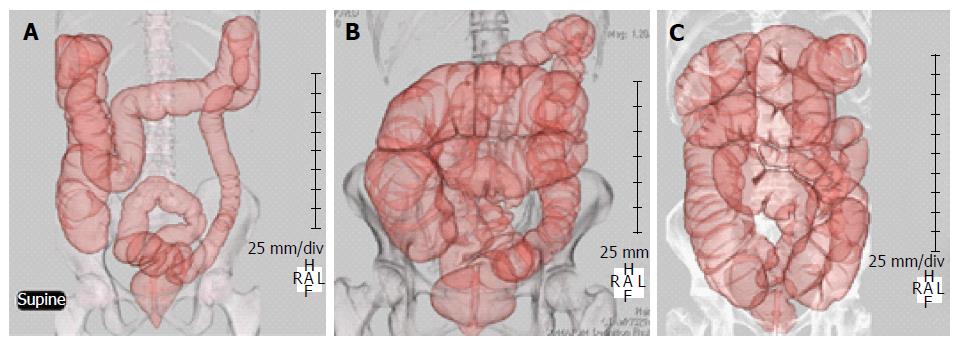
Six male and 8 female patients were enrolled in group C, 10 male and 2 female patients were enrolled in group IBS-D, 6 male and 7 female patients were enrolled in group IBS-C, and 7 male and 5 female patients were enrolled in group FC. The mean age was 64 in group C, 60 in group IBS-D, 61 in group IBS-C and 70 in group FC (Table 1). Examples of CTC findings in IBS-D, IBS-C, and FC patients are shown in Figure 1.
| Group C | Group IBS-D | Group IBS-C | Group FC | |
| Male/female | 6/8 | 10/2 | 6/7 | 7/5 |
| Mean age | 64 | 60 | 61 | 70 |
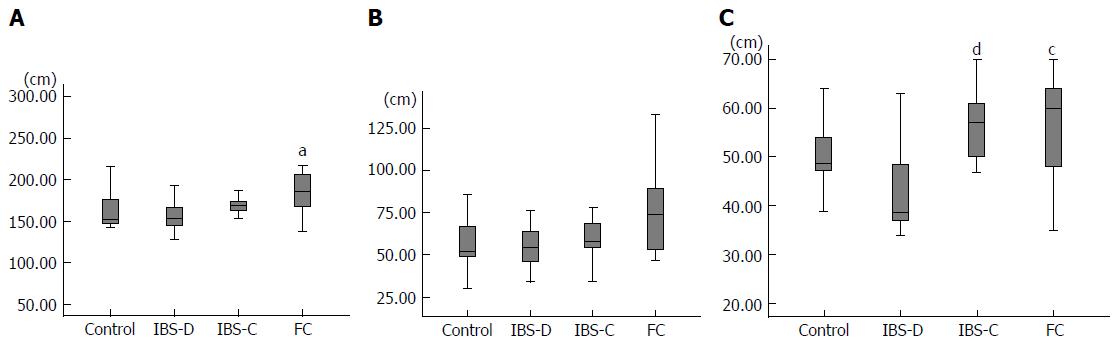
The mean length of the total colon was 156.5 cm in group C, 158.9 cm in group IBS-D, 172.0 cm in group IBS-C, and 188.8cm in group FC (Figure 2A). The total colon in group FC was significantly longer than that in group C (P < 0.05), and the total colon in group FC tended to be longer than that in group IBS-D (P = 0.07). The mean length of the rectosigmoid colon was 56.2 cm in group C, 55.9 cm in group IBS-D, 63.6 cm in group IBS-C, and 77.4 cm in group FC (Figure 2B). The rectosigmoid colon length in group FC tended to be greater than that in group C (P = 0.08) and that in group IBS-D (P = 0.07). The mean length of the transverse colon was 49.9 cm in group C, 43.1 cm in group IBS-D, 57.0 cm in group IBS-C, and 55.0 cm in group FC (Figure 2C). The transverse colon in group IBS-D was significantly shorter than that in group IBS-C (P < 0.01) and that in group FC (P = 0.02).
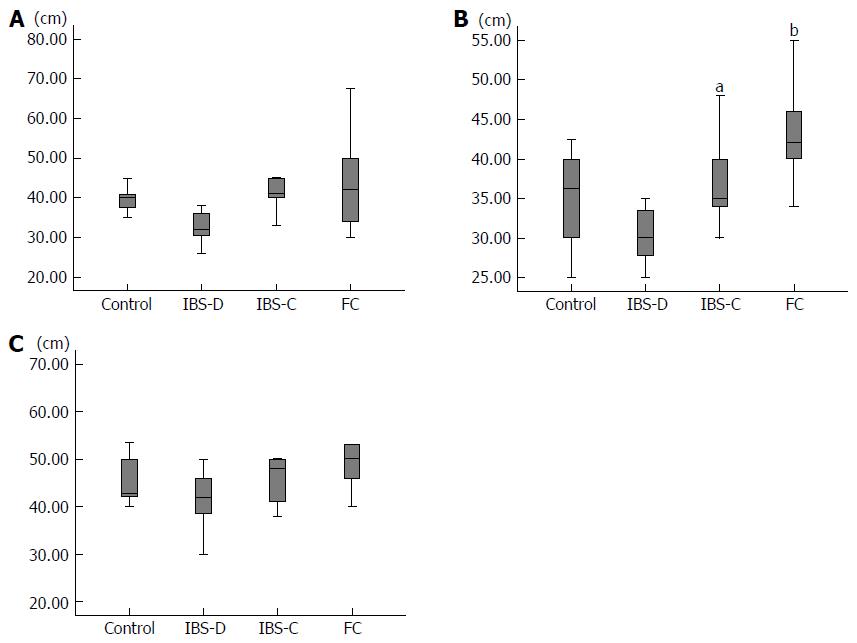
The mean diameter of the rectum was 6.0 cm in group C, 5.4 cm in group IBS-D, 5.8 cm in group IBS-C, and 6.0 cm in group FC. There was no significant difference between the rectum diameters in any two of these groups. The mean diameter of the sigmoid colon was 4.0 cm in group C, 3.3 cm in group IBS-D, 4.2 cm in group IBS-C, and 4.3 cm in group FC (Figure 3A). The sigmoid colon diameter in group IBS-D tended to be less than that in group IBS-C (P = 0.13) and that in group FC (P = 0.07). The mean diameter of the descending colon was 3.6 cm in group C, 3.1 cm in group IBS-D, 3.8cm in group IBS-C, and 4.3 cm in group FC (Figure 3B). The descending colon diameter in group IBS-D was significantly less than that in group IBS-C (P = 0.03) and that in group FC (P < 0.001). The descending colon diameter in group FC was significantly greater than that in group C (P = 0.04). The mean diameter of the transverse colon was 4.4 cm in group C, 3.3 cm in group IBS-D, 4.2 cm in group IBS-C, and 5.0 cm in group FC (Figure 3C). The transverse colon diameter in group IBS-D tended to be less than that in group FC (P = 0.08). The mean diameter of the ascending colon was 5.4 cm in group C, 5.8 cm in group IBS-D, 5.7 cm in group IBS-C, and 6.0 cm in group FC. None of these diameters differed significantly from any of the others.
IBS is a disease based on symptoms: abdominal pain, discomfort and abnormal defecation. IBS is not associated with serious GI diseases such as inflammatory bowel disease, infectious enterocolitis, diverticulitis, and colonic cancer. Ba enema or CTC shows no remarkable findings. Therefore, The Rome criteria were developed as a method to diagnose functional digestive disorders including IBS of FC without the need to subject the patients to invasive, expensive tests or procedures[1]. The diagnosis of IBS is subtyped by the predominant stool pattern: IBS-D, IBS-C, IBS-M, or IBS-U. If a patient meets criteria for IBS-C or D or FC, they should not require colonoscopy or CTC to evaluate the colonic morphology or mucosa. However, several factors are related to IBS - such as psychosocial stress, infection, gut microbiota[7-11] but it is not certain that the causes of each subtype are the same and that each subtype is the same disease. Few studies have investigated typical diagnostic yields using abdominal imagings in IBS patients[15]. In this study the morphology of the colon was examined in IBS-D patients and IBS-C patients, FC patients and control patients. The transverse colon in IBS-D patients was significantly shorter than that in IBS-C patients and FC patients, and the length of the rectosigmoid colon in IBS-D patients tended to be less than that of the rectosigmoid colon in IBS-C patients and FC patients. The diameter of the descending colon in IBS-D patients was significantly smaller than that of the descending colon in IBS-C and FC patients. The diameter of the sigmoid colon in IBS-D patients tended to be smaller than that of the descending colon in IBS-C and FC patients. According to these data, the rectosigmoid colon and transverse colon in IBS-D patients are shorter than that in IBS-C patients and FC patients and the sigmoid colon and descending colon in IBS-D patients has a diameter smaller than that in IBS-C patients and FC patients. The colon morphology in IBS-D might be different from that in IBS-C and FC. On the other hand, neither the length nor diameter of the colon in IBS-C patients differs significantly from that of the colon in FC patients. Therefore, it is supposed that IBS-C and FC are both characterized by a longer and thicker colon.
Many IBS patients have a lowered threshold to pain or discomfort during the rectosigmoid distention[16]. IBS patients experience as painful, rectal sensations that healthy people would regard as nonpainful: this is thought to be part of their hypersensitivity to bodily sensations. A lower tolerance of rectal distension would be expected to be associated with numerous bodily symptoms and more general psychological distress. In our data, IBS-D patients had smaller diameter in the sigmoid colon and descending colon and had a shorter rectosigmoid colon, which might mean they had a lower threshold for pain or discomfort occurring with rectosigmoid distention. However, IBS-C patients also have a lowered threshold for pain or discomfort occurring during rectosigmoid distention, and in this respect they are different from FC patients. The threshold for pain or discomfort during rectosigmoid distention in IBS-D patients might be lower than that in IBS-C patients, but data needed for comparing between IBS-D and IBS-C with regard to the threshold for pain or discomfort during rectosigmoid distention has not been reported yet.
The study by Heredia et al[17] showed that elongation of colon longitudinal muscle results in slow colonic transit in mice and presents a new mechanism of association of elongated colon and poor motility. Colonic elongation was reported as a possible underlying cause for slow colonic transit as observed with experimental stretching of the colon. Their study showed that elongation of the longitudinal muscle triggers inhibition of the colonic migrating motor complex (CMMC), resulting in slow colonic transit. Heredia et al[18] also reported in animal study that partial outlet obstruction caused an elongated impacted large bowel, slowed transit and CMMC. In human, slow colonic transit occurs in patients with chronic constipation and is known as slow transit constipation (STC)[19]. Southwell reported that many patients with STC have an elongated transverse colon and elongated colon often occurs in patients with constipation[20]. Yik et al[21] reported that transverse colon elongation is more common whereas sigmoid colon elongation is not more common in anorectal retention and colonic elongation may be the cause or the result of the underlying slow transit.
Mizukami et al[22-24] reported that abnormal colon morphology is common in IBS patients, and it seems to cause disorders related to defecation. Bowel morphology might be a potentially influential factor on GI symptoms. In our study, the GI morphology of IBS-D is different from that in IBS-C. IBS-D and IBS-C are classified as the same disease symptomatically (abdominal pain with abnormal bowel movement), but, pathophysiologic findings in IBS-D might be different from that in IBS-C. The morphology difference between IBS-D and IBS-C might be one of several causes of IBS. On the other hand, it is also supposed to have arisen from just the results affected by IBS symptoms.
There were several limitations in this study. Two are that it was a retrospective study and the sample size was small. We need to accumulate more clinical data in a prospective study, and a multicenter trial is necessary. Also needs is a control group without colonic polyps and abnormal bowel movements. In our study, colonic morphology in IBS-D and IBS-C were evaluated. The evaluation of colonic morphology not only in IBS-D and IBS-C but also in IBS-M and IBS-U is necessary. The threshold for pain or discomfort during rectosigmoid distention will to be measured in IBS-D patients and IBS-C patients in order to clarify the difference of pathophysiology.
In conclusion, CT colonography might contribute the clarification of subtypes of IBS according to the different morphological findings.
There is a paucity of information regarding the appropriate use of abdominal imaging in patients with irritable bowel syndrome (IBS). The diagnostic threshold of each IBS subtype has not been reported before.
The gastrointestinal (GI) tract morphology revealed using computed tomographic colonography (CTC) in patients with IBS has not been reported.
The colonic morphology in diarrhea type IBS (IBS-D) might be different from that in constipation type IBS (IBS-C) and functional constipation (FC).
CTC might contribute the clarification of IBS.
The authors evaluated the GI tract morphology revealed using CTC in patients with IBS-D, IBS-C and FC. The colonic morphology in IBS-D might be different from that in IBS-C and FC. Further clinical trials in a prospective study and a multicenter trial will be necessary.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Malowitz S S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1467] [Cited by in F6Publishing: 1420] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 2. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3260] [Article Influence: 181.1] [Reference Citation Analysis (1)] |
| 3. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1292] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 4. | Miwa H. Prevalence of irritable bowel syndrome in Japan: Internet survey using Rome III criteria. Patient Prefer Adherence. 2008;2:143-147. [PubMed] [Cited in This Article: ] |
| 5. | Agrawal A, Houghton LA, Lea R, Morris J, Reilly B, Whorwell PJ. Bloating and distention in irritable bowel syndrome: the role of visceral sensation. Gastroenterology. 2008;134:1882-1889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:119-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 559] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 8. | Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41-47. [PubMed] [Cited in This Article: ] |
| 9. | Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60:817-827. [PubMed] [Cited in This Article: ] |
| 11. | Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435-1446. [PubMed] [Cited in This Article: ] |
| 12. | Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, Nikolaou K, Lottes A, Geisbüsch S, Kramer H. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 670] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 14. | de Haan MC, Pickhardt PJ, Stoker J. CT colonography: accuracy, acceptance, safety and position in organised population screening. Gut. 2015;64:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | O'Connor OJ, McSweeney SE, McWilliams S, O’Neill S, Shanahan F, Quigley EM, Maher MM. Role of radiologic imaging in irritable bowel syndrome: evidence-based review. Radiology. 2012;262:485-494. [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Guthrie E, Barlow J, Fernandes L, Ratcliffe J, Read N, Thompson DG, Tomenson B, Creed F; North of England IBS Research Group. Changes in tolerance to rectal distension correlate with changes in psychological state in patients with severe irritable bowel syndrome. Psychosom Med. 2004;66:578-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J Physiol. 2010;588:2919-2934. [PubMed] [Cited in This Article: ] |
| 18. | Heredia DJ, Grainger N, McCann CJ, Smith TK. Insights from a novel model of slow-transit constipation generated by partial outlet obstruction in the murine large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1004-G1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Hutson JM, Chase JW, Clarke MC, King SK, Sutcliffe J, Gibb S, Catto-Smith AG, Robertson VJ, Southwell BR. Slow-transit constipation in children: our experience. Pediatr Surg Int. 2009;25:403-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Southwell BR. Colon lengthening slows transit: is this the mechanism underlying redundant colon or slow transit constipation? J Physiol. 2010;588:3343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Yik YI, Cook DJ, Veysey DM, Tudball CF, Cain TM, Southwell BR, Hutson JM. How common is colonic elongation in children with slow-transit constipation or anorectal retention? J Pediatr Surg. 2012;47:1414-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sugimoto S, Mizukami T. Diagnostic and therapeutic applications of water-immersion colonoscopy. World J Gastroenterol. 2015;21:6451-6459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Mizukami T, Horiuchi Y, Mori N. Endoscopic evaluation of colonic movement after the administration of a spasmolytic drug to irritable bowel syndrome patients: application to diagnosis and therapy. J Psychosom Digest Dis. 2009;16:91-97. [Cited in This Article: ] |
| 24. | Mizukami T, Suzuki H, Hibi T. Colonoscopic examination on Irritable Bowel Syndrome (IBS) patients: Abnormal bowel motility type and abnormal bowel morphology type. J Psychosom Digest Dis. 2010;17:33-39. [Cited in This Article: ] |