Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2582
Peer-review started: October 4, 2014
First decision: October 29, 2014
Revised: November 15, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: March 7, 2015
Hepatosplanchnic circulation receives almost half of cardiac output and is essential to physiologic homeostasis. Liver cirrhosis is estimated to affect up to 1% of populations worldwide, including 1.5% to 3.3% of intensive care unit patients. Cirrhosis leads to hepatosplanchnic circulatory abnormalities and end-organ damage. Sepsis and cirrhosis result in similar circulatory changes and resultant multi-organ dysfunction. This review provides an overview of the hepatosplanchnic circulation in the healthy state and in cirrhosis, examines the signaling pathways that may play a role in the physiology of cirrhosis, discusses the physiology common to cirrhosis and sepsis, and reviews important issues in management.
Core tip: The prevalence of cirrhosis in critically ill patients is increasing worldwide. Cirrhosis leads to hepatosplanchnic circulatory abnormalities and end-organ damage, which resemble the clinical syndrome of patients with sepsis. The pathophysiology of cirrhosis can both predispose patients to, and exacerbate, sepsis. An understanding of this pathophysiology may assist critical care providers in the development and application of treatment modalities.
- Citation: Prin M, Bakker J, Wagener G. Hepatosplanchnic circulation in cirrhosis and sepsis. World J Gastroenterol 2015; 21(9): 2582-2592
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2582
The prevalence of severe liver disease among intensive care unit (ICU) patients ranges from 1.35%-3.3%[1-3] and is increasing worldwide. A study of 174 ICUs in the United Kingdom reported that the number of patients admitted to ICU with alcoholic liver disease tripled from 1995 to 2005[1]. The ICU mortality for patients with liver disease is high, ranging from 36.6% to 73.6%[4-8], and the one-year mortality for ICU survivors is as high as 68%[6].
Liver disease exacerbates coexisting diseases. Hepatic cirrhosis is associated with an increased risk for ICU-associated pneumonia, respiratory failure, and death[9,10]. In both the United Kingdom and the United States the proportion of sepsis in the setting of liver disease is increasing and patients with cirrhosis are more likely to die from sepsis[11,12]. The systemic effects of cirrhosis also increase the morbidity and mortality of surgery[13,14].
Given the high morbidity of severe liver disease in critical care, an appreciation of hepatosplanchnic physiology may help guide intensivists, particularly those who care for patients with sepsis. This review provides an overview of the hepatic and splanchnic circulatory anatomy, examines the factors that contribute to the circulatory changes of cirrhosis, reviews the pathophysiology common to cirrhosis and sepsis, and discusses clinical management in the ICU.
Cirrhosis may be the result of infectious, autoimmune, vascular, hereditary, or toxic factors. In Europe and the United States it is primarily caused by either alcohol use or infection with hepatitis C virus, while in Asia and sub-Saharan Africa the most common cause is infection with hepatitis B virus[15]. Observational studies in the United Kingdom and France showed that the most common cause of cirrhosis among patients admitted to ICUs was alcoholic hepatitis (43%-78%) followed by viral hepatitis (10%-19%)[4,6,16].
Although the precise global prevalence is unknown because compensated disease can remain undetected for many years, up to 1% of populations worldwide may have histological cirrhosis[17]. In the United States the prevalence of cirrhosis is estimated at 0.15%[18].
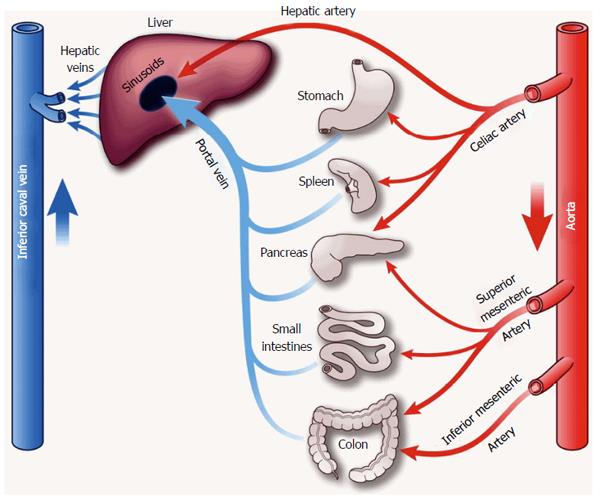
The liver receives 20% of cardiac output[19]. Total liver blood flow is approximately 100 mL/min per 100 g liver tissue, or 800-1200 mL/min[20]. The liver has a dual blood supply with blood from the hepatic artery and portal vein, which together with the bile duct form the hepatic triad. The hepatic artery is a branch of the celiac artery, with a pressure similar to aortic pressure (mean 60-80 mmHg). It carries well-oxygenated blood to the liver, providing approximately 30% of hepatic blood flow. The valveless portal vein is a low-pressure/low-resistance system that provides partially deoxygenated blood from the intestinal bed to the liver, accounting for 70% of hepatic blood supply. Normal mean portal pressures range from 5-10 mmHg[21] (Figure 1). Oxygen delivery to hepatocytes does not depend on the proportion of portal versus arterial blood flow[22]. Animal models have demonstrated that normal hepatocyte oxygen supply is approximately 16 mL/min per 100 g liver tissue with an extraction ratio of 35%[22]. Oxygen extraction changes with variations in demand; as oxygen supply decreases the extraction can approach 100%[22].
The unique interaction between the hepatic artery and portal vein flow, termed the hepatic artery buffer response (HABR)[23], is essential to maintenance of hepatic blood flow. The hepatic artery flow increases in response to decreases in portal venous flow[24,25]. This relationship is unilateral; portal vein flow does not change in response to alterations of hepatic artery flow. The HABR is capable of offsetting a 25%-60% decrease in portal vein flow[26,27].
The HABR is the primary regulator of hepatic artery flow. Flow does not change in response to metabolic activity or blood oxygen content[28] and myogenic autoregulation plays a relatively small role[29]. The physiologic purpose of HABR is unclear, as hepatic oxygen supply exceeds demand and oxygen extraction can increase in response to metabolic changes or decreased blood supply. Further, the underlying physiology remains unclear. The role of nitric oxide synthase (NOS) has been investigated but animal models have not shown a major contribution to the HABR[30]. The HABR is likely regulated by washout of adenosine, mediated through P1-purinoceptors[26,31,32].
The splanchnic vasculature, comprised of gastric, small intestinal, colonic, pancreatic, and splenic vessels arranged in parallel, receives approximately 25% of cardiac output at rest[33] and more during digestion. The major supplying arteries are the celiac, superior and inferior mesenteric. The capillary beds of this system form extensive anastamoses. Human studies of splanchnic blood flow are scarce because direct measurement of splanchnic vasculature is almost impossible without surgery. Most studies rely on indirect measurements and extrapolation from experimental models.
Splanchnic blood flow is regulated by a combination of local and systemic factors including paracrine and endocrine signaling, vasoactive substances, and sympathetic innervation. Autonomic regulation is a weak contributor, although it is enhanced in the fed state compared to the starved state[34]. In a low-flow state, splanchnic blood flow decreases in order to maintain vital cardiac and cerebral blood supply[35]. This response occurs even after small-volume hemorrhage[36]. The splanchnic organs do not produce lactate early in low-flow states because oxygenation is preserved due to high baseline supply[35]. However, recovery of splanchnic flow is protracted even after adequate volume resuscitation[37].
The hepatosplanchnic vasculature’s active response to systemic bloodflow contributes to its role as a blood volume reservoir, and its anatomic position just distal to the inferior vena cava make it a significant component of cardiovascular preload. As a capacitance vessel, it has been shown to pool 2.5% or mobilize up to 5%-6% of total blood volume in response to physiologic challenges[38-40].
Liver cirrhosis is the end-stage of chronic liver disease characterized by replacement of hepatic tissue with fibrosis and regenerative nodules (structurally abnormal areas of attempted tissue repair), and impaired liver function. The altered hepatic architecture in cirrhosis leads to circulatory abnormalities, namely portal hypertension, splanchnic vasodilation, and hyperdynamic circulation.
Portal hypertension is a pathognomic feature of liver cirrhosis, defined as an increase in the hepatic venous pressure gradient (an indirect reflection of the portocaval gradient in patients with cirrhosis) of more than 10 mmHg[41]. Portal hypertension can also be diagnosed ultrasonographically: hepatic vein pulsatility flattens from triphasic to monophasic secondary to histologic reductions in hepatic vein compliance. This is accompanied by a decrease in portal vein flow and an increase in the hepatic artery pulsatility index, due to the HABR[42]. Portal hypertension can be diagnosed clinically by the presence of esophageal varices, patency of the umbilical vein, and the presence of portocaval shunts (e.g., splenorenal shunts).
The development of portal hypertension is multifactorial. Hepatic fibrosis plays a role by disrupting hepatocyte architecture and increasing resistance to bloodflow. Hepatic sinusoidal pressure is negatively correlated with the percentage of un-fibrosed portal spaces, or “residual portal spaces”[43]. Hepatic fibrosis is largely caused by hepatic stellate cell injury. When injured by toxins (e.g., alcohol, hepatitis virus, infection, acetaminophen) or exposed to platelet activating factor (PAF), hepatic stellate cells transform into myofibroblast-like cells, releasing collagen I and III[44]. Other cell types implicated in fibrosis include myofibroblasts derived from portal vessels[45] and hematopoietic stem cells[46]. Fibrosis is also stimulated by inflammatory cytokines and vasoactive molecules, including chemotactic protein 1, transforming growth factor-β1, nitric oxide, endothelin-1 and angiotensin II[47,48]. These mediators are increased in liver disease and can further upregulate their own release, thereby accelerating an inflammatory cycle.
Circulatory system changes may also contribute to the development of portal hypertension. Direct intraoperative measurements have demonstrated that, in cirrhosis, a basal HABR is continuously activated but the acute HABR is impaired[49]. While recent data suggests that angiotensin II is a primary mediator of the progression from hepatic inflammation to fibrosis[48], the entire renin-angiotensin-aldosterone system (RAAS) may also play a role[50].
Splanchnic arteriolar vasodilation with hyperdynamic flow has been demonstrated in liver disease by observation of shortened albumin transit times through the splanchnic circulation[51], increased splenic and mesenteric bloodflow[52], and decreased measured superior mesenteric artery impedance[53]. Decreased mesenteric artery impedance begins early in liver disease and worsens with the progression to cirrhosis[53]. Splanchnic vasodilation is multifactorial and not completely understood. The pathogenesis is partly explained by increased resistance to portal outflow, but activation of other mediators including the RAAS, nitric oxide, PAF, vasopressin, and inflammatory molecules likely plays a role.
Early in liver disease, total blood volume increases but is largely sequestered in the splanchnic vascular bed, leading to “splanchnic steal” and systemic hypovolemia[54-56]. Animal models have shown that this occurs before the development of portal hypertension or splanchnic vasodilation[57]. Splanchnic steal is likely mediated by the RAAS[58], a hormone cascade which leads to volume loading through modulation of renal sodium retention. Recently an alternate RAAS pathway, angiotensin-converting-enzyme-2 (ACE-2) has also been investigated for its role in liver disease. ACE-2 levels are upregulated in cirrhosis, and expression is directly related to hepatocyte hypoxia[59,60]. The ACE-2 system acts downstream at the Mas receptor, which vasodilates splanchnic vessels. In cirrhosis, blockade of this receptor reduces portal pressure[60].
Nitric oxide (NO) is an endothelial-derived relaxing factor. Cirrhotic patients not only have increased expression of NO, but also show increased sensitivity to NO-mediated vasodilation[61]. NO causes vasodilation by stimulating soluble guanylate cyclase to generate cyclic guanosine monophosphate in vascular smooth muscle[62]. It also decreases vascular response to vasoconstrictors[63]. In animal models this vasoplegia is completely reversed by removal of the endothelium[64]. The constitutively expressed endothelial isoform of NOS has been implicated as a major contributor to splanchnic vascular overexpression of NO and its activity precedes splanchnic vasodilatation in rats[65]. Neuronal NOS is also upregulated in experimental models of cirrhosis[66,67]. In addition to nitric oxide, other vasodilators suggested to play a role in splanchnic dilation include carbon monoxide[68], plasma calcitonin gene related peptide[69], eicosanoids, bile salts, adenosine and substance P[41].
PAF is a pro-inflammatory molecule that affects platelet aggregation, vascular permeability, and vascular tone. Hepatic concentrations of PAF are increased in cirrhosis[70]. The effect of PAF on vasculature tone is regional, and exogenous PAF increases portal pressure but decreases systemic arterial blood pressure[71]. PAF is also neoangiogenic, and may play a role in the development of the arteriovenous and portocaval shunts common to cirrhosis.
Endothelin is a paracrine vasoconstrictor, released by vascular endothelial cells, which is increased in cirrhosis[72]. It stimulates hepatic sinusoidal macrophages to produce PAF[73], particularly in the setting of cirrhosis[74]. There are least four endothelin receptors (ETA, ETB1, ETB2, ETC). Endothelin-1 mediated vasoconstriction occurs through the activation of the ETA receptor, and the ETB1 receptor stimulates the release of nitric oxide[75]. Endothelin-1 also stimulates catecholamine release[76,77] which may contribute to the elevated levels seen in cirrhosis. In vitro models have shown a direct relationship between ETA and ETB expression and portal pressure[78]. It is unclear if endothelin is increased in cirrhosis as a consequence or pathogenic response to splanchnic dilation.
Vasopressin is a neurohypophyseal hormone that regulates plasma osmolality and increases vascular resistance in vasodilated states. Cirrhotic patients are vasopressin deficient, but respond to exogenous vasopressin (and its analogues terlipressin or ornipressin) with increased blood pressure[79]. It is unclear if vasopressin deficiency precedes or causes splanchnic dilation.
The hyperdynamic circulation associated with cirrhosis is characterized by increased heart rate, increased cardiac output, and systemic hypotension[80]. The etiology of hyperdynamic circulation is unclear but may include (1) desensitization of myocardial β-receptors in the setting of an activated sympathetic nervous system[81,82]; (2) splanchnic steal that decreases the effective circulating volume; (3) anemia[83]; and (4) cardiodepressants such as nitric oxide[84,85] and endogenous cannibinoids[86]. Hyperdynamic circulation is exacerbated by systemic and intrahepatic angiogenesis, which is mediated by hypoxia, PAF, vascular endothelial growth factor, and transforming growth factor[87]. Portal hypertension-mediated engorgement of collateral veins (i.e., esophageal varices, hemorrhoids, caput medusae) also increases the circulatory surface area.
Observational echocardiographic studies of cirrhotic patients have noted normal baseline cardiac contractility but attenuated stress-response, with disturbances of left diastolic function[88,89]. This dysfunction is termed cirrhotic cardiomyopathy. Cirrhotic cardiomyopathy is distinct from alcoholic cardiomyopathy which is characterized by reduced left ventricular contractility at baseline[90]. Overt heart failure is rare in cirrhotic cardiomyopathy. The splanchnic sequestration of blood volume reduces the cardiac workload and disguises the symptoms of heart failure; these can be unmasked by physical or pharmacologic stress (e.g., surgery). The pathophysiology of cirrhotic cardiomyopathy is not completely understood but endocannibinoid-receptor antagonists have improved cardiac contractility in animal models, suggesting a role for endocannibinoids in the pathogenesis of cardiac dysfunction[91].
The circulatory changes in cirrhosis lead to a clinical syndrome that resembles sepsis (Table 1). Both sepsis and cirrhosis feature a vasodilated state despite high levels of endogenous catecholamines, a compensatory hyperdynamic state with the possibility of cardiac dysfunction, and splanchnic hyperemia with systemic hypovolemia. Both cirrhotic and septic patients frequently present with signs of systemic inflammatory response syndrome including elevated heart rate, elevated respiratory rate, and blunted temperature regulation.
| Cirrhosis | Sepsis1 | |
| Prevalence in ICU population[1-3,124-126] | 1%-3% | 11%-33% |
| ICU mortality[4-8,127,128] | 37%-74% | 18%-61% |
| Clinical presentation[129] | Jaundice Enlarged collateral veins (i.e., esophageal varices, hemorrhoids, caput medusae) SIRS like presentation possible | Systemic inflammatory response syndrome (SIRS): Temp < 36 °C/> 38 °C Heart rate > 90 RR > 20 or PaCO2 < 32 WBC < 4 k or > 12 k and infection |
| Laboratory findings[129,130] | ↓ Polymorphonuclear cells Thrombocytopenia Hypoalbuminemia Increased PT, INR Hyperlactatemia Hypoglycemia | ↓ or ↑ polymorphonuclear cells Thrombocytopenia Increased PT, aPTT, INR Decreased fibrinogen |
| Bacteremia[94,131-134] | 32%-41% | 27%-31% |
| Sources of infection[6,128,135,136] | ||
| Respiratory | 9%-61% | 60%-64% |
| Abdominal | 8%-30% | 15%-19% |
| Bloodstream | 17%-72% | 13%-15% |
| Renal/urinary | 7%-11% | 11%-14% |
| Skin | 7.1% | 7% |
| Catheter-related | 4%-5% | 5% |
| Microbiology[6,128,135,136] | ||
| Gram-negative | 52.7%-64% | 49%-63% |
| Gram-positive | 30%-56% | 40%-47% |
| Fungi | 10%-25% | 10%-19% |
| Mediators associated with disease progression[47,61,71,72,105,106,137] | Endothelin, angiotensin, PAF, NO, TNF-α and HLA-DR, bacterial DNA, IL-1β, IL-2R, IL-6, IL-8 and IL-10 | Endothelin, NO, LPS, LTA, lipoproteins, sTNF, bacterial DNA, peptidoglycans, IL-6, IL-8, IL-4, IL-10 |
| Endotoxin levels[138,139] | ↑↑ | ↑↑ |
| Protein C activity[140,141] | ↓↓ | Predicts outcome |
| Mesenteric lymph nodes[95,142] | + | + |
Bacterial infections are a common complication of cirrhosis[92,93], and are the most common independent risk factor for death[94,95]. A multicenter prospective cohort study found the highest case fatality rate was from infections with Clostridium dificile (40%), respiratory infections (37.5%), and spontaneous bacteremia (37%)[94]. In a large epidemiologic survey, Foreman et al[9] noted that cirrhosis more than doubles both the risk of being hospitalized with sepsis and the risk of death from sepsis.
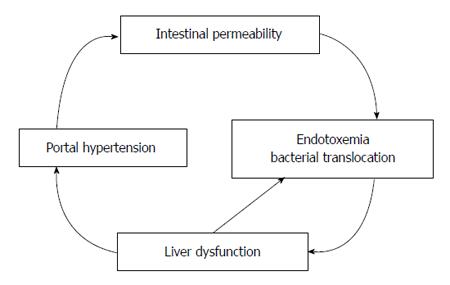
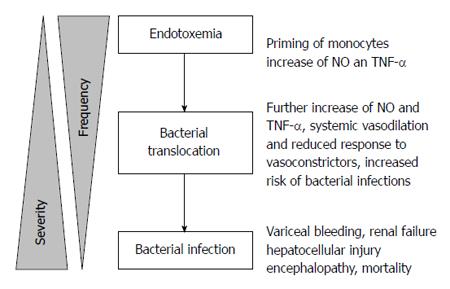
The increased risk for sepsis may be attributed to bacteremia, particularly from intestinal bacterial translocation. Bacteremia increases the risk of spontaneous bacterial peritonitis which is found in up to 15% of hospitalized cirrhotic patients[96] and 3%-3.5% of asymptomatic outpatients with cirrhosis[97,98], and it increases the risk of variceal hemorrhage[99]. Bacterial translocation occurs in the setting of splanchnic vasodilation (which increases intestinal mucosal permeability)[100], bacterial overgrowth secondary to delayed intestinal transit time[101,102] and structural damage of intestinal epithelial cells[103] (Figure 2). iNOS knockout mice are resistant to bacterial translocation, suggesting a link between NO and bacterial translocation[104] (Figure 3). Bacterial translocation also stimulates the release of inflammatory mediators, and severity of liver disease correlates with levels of these mediators, including interleukin (IL)-1β, IL-2R, IL-6, IL-8 and IL-10[105,106].
The risk for developing sepsis may also be secondary to impaired cellular immunity. In a single-center study, decreased levels of tumor necrosis factor (TNF-α) and human leukocyte antigen-DR were found in patients with acute liver failure and patients with sepsis, in comparison to patients with stable cirrhosis[106]. Changes in cellular immunity may alter the secretion of inflammatory mediators and the vulnerability to infection.
Liver disease increases the susceptibility for sepsis, but sepsis also aggravates liver disease. In animal models of sepsis, portal vein and overall hepatic flow decreases and angiotensin II has been implicated[107,108]. Up to one third of patients with late septic shock have depleted vasopressin levels[109] resulting in hypotension, vasoplegia, and catecholamine resistance. These circulatory changes may affect hepatic blood flow and function. In septic patients with no previous history of liver disease, postmortem histopathologic hepatic changes were found, including portal inflammation, centrilobular necrosis, and hepatocellular apoptosis[110]. Human studies of hepatosplanchnic flow in sepsis remain scarce and it is important to note that animal studies do not always include volume-resuscitated arms, which would increase their clinical relevance.
Patients with cirrhosis may be admitted to the ICU with decompensated disease, after surgery, or with infection and sepsis. Although the Child-Turcotte-Pugh score[111,112] has traditionally been used for risk assessment, the Model for End Stage Liver Disease (MELD) score[113] is now commonly used to assess liver disease and rank-list patients for liver transplantation. While the MELD score is an excellent tool for predicting short-term mortality amongst cirrhotic patients awaiting liver transplantation[114], data regarding its predictive power for mortality in hospitalized cirrhotic patients has been inconsistent. Teh et al[115] retrospectively demonstrated increased mortality in postoperative cirrhotic patients with MELD greater than 20, while Oberkofler et al[116] found no mortality prediction in a cohort of liver transplant recipients.
ICU scoring systems (e.g., Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score II) have demonstrated superior mortality prediction in cirrhotic patients in the ICU[117,118]. Recently, two new scores have been developed for mortality prediction: a modified SOFA score for Chronic Liver Failure (CLIF-SOFA) and the Royal Free Hospital Score[119,120]. Single biomarkers have also shown prognostic value. In developing the CLIF-SOFA score, Moreau et al[119] demonstrated that leukocyte count was independently associated with acute-on-chronic liver failure and associated 28-d mortality. Furthermore, in an effort to identify patients at risk for imminent decompensation, López-Velázquez et al[121] found that bilirubin concentration alone was an independent predictor of 7-d mortality.
Beyond scoring systems, multiorgan dysfunction in cirrhosis has been correlated with hospital mortality: a prospective study of ICU patients with cirrhosis found that coma and acute renal failure were independent predictors of mortality[8]. While organ dysfunction is reflected in scoring systems, these findings highlight the importance of assessing patients for clinical markers of dysfunction other than those included in scores. Recently a novel method of transient elastography has been used to measure liver stiffness, a metric associated with hepatic fibrosis. In a prospective study of ICU patients, liver stiffness was highest in patients with decompensated cirrhosis (compared to other critical illnesses or comorbidities), and was associated with increased ICU- and post-discharge-mortality[122]. Transient elastography may serve as a useful triage tool for critically ill patients with liver disease.
As noted, the circulatory abnormalities of cirrhosis predispose patients to multiorgan dysfunction including heart failure, renal dysfunction, and hemodynamic instability. Monitoring to predict or prevent this morbidity has not been identified, nor has the optimal treatment regimen. Notably, a prospective study of ICU patients with cirrhosis demonstrated 100% mortality for those with pulmonary artery catheters, 84% mortality for patients requiring mechanical ventilation, and 89% mortality for those requiring renal replacement therapy[8]. These mortality rates likely reflect a high severity of disease rather than adverse effects of the monitors themselves. Studies are needed to determine the most appropriate monitoring and interventions for ICU patients with cirrhosis.
Given the morbidity and mortality attributable to sepsis for cirrhotic patients in the ICU, intensivists should maintain a high index of suspicion for infection. Early prophylactic antibiotics for patients with cirrhosis may reduce the incidence of bacterial translocation, sepsis, and variceal hemorrhage[123]. Studies focused on immune system function and inflammatory mediators may clarify the pathophysiology common to cirrhosis and sepsis, and suggest novel therapeutic interventions.
The hepatosplanchnic circulatory system is the largest blood reservoir in the human body and is essential to multiple aspects of homeostasis, including nutrient absorption, endocrine function, and toxin metabolism. Pathologic splanchnic vasodilation in cirrhosis leads to hyperdynamic circulation and blunting of the HABR. These alterations contribute to systemic disease and perioperative mortality, and resemble pathophysiologic changes seen in sepsis. Cirrhosis increases the risk of developing sepsis, and sepsis may exacerbate cirrhosis. A better comprehension of circulatory changes in cirrhosis may lead to therapeutic modalities that improve intensive care management.
P- Reviewer: Carulli L, Cucchetti A, Kitade M, Reshetnyak VI S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Welch C, Harrison D, Short A, Rowan K. The increasing burden of alcoholic liver disease on United Kingdom critical care units: secondary analysis of a high quality clinical database. J Health Serv Res Policy. 2008;13 Suppl 2:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Fuchs L, Chronaki CE, Park S, Novack V, Baumfeld Y, Scott D, McLennan S, Talmor D, Celi L. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Med. 2012;38:1654-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Thomson SJ, Moran C, Cowan ML, Musa S, Beale R, Treacher D, Hamilton M, Grounds RM, Rahman TM. Outcomes of critically ill patients with cirrhosis admitted to intensive care: an important perspective from the non-transplant setting. Aliment Pharmacol Ther. 2010;32:233-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Das V, Boelle PY, Galbois A, Guidet B, Maury E, Carbonell N, Moreau R, Offenstadt G. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38:2108-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Cholongitas E, Calvaruso V, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24:1639-1647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Aggarwal A, Ong JP, Younossi ZM, Nelson DR, Hoffman-Hogg L, Arroliga AC. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest. 2001;119:1489-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Arabi Y, Ahmed QA, Haddad S, Aljumah A, Al-Shimemeri A. Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol. 2004;16:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Di Pasquale M, Esperatti M, Crisafulli E, Ferrer M, Bassi GL, Rinaudo M, Escorsell A, Fernandez J, Mas A, Blasi F. Impact of chronic liver disease in intensive care unit acquired pneumonia: a prospective study. Intensive Care Med. 2013;39:1776-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Osmon S, Warren D, Seiler SM, Shannon W, Fraser VJ, Kollef MH. The influence of infection on hospital mortality for patients requiring & gt; 48 h of intensive care. Chest. 2003;124:1021-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999;90:42-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 275] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | de Goede B, Klitsie PJ, Lange JF, Metselaar HJ, Kazemier G. Morbidity and mortality related to non-hepatic surgery in patients with liver cirrhosis: a systematic review. Best Pract Res Clin Gastroenterol. 2012;26:47-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Cowie B, MacLachlan J. The global burden of liver disease attributable to hepatitis B, hepatitis C, and alcohol: increasing mortality, differing causes. 64th Annual Meeting of the American Association for the Study of Liver Diseases. Washington, DC: AASLD 2013; . [Cited in This Article: ] |
| 16. | McPhail MJ, Shawcross DL, Abeles RD, Huei-Lee G, Abdulla M, Chang T, Willars C, Sizer E, Auzinger G, Bernal W. Changing outcomes in patients with chronic liver disease in intensive care: a decade of experience. Critical Care. 2012;16:393. [Cited in This Article: ] |
| 17. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1428] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 18. | Everhart JE; National Institutes of Health. Digestive diseases in the United States: Epidemiology and Impact. Bethesda, MD: National Institutes of Health 1994; . [Cited in This Article: ] |
| 19. | Bennett TD, Rothe CF. Hepatic capacitance responses to changes in flow and hepatic venous pressure in dogs. Am J Physiol. 1981;240:H18-H28. [PubMed] [Cited in This Article: ] |
| 20. | Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23-65. [PubMed] [Cited in This Article: ] |
| 21. | Womack NA, Peters RM. An investigation of the relationship between portal venous pressure and inferior vena caval and portal venous oxygen saturations. Ann Surg. 1957;146:691-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 33] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Lutz J, Henrich H, Bauereisen E. Oxygen supply and uptake in the liver and the intestine. Pflugers Arch. 1975;360:7-15. [PubMed] [Cited in This Article: ] |
| 23. | Role and control of the hepatic artery. In: Lautt WW, editor. Hepatic circulation in health and disease. New York: Raven Press 1981; 203-226. [Cited in This Article: ] |
| 24. | Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. 1990;98:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Jakab F, Ráth Z, Schmal F, Nagy P, Faller J. The interaction between hepatic arterial and portal venous blood flows; simultaneous measurement by transit time ultrasonic volume flowmetry. Hepatogastroenterology. 1995;42:18-21. [PubMed] [Cited in This Article: ] |
| 26. | Lautt WW, Legare DJ, d’Almeida MS. Adenosine as putative regulator of hepatic arterial flow (the buffer response). Am J Physiol. 1985;248:H331-H338. [PubMed] [Cited in This Article: ] |
| 27. | Lautt WW. Relationship between hepatic blood flow and overall metabolism: the hepatic arterial buffer response. Fed Proc. 1983;42:1662-1666. [PubMed] [Cited in This Article: ] |
| 28. | Lautt WW. Control of hepatic arterial blood flow: independence from liver metabolic activity. Am J Physiol. 1980;239:H559-H564. [PubMed] [Cited in This Article: ] |
| 29. | Torrance HB. The control of the hepatic arterial circulation. J Physiol. 1961;158:39-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Grund F, Sommerschild HT, Winecoff A, Ujhelyi MR, Tønnessen T, Kirkebøen KA, Rutlen DL, Ilebekk A. Importance of nitric oxide in hepatic arterial blood flow and total hepatic blood volume regulation in pigs. Acta Physiol Scand. 1997;161:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Browse DJ, Mathie RT, Benjamin IS, Alexander B. The role of ATP and adenosine in the control of hepatic blood flow in the rabbit liver in vivo. Comp Hepatol. 2003;2:9. [PubMed] [Cited in This Article: ] |
| 32. | Lautt WW, McQuaker JE. Maintenance of hepatic arterial blood flow during hemorrhage is mediated by adenosine. Can J Physiol Pharmacol. 1989;67:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Donald D. Splanchnic circulation. Handbook of Physiology. part 2 ed. Bethesda, MD: American Physiological Society 1983; 219-240. [Cited in This Article: ] |
| 34. | Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Jakob SM, Tenhunen JJ, Laitinen S, Heino A, Alhava E, Takala J. Effects of systemic arterial hypoperfusion on splanchnic hemodynamics and hepatic arterial buffer response in pigs. Am J Physiol Gastrointest Liver Physiol. 2001;280:G819-G827. [PubMed] [Cited in This Article: ] |
| 36. | Dalton JM, Gore DC, Makhoul RG, Fisher MR, DeMaria EJ. Decreased splanchnic perfusion measured by duplex ultrasound in humans undergoing small volume hemorrhage. Crit Care Med. 1995;23:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Edouard AR, Degrémont AC, Duranteau J, Pussard E, Berdeaux A, Samii K. Heterogeneous regional vascular responses to simulated transient hypovolemia in man. Intensive Care Med. 1994;20:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Kjekshus H, Risoe C, Scholz T, Smiseth OA. Regulation of hepatic vascular volume: contributions from active and passive mechanisms during catecholamine and sodium nitroprusside infusion. Circulation. 1997;96:4415-4423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Lautt WW. Regulatory processes interacting to maintain hepatic blood flow constancy: Vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res. 2007;37:891-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Greenway CV, Innes IR. Effects of splanchnic nerve stimulation on cardiac preload, afterload, and output in cats. Circ Res. 1980;46:181-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Zhang L, Yin J, Duan Y, Yang Y, Yuan L, Cao T. Assessment of intrahepatic blood flow by Doppler ultrasonography: relationship between the hepatic vein, portal vein, hepatic artery and portal pressure measured intraoperatively in patients with portal hypertension. BMC Gastroenterol. 2011;11:84. [PubMed] [Cited in This Article: ] |
| 43. | Picchiotti R, Mingazzini PL, Scucchi L, Bressan M, Di Stefano D, Donnetti M, Feroci L. Correlations between sinusoidal pressure and liver morphology in cirrhosis. J Hepatol. 1994;20:364-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069-11076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 45. | Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:d496-d503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 296] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 47. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3795] [Article Influence: 199.7] [Reference Citation Analysis (3)] |
| 48. | Matthew Morris E, Fletcher JA, Thyfault JP, Rector RS. The role of angiotensin II in nonalcoholic steatohepatitis. Mol Cell Endocrinol. 2013;378:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Aoki T, Imamura H, Kaneko J, Sakamoto Y, Matsuyama Y, Kokudo N, Sugawara Y, Makuuchi M. Intraoperative direct measurement of hepatic arterial buffer response in patients with or without cirrhosis. Liver Transpl. 2005;11:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol. 2010;53:273-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Kotelanski B, Groszmann R, Cohn JN. Circulation times in the splanchnic and hepatic beds in alcoholic liver disease. Gastroenterology. 1972;63:102-111. [PubMed] [Cited in This Article: ] |
| 52. | Williams R, Condon RE, Williams HS, Blendis LM, Kreel L. Splenic blood flow in cirrhosis and portal hypertension. Clin Sci. 1968;34:441-452. [PubMed] [Cited in This Article: ] |
| 53. | Piscaglia F, Gaiani S, Gramantieri L, Zironi G, Siringo S, Bolondi L. Superior mesenteric artery impedance in chronic liver diseases: relationship with disease severity and portal circulation. Am J Gastroenterol. 1998;93:1925-1930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121-S131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 374] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 55. | Kiszka-Kanowitz M, Henriksen JH, Møller S, Bendtsen F. Blood volume distribution in patients with cirrhosis: aspects of the dual-head gamma-camera technique. J Hepatol. 2001;35:605-612. [PubMed] [Cited in This Article: ] |
| 56. | Møller S, Bendtsen F, Henriksen JH. Effect of volume expansion on systemic hemodynamics and central and arterial blood volume in cirrhosis. Gastroenterology. 1995;109:1917-1925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Levy M, Wexler MJ. Renal sodium retention and ascites formation in dogs with experimental cirrhosis but without portal hypertension or increased splanchnic vascular capacity. J Lab Clin Med. 1978;91:520-536. [PubMed] [Cited in This Article: ] |
| 58. | Bernardi M, Trevisani F, Santini C, De Palma R, Gasbarrini G. Aldosterone related blood volume expansion in cirrhosis before and during the early phase of ascites formation. Gut. 1983;24:761-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, Walther T, Sauerbruch T, Burrell LM, Angus PW. Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874-884.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Helmy A, Jalan R, Newby DE, Johnston NR, Hayes PC, Webb DJ. Altered peripheral vascular responses to exogenous and endogenous endothelin-1 in patients with well-compensated cirrhosis. Hepatology. 2001;33:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Ignarro LJ, Byrns RE, Wood KS. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987;60:82-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Lee FY, Albillos A, Colombato LA, Groszmann RJ. The role of nitric oxide in the vascular hyporesponsiveness to methoxamine in portal hypertensive rats. Hepatology. 1992;16:1043-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 116] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Atucha NM, Shah V, García-Cardeña G, Sessa WE, Groszmann RJ. Role of endothelium in the abnormal response of mesenteric vessels in rats with portal hypertension and liver cirrhosis. Gastroenterology. 1996;111:1627-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Wiest R, Shah V, Sessa WC, Groszmann RJ. NO overproduction by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. Am J Physiol. 1999;276:G1043-G1051. [PubMed] [Cited in This Article: ] |
| 66. | Jurzik L, Froh M, Straub RH, Schölmerich J, Wiest R. Up-regulation of nNOS and associated increase in nitrergic vasodilation in superior mesenteric arteries in pre-hepatic portal hypertension. J Hepatol. 2005;43:258-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Xu L, Carter EP, Ohara M, Martin PY, Rogachev B, Morris K, Cadnapaphornchai M, Knotek M, Schrier RW. Neuronal nitric oxide synthase and systemic vasodilation in rats with cirrhosis. Am J Physiol Renal Physiol. 2000;279:F1110-F1115. [PubMed] [Cited in This Article: ] |
| 68. | De las Heras D, Fernández J, Ginès P, Cárdenas A, Ortega R, Navasa M, Barberá JA, Calahorra B, Guevara M, Bataller R. Increased carbon monoxide production in patients with cirrhosis with and without spontaneous bacterial peritonitis. Hepatology. 2003;38:452-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Henriksen JH, Møller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to low adrenaline and elevated circulating calcitonin gene related peptide but not to activated vasoconstrictor systems. Gut. 2001;49:112-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Yang Y, Nemoto EM, Harvey SA, Subbotin VM, Gandhi CR. Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis. Gut. 2004;53:877-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Kleber G, Braillon A, Gaudin C, Champigneulle B, Cailmail S, Lebrec D. Hemodynamic effects of endotoxin and platelet activating factor in cirrhotic rats. Gastroenterology. 1992;103:282-288. [PubMed] [Cited in This Article: ] |
| 72. | Bruno CM, Neri S, Sciacca C, Caruso L. Plasma endothelin-1 levels in liver cirrhosis. Int J Clin Lab Res. 2000;30:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Mustafa SB, Gandhi CR, Harvey SA, Olson MS. Endothelin stimulates platelet-activating factor synthesis by cultured rat Kupffer cells. Hepatology. 1995;21:545-553. [PubMed] [Cited in This Article: ] |
| 74. | Yang Y, Harvey SA, Gandhi CR. Kupffer cells are a major source of increased platelet activating factor in the CCl4-induced cirrhotic rat liver. J Hepatol. 2003;39:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Helmy A, Newby DE, Jalan R, Hayes PC, Webb DJ. Enhanced vasodilatation to endothelin antagonism in patients with compensated cirrhosis and the role of nitric oxide. Gut. 2003;52:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Yamaguchi N. Role of ET(A) and ET(B) receptors in endothelin-1-induced adrenal catecholamine secretion in vivo. Am J Physiol. 1997;272:R1290-R1297. [PubMed] [Cited in This Article: ] |
| 77. | Yamaguchi N. Implication of L-type Ca2+ channels in noncholinergic adrenal catecholamine secretion by endothelin-1 in vivo. Am J Physiol. 1995;269:R287-R293. [PubMed] [Cited in This Article: ] |
| 78. | Cahill PA, Foster C, Redmond EM, Gingalewski C, Wu Y, Sitzmann JV. Enhanced nitric oxide synthase activity in portal hypertensive rabbits. Hepatology. 1995;22:598-606. [PubMed] [Cited in This Article: ] |
| 79. | Wagener G, Kovalevskaya G, Minhaz M, Mattis F, Emond JC, Landry DW. Vasopressin deficiency and vasodilatory state in end-stage liver disease. J Cardiothorac Vasc Anesth. 2011;25:665-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 81. | Gerbes AL, Remien J, Jüngst D, Sauerbruch T, Paumgartner G. Evidence for down-regulation of beta-2-adrenoceptors in cirrhotic patients with severe ascites. Lancet. 1986;1:1409-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Lee SS, Marty J, Mantz J, Samain E, Braillon A, Lebrec D. Desensitization of myocardial beta-adrenergic receptors in cirrhotic rats. Hepatology. 1990;12:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Cirera I, Elizalde JI, Piqué JM, Feu F, Casadevall M, Goldin E, Terés J, Bosch J, Rodés J. Anemia worsens hyperdynamic circulation of patients with cirrhosis and portal hypertension. Dig Dis Sci. 1997;42:1697-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Castro A, Jiménez W, Clària J, Ros J, Martínez JM, Bosch M, Arroyo V, Piulats J, Rivera F, Rodés J. Impaired responsiveness to angiotensin II in experimental cirrhosis: role of nitric oxide. Hepatology. 1993;18:367-372. [PubMed] [Cited in This Article: ] |
| 85. | Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology. 2000;118:937-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315-323. [PubMed] [Cited in This Article: ] |
| 87. | Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 88. | Bernal V, Pascual I, Esquivias P, García-Gil A, Fernández C, Mateo JM, González M, Simón MA. Cardiac hemodynamic profiles and pro-B-type natriuretic Peptide in cirrhotic patients undergoing liver transplantation. Transplant Proc. 2009;41:985-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Poliwczak AR, Białkowska J, Broncel M, Koziróg M, Dworniak K, Kotecka K, Jabłkowski M. Heart rhythm turbulence and NT-proBNP in decompensated liver cirrhosis--a pilot study. Med Sci Monit. 2011;17:PR5-P11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | George A, Figueredo VM. Alcoholic cardiomyopathy: a review. J Card Fail. 2011;17:844-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. 2007;293:H1689-H1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41-48. [PubMed] [Cited in This Article: ] |
| 93. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 661] [Cited by in F6Publishing: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 94. | Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 95. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [PubMed] [Cited in This Article: ] |
| 96. | Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 2001;8:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 279] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Castellote J, Girbau A, Maisterra S, Charhi N, Ballester R, Xiol X. Spontaneous bacterial peritonitis and bacterascites prevalence in asymptomatic cirrhotic outpatients undergoing large-volume paracentesis. J Gastroenterol Hepatol. 2008;23:256-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 100. | Khanna A, Rossman JE, Fung HL, Caty MG. Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J Surg Res. 2001;99:114-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 101. | Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 102. | Zhang SC, Wang W, Ren WY, He BM, Zhou K, Zhu WN. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol. 2003;9:534-538. [PubMed] [Cited in This Article: ] |
| 103. | Such J, Guardiola JV, de Juan J, Casellas JA, Pascual S, Aparicio JR, Solá-Vera J, Pérez-Mateo M. Ultrastructural characteristics of distal duodenum mucosa in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2002;14:371-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Suzuki Y, Deitch EA, Mishima S, Lu Q, Xu D. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia-reperfusion injury. Crit Care Med. 2000;28:3692-3696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Goral V, Atayan Y, Kaplan A. The relation between pathogenesis of liver cirrhosis, hepatic encephalopathy and serum cytokine levels: what is the role of tumor necrosis factor α? Hepatogastroenterology. 2011;58:943-948. [PubMed] [Cited in This Article: ] |
| 106. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195-201. [PubMed] [Cited in This Article: ] |
| 107. | Dahm PL, Thörne J, Myhre E, Grins E, Mårtensson L, Blomquist S. Intestinal and hepatic perfusion and metabolism in hypodynamic endotoxic shock. Effects of nitric oxide synthase inhibition. Acta Anaesthesiol Scand. 1999;43:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Tadros T, Traber DL, Herndon DN. Trauma- and sepsis-induced hepatic ischemia and reperfusion injury: role of angiotensin II. Arch Surg. 2000;135:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31:1752-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 110. | Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, Betrosian A. Liver histology in ICU patients dying from sepsis: a clinico-pathological study. World J Gastroenterol. 2008;14:1389-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 84] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Child III C, Turcotte JG. Surgery and portal hypertension. The Liver and Portal Hypertension. Philadelphia: Saunders 1964; 50. [Cited in This Article: ] |
| 112. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5490] [Cited by in F6Publishing: 5498] [Article Influence: 107.8] [Reference Citation Analysis (1)] |
| 113. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 1911] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 114. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1825] [Cited by in F6Publishing: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 115. | Teh SH, Nagorney DM, Stevens SR, Offord KP, Therneau TM, Plevak DJ, Talwalkar JA, Kim WR, Kamath PS. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 116. | Oberkofler CE, Dutkowski P, Stocker R, Schuepbach RA, Stover JF, Clavien PA, Béchir M. Model of end stage liver disease (MELD) score greater than 23 predicts length of stay in the ICU but not mortality in liver transplant recipients. Crit Care. 2010;14:R117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Saliba F, Ichai P, Levesque E, Samuel D. Cirrhotic patients in the ICU: prognostic markers and outcome. Curr Opin Crit Care. 2013;19:154-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 118. | Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 119. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1860] [Article Influence: 169.1] [Reference Citation Analysis (3)] |
| 120. | Theocharidou E, Pieri G, Mohammad AO, Cheung M, Cholongitas E, Agarwal B, Burroughs AK. The Royal Free Hospital score: a calibrated prognostic model for patients with cirrhosis admitted to intensive care unit. Comparison with current models and CLIF-SOFA score. Am J Gastroenterol. 2014;109:554-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | López-Velázquez JA, Chávez-Tapia NC, Ponciano-Rodríguez G, Sánchez-Valle V, Caldwell SH, Uribe M, Méndez-Sánchez N. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: an important prognostic indicator. Ann Hepatol. 2013;13:98-104. [PubMed] [Cited in This Article: ] |
| 122. | Koch A, Horn A, Dückers H, Yagmur E, Sanson E, Bruensing J, Buendgens L, Voigt S, Trautwein C, Tacke F. Increased liver stiffness denotes hepatic dysfunction and mortality risk in critically ill non-cirrhotic patients at a medical ICU. Crit Care. 2011;15:R266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 123. | Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 124. | van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care. 2004;8:R153-R162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 125. | Ponce de León-Rosales SP, Molinar-Ramos F, Domínguez-Cherit G, Rangel-Frausto MS, Vázquez-Ramos VG. Prevalence of infections in intensive care units in Mexico: a multicenter study. Crit Care Med. 2000;28:1316-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 778] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 127. | Singh N, Gayowski T, Wagener MM, Marino IR. Outcome of patients with cirrhosis requiring intensive care unit support: prospective assessment of predictors of mortality. J Gastroenterol. 1998;33:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 551] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 129. | Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787-799, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2003;497-519. [PubMed] [Cited in This Article: ] |
| 131. | Francés R, Benlloch S, Zapater P, González JM, Lozano B, Muñoz C, Pascual S, Casellas JA, Uceda F, Palazón JM. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 133. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 134. | Bates DW, Sands K, Miller E, Lanken PN, Hibberd PL, Graman PS, Schwartz JS, Kahn K, Snydman DR, Parsonnet J. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176:1538-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 135. | Gustot T, Felleiter P, Pickkers P, Sakr Y, Rello J, Velissaris D, Pierrakos C, Taccone FS, Sevcik P, Moreno C. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 2014;34:1496-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 136. | Bartoletti M, Giannella M, Caraceni P, Domenicali M, Ambretti S, Tedeschi S, Verucchi G, Badia L, Lewis RE, Bernardi M. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 137. | Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 138. | Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, Tseng SC, Lin WP, Chen WT, Sheen IS. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol. 2007;46:816-826. [PubMed] [Cited in This Article: ] |
| 139. | Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6:342-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 140. | Wagener G, Diaz G, Guarrera JV, Minhaz M, Renz JF, Sladen RN. Protein C activity and postoperative metabolic liver function after liver transplantation. Transplant Proc. 2012;44:1336-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Shorr AF, Bernard GR, Dhainaut JF, Russell JR, Macias WL, Nelson DR, Sundin DP. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10:R92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |