Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.9900
Peer-review started: February 27, 2015
First decision: March 26, 2015
Revised: April 11, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: September 14, 2015
AIM: To reveal the functions of microRNAs (miRNAs) with respect to hepatic stellate cells (HSCs) in response to portal hypertension.
METHODS: Primary rat HSCs were exposed to static water pressure (10 mmHg, 1 h) and the pressure-induced miRNA expression profile was detected by next-generation sequencing. Quantitative real-time polymerase chain reaction was used to verify the expression of miRNAs. A potential target of MiR-9a-5p was measured by a luciferase reporter assay and Western blot. CCK-8 assay and Transwell assay were used to detect the proliferation and migration of HSCs under pressure.
RESULTS: According to the profile, the expression of miR-9a-5p was further confirmed to be significantly increased after pressure overload in HSCs (3.70 ± 0.61 vs 0.97 ± 0.15, P = 0.0226), which resulted in the proliferation, migration and activation of HSCs. In vivo, the up-regulation of miR-9a-5p (2.09 ± 0.91 vs 4.27 ± 1.74, P = 0.0025) and the down-regulation of Sirt1 (2.41 ± 0.51 vs 1.13 ± 0.11, P = 0.0006) were observed in rat fibrotic liver with portal hypertension. Sirt1 was a potential target gene of miR-9a-5p. Through restoring the expression of Sirt1 in miR-9a-5p transfected HSCs on pressure overload, we found that overexpression of Sirt1 could partially abrogate the miR-9a-5p mediated suppression of the proliferation, migration and activation of HSCs.
CONCLUSION: Our results suggest that during liver fibrosis, portal hypertension may induce the proliferation, migration and activation of HSCs through the up-regulation of miR-9a-5p, which targets Sirt1.
Core tip: Portal hypertension is a syndrome as the main characteristics of the portal system hemodynamics change. Hepatic stellate cell (HSC) activation is the key factor of promoting the development of the occurrence of liver cirrhosis and portal hypertension. We determined the levels of miR-9a-5p in HSCs under pressure. We found that higher levels of miR-9a-5p, which targeted Sirt1, were expressed in vivo. The restored expression of miR-9a-5p and Sirt1 could significantly suppress the proliferation, migration and activation of HSCs.
- Citation: Qi F, Hu JF, Liu BH, Wu CQ, Yu HY, Yao DK, Zhu L. MiR-9a-5p regulates proliferation and migration of hepatic stellate cells under pressure through inhibition of Sirt1. World J Gastroenterol 2015; 21(34): 9900-9915
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/9900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.9900
Portal hypertension is the consequence of an increase in both intrahepatic vascular resistance and portal blood flow[1]. In the normal liver, hepatic stellate cells (HSCs) reside in the space of Disse. Under physiological conditions, the main function of HSCs is the storage of vitamin A and other retinoids. In response to liver injury, HSCs gradually transdifferentiate to an activated α-SMA-positive phenotype through extensive proliferation and are considered to be the major source of fibrillar collagen production[2,3]. It has been widely accepted that the activation of HSCs is a key factor in the pathogenesis of liver fibrosis and portal hypertension[4]. Moreover, the increased portal pressure can promote the activation and migration of HSCs which further exacerbate liver fibrosis and cirrhosis[5-7]. However, whether HSCs are regulated by elevated portal pressure has received little attention.
MicroRNAs (miRNAs), which were discovered in recent years, are small noncoding RNAs 21-25 nucleotides in length that regulate gene expression by binding to the 3′-untranslated region (UTR) of mRNAs; this in turn causes the degradation or translational repression of the corresponding mRNAs[8,9]. According to a study of liver fibrosis, miRNAs play an important role in the regulation of the differentiation, proliferation and apoptosis of HSCs[10]. In one study, miR-15b and miR-16 promoted apoptosis of HSCs via the inhibition of Bcl-2 protein expression[11]. In another study, miR-335 was significantly reduced during the activation of HSCs, and the restoration of miR-335 could inhibit the activation and migration of HSCs[12]. The overexpression of miR-19b inhibited the activation of HSCs and negatively regulated profibrotic TGF-β signaling[13]. However, little is known about whether miRNAs play a role in the response of HSCs to the portal vein pressure.
In this study, an HSC pressure-loading apparatus was applied followed by a screening of the increased pressure-induced miRNA expression profile in HSCs by next-generation sequencing (after 14 d when HSCs were fully activated)[14]. Among these miRNAs, miR-9a-5p was validated to be significantly upregulated by real-time quantitative polymerase chain reaction (qRT-PCR) during HSC pressure overload. Meanwhile, miR-9a-5p was upregulated and Sirt1 was downregulated following fibrotic liver injury in vivo. We also found that the inhibition of miR-9a-5p could significantly suppress the proliferation, migration and activation of HSCs. Furthermore, our findings indicated that miR-9a-5p might function by directly binding to the 3’-UTR of Sirt1. Collectively, these results revealed that miR-9a-5p, as a regulator of Sirt1, influences the activation, proliferation and migration of HSCs.
Ten male rats (180-200 g) received an intraperitoneal injection of CCl4 (2 mL/kg body weight) mixed with olive oil (40% CCl4), and then the first dosage was doubled. In order to induce liver fibrosis, CCl4 was injected twice a week for 8 wk. Ten control animals were injected with an equal volume of olive oil at the same time intervals. Rats were sacrificed 48 h after the last injection. The method of sacrifice entailed the use of 2 mL of 10% chloral hydrate that was injected into the heart. The experimental procedures were approved by the Committee on the Ethics of Animal Experiments of the Second Military Medical University animal center. All surgeries were performed under sodium pentobarbital anesthesia to minimize pain.
Primary HSCs were extracted from male Sprague-Dawley rats (with a weight of approximately 400-600 g) that were purchased from the animal experiment center of the Second Military Medical University. The method of isolation of primary rat HSCs was described previously[15].
Pressure loading was performed as described previously with some amendments[16]. During the progression from chronic liver disease (CLD) to cirrhosis of the liver, the activation of HSCs plays a more important role. Therefore, 14 d was the time frame in which the primary HSCs were cultured before the pressure device was applied. This device was connected to nitrogen and to a hematomanometer; the hematomanometer was opened, and nitrogen was slowly injected. When the pressure rose to 10 mmHg, the valve was closed, which sealed the container so that it could be used in subsequent experiments after loading for 1 h (as reported previously).
Total RNA was isolated with TRIzol (Invitrogen) and was treated with DNase to remove potential genomic DNA contamination, according to the manufacturer’s protocol. The quality of the RNA was analyzed with an Agilent 2100 Bioanalyzer (Agilent Technologies). Adapters were ligated to the 5’ and 3’ ends of the isolated small RNAs. Reverse transcription followed by PCR was then used to create cDNA constructs based on the small RNA ligated with the 5’ and 3’ adapters. The cDNA was then sequenced with an ABI Proton. Reads Per Kilobase of exon model per Million mapped reads (RPKM) was adopted to quantitatively express the miRNA[17] with DEG-Seq to compare the pressure group and the control group. The following criteria were used to screen for different miRNAs: fold change ≥ 2, fold change ≤ 0.5, and false discovery rate (FDR) < 0.05[18]. The original data have been submitted to Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63783).
Gene ontology (GO) analysis was applied to analyze the main function of the differential expression of genes according to the GO which is the key functional classification of NCBI. Generally, Fisher’s exact test and χ2 test were used to classify the GO category, and the FDR was calculated to correct the P-value; the smaller the FDR, the smaller the error in judging the P-value. The FDR was defined as FDR = Nk/T, where Nk refers to the number of Fisher’s test P-values less than χ2 test P-values. We computed P-values for the GOs of all the differential genes.
Pathway analysis was used to find out the significant pathways of the differential genes. Pathway annotations of microarray genes were downloaded from KEGG (http://www.genome.jp/kegg/). A Fisher exact test was used to find the significant enrichment pathways. The resulting P-values were adjusted using the BH FDR algorithm. Pathway categories with an FDR < 0.05 were reported. Enrichment provides a measure of the significance of the function: as the enrichment increases, the corresponding function is more specific, which helps us to find those more significant pathways in the experiment. The enrichment was given by: enrichment = (ng/na)/(Ng/Na). Where ng is the number of differential genes within the particular pathway, na is the total number of genes within the same pathway, Ng is the number of differential genes which have at least one pathway annotation, and Na is the number of genes which have at least one pathway annotation in the entire microarray.
HSCs were exposed to the indicated pressures. The concentration of the sample RNA was detected with a spectrophotometer: RNA concentration (μg/μL) = A260 value × dilution ratio × 40/1000. The amplification primers of α-SMA, collagen type I (Col1) and Sirt1 were then designed (Table 1). The primers of miRNAs were designed by Guangzhou RiboBio Co., Ltd. Then, 20 μL were obtained from the experimental group and the control group, respectively, for reverse transcription. According to the instructions included in the TaKaRa kit, the reverse transcription reaction was performed for the mRNA (37 °C for 15 min or 85 °C for 5 s) and miRNA (42 °C for 60 min or 70 °C for 10 min). Gene transcripts were quantified on a 7900HT Fast Real-Time PCR System (Life Technologies Corporation, United States) with SYBR green dye and were normalized to GAPDH and U6. The experiments for each sample were repeated 3 times.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) | Product size (bp) |
| α-SMA | GCCACTGCTGCTTCCTCTTCTT | CCGCCGACTCCATTCCAATGAA | 136 |
| Col1 | GCTCGTGGATTGCCTGGAACAG | CACCGACAGCACCATCGTTACC | 235 |
| Sirt1 | TGTGGGTCTATGCCTTTCTGGGT | GCTTGCGTGTGATGCTCTGTC | 239 |
| GAPDH | ACGGCAAGTTCAACGGCACAG | CCACGACATACTCAGCACCAGC | 132 |
Antibodies against the following proteins were used for Western blot analysis: α-SMA (1:1000; Abcam, United States), Col1 (1:5000; Sigma, United States), β-actin (1:1000; CST, United States) and Sirt1 (1:1000; CST, United States). Lysed protein samples were electrophoresed by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore Co. United States). The membranes were first incubated with the primary antibodies and were then incubated with the secondary antibodies. After washing with TBST 3 times for 10 min each, the membranes were developed with an enhanced chemiluminescent ECL assay kit (Santa Cruz Biotechnology, United States).
Cells were transfected using a ribo FECT™ CP Transfection Kit (Guangzhou RiboBio Co., Ltd) according to the manufacturer’s instructions. Control miR-9a-5p, an miR-9a-5p inhibitor and an miR-9a-5p mimic were purchased from Guangzhou RiboBio Co., Ltd. The miRNA inhibitor and the miRNA mimic were transfected into the cells at a density of 100 nmol/L and 50 nmol/L, respectively; the cells were collected after 24-48 h.
After the transfection with the miRNA inhibitor and the subsequent pressure loading (10 mmHg, 1 h), the cells were fostered in 96-well plates and cultivated for 24, 48 or 72 h. A Cell Counting Kit 8 (Weiao, Shanghai, China) and an EL × 800 microplate reader (BioTek Instruments Inc., Winooski, Vermont, United States) (absorbance at 450 nm) were used to determine the cell proliferation rate.
A total of 1 × 106 cells that were transfected with the miRNA inhibitor were grown in a 6-cm Petri dish under the appropriate pressure (10 mmHg, 1 h). After the cells were collected, they were mixed with ice-cold 70% ethanol, washed with PBS, and resuspended in 0.5 mL PBS with propidium iodide (0.5 mg/mL in PBS with 0.1% sodium azide) and RNase A (1 mg/mL). Finally, the cells were incubated at 37 °C for 30 min. The results were obtained with a FACS Calibur flow cytometer (Becton–Dickinson, CA, United States) and FlowJo software (Tree Star, Ashland, OR, United States).
Transwell was used for migration assay experiments. HSCs were transfected and given pressure loading (10 mmHg, 1 h) as described previously. HSCs were inoculated at a density of 5 × 103 cells/well with 100 μL cell suspension of each small chamber. After migration for 24 h, the downside cells were fixed and stained. Five fields (× 200) were randomly observed and the bottom of the filter was visualized for counting the number of cells on each surface. This experiment was repeated 3 times.
Liver tissues were obtained from the rats with CCl4-induced liver fibrosis[19,20]. Tissues were fixed in 4% paraformaldehyde and stained with anti-Sirt1 antibody as previously described. Sections (6 μm in thickness) were cut from paraffin embedded tissues.
Sirt1 3′-UTR constructs were PCR amplified (Primer sequence, forward: 5′-CATGAGCTCCACTATTGAAGCTGTCCGGATTC-3′; reverse: 5′-GAGCTGAAAACAGTCTTCACTG-3′) and cloned into pRL-CMV-Basic vector for Luciferase reporter gene assay. The detailed sequence information was reported previously[21]. The construction of the Sirt1 3′-UTR mutant was done using the Quickchange site directed mutagenesis kit (Qiagen, United States) (Primer sequence, forward: 5′-GAACAGCTTATCTAGACCGCGGAATGGTATTTCACACTTT-3′; reverse: 5′-AAAGTGTGAAATACCATTCCGCGGTCTAGATAAGCTGTTC-3′).
HEK293T cells were transfected with pmir-Report-Sirt1 3’-UTR (wild-type or mutant), miR-9a-5p mimic and the control. The cells were harvested 24 h later and a luciferase assay was performed with the Stratagene Luciferase Assay kit (Infinite M1000, TECAN) according to the manufacturer’s instructions. The luciferase activity was determined by a dual luciferase assay and is expressed as a percentage of the control.
All data are expressed as the mean ± SD of three independent experiments. Student’s t-test was performed when the data consisted of only two groups. One-way ANOVA was performed when three or more groups were compared. SPSS software, version 16.0, was used for all of the statistical analyses, and P values ≤ 0.05 were considered statistically significant.
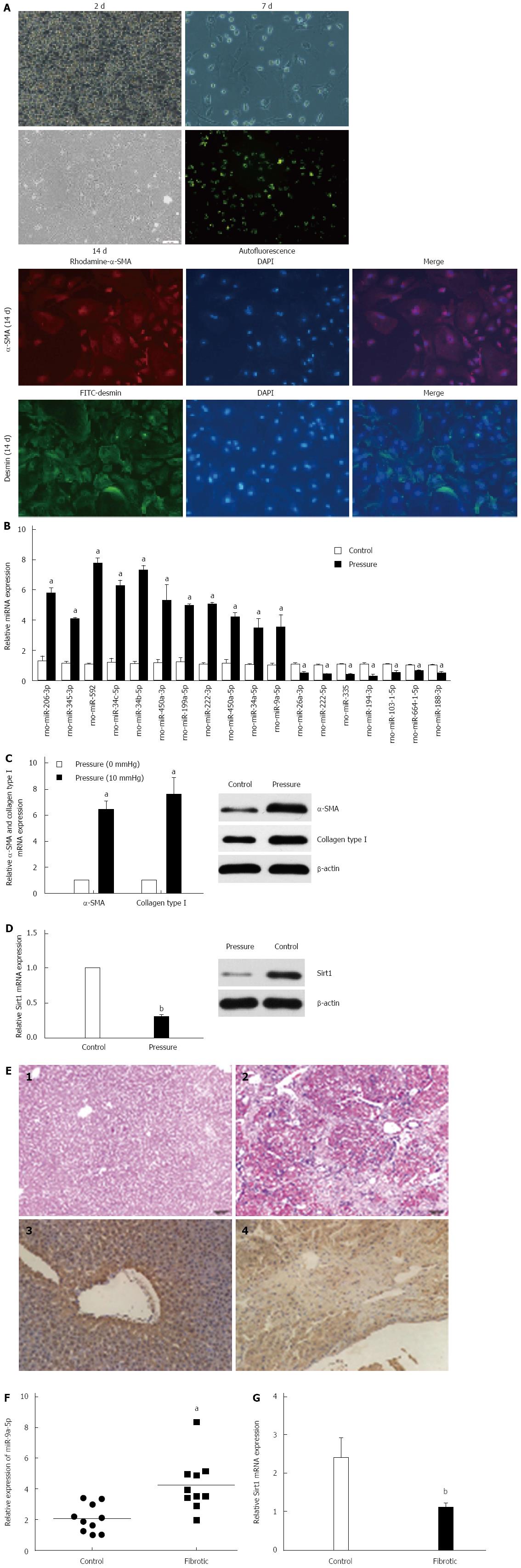
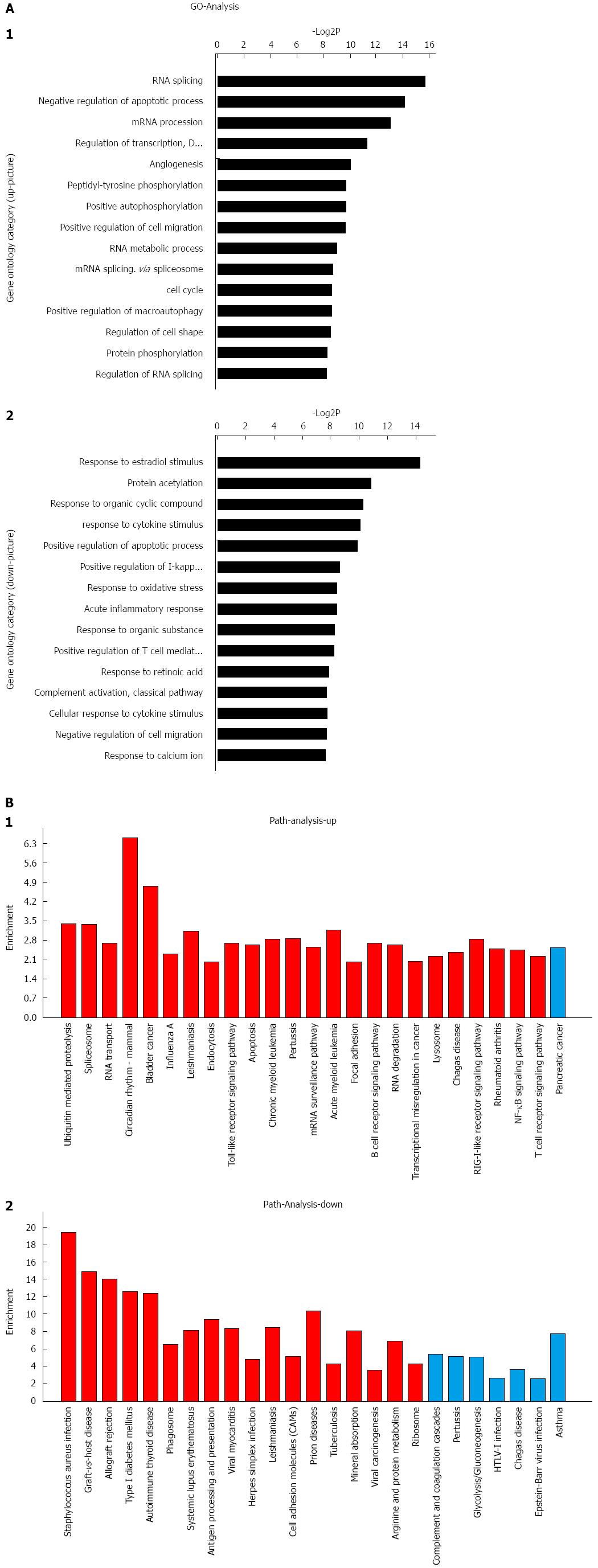
The purity of primary HSCs was determined by autofluorescence and immunohistochemistry with antibodies against desmin (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, United States) and α-SMA (1:100, Abcam, CA, United States) (Figure 1A). A total of 18 significantly abnormally expressed miRNAs were discovered by next-generation sequencing among zero pressure-loaded HSCs and pressure-overloaded HSCs. According to the screening criteria, 11 of these miRNAs were upregulated and the rest were downregulated (Table 2). qRT-PCR was used to confirm these data (Figure 1B). According to the results of the data mining, different GOs were controlled by upregulated miRNAs and downregulated miRNAs, such as cell cycle, negative regulation of fibroblast proliferation, positive regulation of macroautophagy, response to retinoic acid, protein K48-linked deubiquitination, protein K63-linked deubiquitination and negative regulation of gene expression (Figure 2A) (Supplementary material). Meanwhile, miRNAs regulated critical pathways involved in RNA transport, focal adhesion, NF-κB signaling, and cell adhesion, among others (Figure 2B) (Supplementary material).
| miRNA | Log2FC | Fold change | FDR |
| Upregulated microRNAs | |||
| rno-miR-206-3p | 3.347 | 10.176 | 2.22E-05 |
| rno-miR-210-3p | 1.903 | 3.740 | 9.61E-05 |
| rno-miR-494-5p | 1.691 | 3.229 | 0.001401 |
| rno-miR-345-3p | 3.127 | 8.736 | 0.001984 |
| rno-miR-592 | 2.990 | 7.945 | 0.002623 |
| rno-miR-124-3p | 1.656 | 3.151 | 0.002647 |
| rno-miR-429 | 1.862 | 3.635 | 0.003247 |
| rno-miR-34c-5p | 2.612 | 6.113 | 0.007838 |
| rno-miR-34b-5p | 2.482 | 5.587 | 0.008211 |
| rno-miR-34a-3p | 2.434 | 5.404 | 0.008598 |
| rno-miR-450a-3p | 2.309 | 4.955 | 0.008823 |
| rno-miR-199a-5p | 2.091 | 4.260 | 0.009277 |
| rno-miR-222-3p | 2.608 | 6.097 | 0.009440 |
| rno-miR-450a-5p | 2.373 | 5.180 | 0.009558 |
| rno-miR-34a-5p | 2.319 | 4.990 | 0.009845 |
| rno-miR-494-3p | 1.671 | 3.184 | 0.019836 |
| rno-miR-377-5p | 1.877 | 3.673 | 0.037294 |
| rno-miR-9a-5p | 2.124 | 4.359 | 1.23E-05 |
| Downregulated miRNAs | |||
| rno-miR-26a-3p | -3.412 | 0.094 | 1.54E-04 |
| rno-miR-222-5p | -2.740 | 0.150 | 0.007077 |
| rno-miR-335 | -2.428 | 0.186 | 0.009698 |
| rno-miR-194-3p | -2.785 | 0.145 | 0.021509 |
| rno-miR-103-1-5p | -2.555 | 0.170 | 0.028431 |
| rno-miR-664-1-5p | -1.972 | 0.255 | 0.041855 |
| rno-miR-188-3p | -2.472 | 0.180 | 0.043457 |
The effects of pressure on HSC activation and ECM protein production were identified. Pressure overload in HSCs resulted in an increase in the levels of α-SMA and Col1 compared with cells in the control group as determined by qRT-PCR and Western blot (Figure 1C). Sirt1 was dramatically down-regulated in response to pressure overload by qRT-PCR and Western blot (Figure 1D). MiR-9a-5p expression was also increased in a rat model of hepatic fibrosis compared with normal rats. H&E staining was used to determine the degree of hepatic fibrosis in the rats. Through qRT-PCR and immunohistochemistry experiments, we verified that the expression of Sirt1 was also decreased in fibrotic liver tissue (Figure 1E and F).
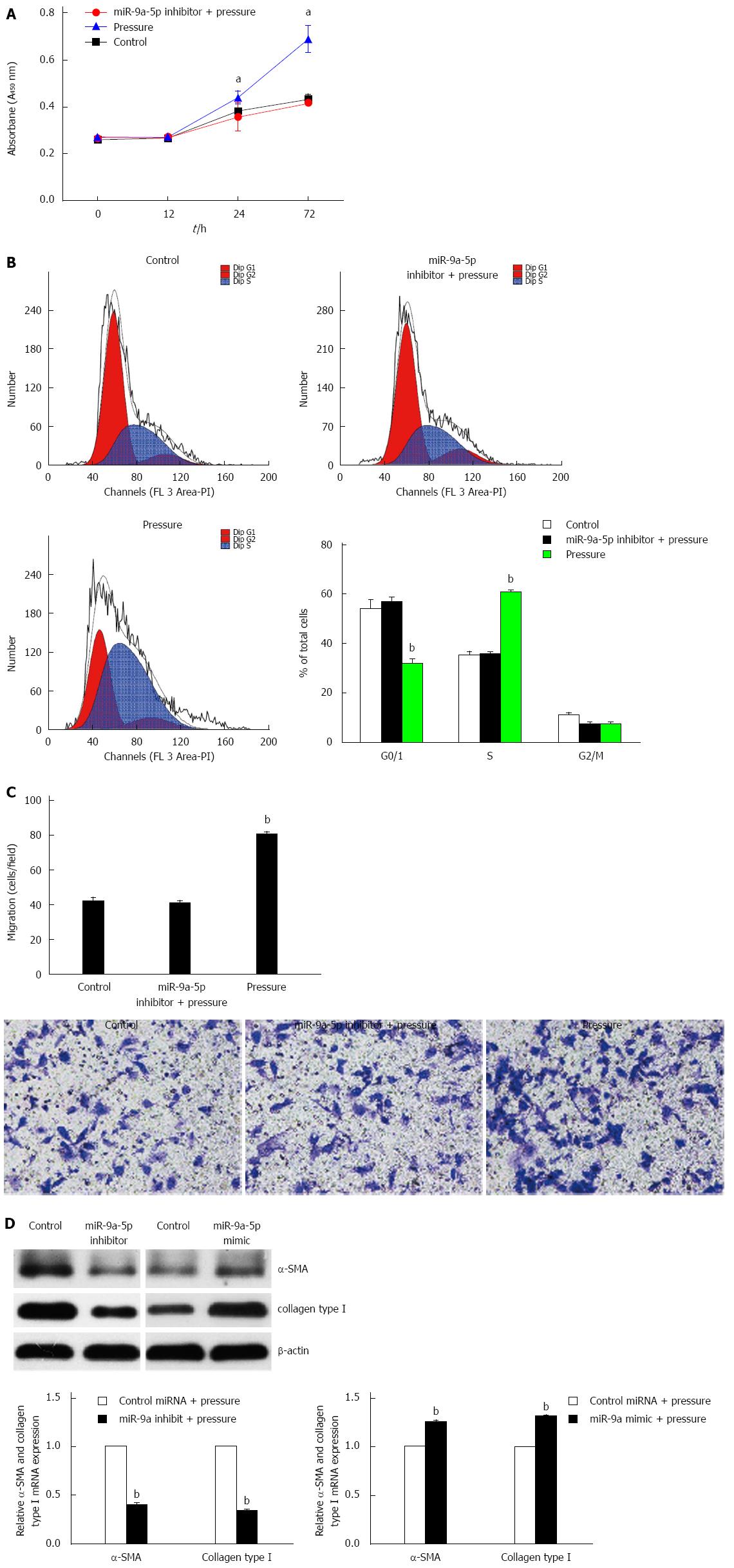
We examined the proliferation rate and migration of pressure-overloaded HSCs in response to a miR-9a-5p inhibitor. CCK-8 and transwell assays showed that the proliferation rate and migration of HSCs were decreased upon pressure overload (Figure 3A and C). According to the fluorescence activated cell sorting (FACS) analyses of propidium iodine-stained nuclear DNA, we found that in the control group, 35.27% and 53.83% of zero pressure-loaded cells were in the S phase and G0/1 phase, respectively. In the pressure-overload group, 60.76% of the HSCs were in the S phase of the cell cycle, while 31.77% of the HSCs were in the G0/1 phase. In contrast, approximately 35.70% and 56.80% of HSCs were in the S phase and G0/1 phase, respectively, when miR-9a-5p expression was inhibited (Figure 3B). Through transfection of a miR-9a-5p inhibitor or a mimic into activated HSCs that were cultured for 14 d, and after pressure application for 1 h, the expression of α-SMA and Col1 was decreased and increased, respectively, compared with HSCs that were transfected with control-miRNA (Figure 3D). In summary, the results showed that miR-9a-5p suppresses cell proliferation, migration and activation in S phase of the cell cycle.
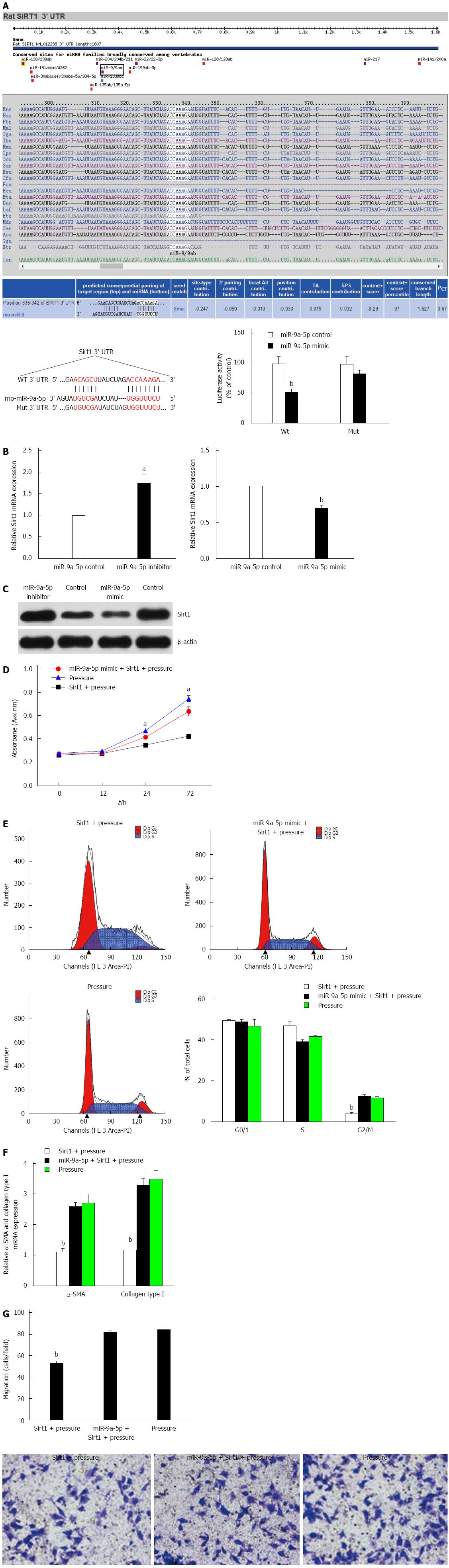
Sirt1 is involved in the regulation of many physiological functions in the body, including gene transcription, energy metabolism and the regulation of the cell aging process. Sirt1 has been reported to be a potential target of miR-9 in studies of insulin secretion[21]. Moreover, with the online prediction tools Pictar and Targetscan (Supplementary material), we found that miR-9a-5p binds to two different sites in the 3′-UTR of Sirt. In order to assess the role of this miRNA, a dual-luciferase reporter system was adopted. Luciferase reporters that contain a wild-type (WT) Sirt1 3′-UTR and a Sirt1 3′-UTR, including mutant sequences in the two possible binding sites, were constructed. The results showed that the luciferase activity of the WT Sirt1 3′-UTR in cells that were treated with a miR-9a-5p mimic was significantly decreased. However, the activity was restored in the mutant 3′-UTR construct (Figure 4A).
Next, we found that the mRNA and protein levels of Sirt1 were increased in HSCs upon application of a miR-9a-5p inhibitor. Following transfection with a miR-9a-5p mimic, Sirt1 expression was decreased (Figure 4B and C). Taken together, it was demonstrated that miR-9a-5p is able to target Sirt1 and negatively regulate its expression.
To elucidate whether miR-9a-5p regulates HSC proliferation, migration and activation by targeting Sirt1, we examined the proliferation, migration and activation of pressure-overloaded HSCs in response to overexpression of Sirt1. We found that Sirt1 overexpression could inhibit the proliferation, migration and activation of HSCs compared with overexpression of miR-9a-5p and Sirt1 simultaneously (Figure 4D, F and G). We also found that in the pressure-overload group, 41.92% and 11.48% of cells were in the S phase and G2 phase, respectively. In the Sirt1 overexpression group, 46.90% of HSCs were in the S phase of the cell cycle, while 3.68% of the HSCs were in the G2 phase. In the miR-9a-5p and Sirt1 overexpression group, approximately 39.15% and 12.15% of HSCs were in the S phase and G2 phase, respectively (Figure 4E). The results showed that increased expression of Sirt1 can restore the proliferation, migration and activation of HSCs.
Continuous activation of HSCs is the key factor in the promotion of the development of liver fibrosis, which may also cause an increase in portal pressure. Our previous study results showed that the liver sinus pressure also promotes the activation of HSCs. Activated HSCs secrete a variety of fibrotic factors such as vascular endothelial growth factor, transforming growth factor, Dl and collagen I, which cause collagen deposition in the liver sinus; these factors also impede blood flow, which promotes the anatomical features of portal hypertension. Therefore, a vicious cycle exists among the development of liver cirrhosis, portal hypertension, the liver sinus pressure and activated HSCs. The experimental data also show that pressure can obviously promote the proliferation of HSCs and that this mechanism regulates cell proliferation in the S phase of the cell cycle.
MiRNAs play a very important role in various liver diseases such as viral hepatitis, fatty liver disease, liver fibrosis and hepatocellular carcinoma. MiR-122 is associated with viral infection and replication. After miR-122 is silenced, HCV RNA is decreased by 64%, which indicates that miR-122 is expected to become one of the targets of anti-HCV therapy[22,23]. MiR-14 is also associated with lipid metabolism, since miR-14 knockout animals demonstrate an increase in the levels of triglyceride and diacylglycerol[24].
Recent studies also suggest that miRNAs are very closely linked with biomechanical responses. It has been reported that miR-34a plays an important role in the regulation of cirrhosis with portal hypertension[25]. MiR-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells[26]. In our study, we screened the pressurization-induced miRNA expression profile in HSCs by next-generation sequencing. The expression of miR-9a-5p is obviously differentially expressed. qRT-PCR results showed that miR-9a-5p also significantly increased under increased pressure, which is consistent with its expression in quiescent HSCs vs activated HSCs[27]. In this study, we found that miR-9a-5p directly reduced the expression of Sirt1. Recent research also suggests that Sirt1 is a target gene of miR-9a-5p in the beta cells of pancreatic islets[21]. In addition, some studies have shown that Sirt1 participates in hepatic steatosis[28,29]. We also found that a miR-9a-5p inhibitor could inhibit HSC proliferation and migration. Compared with the control group, HSC proliferative and migration activity was significantly inhibited in the miR-9a-5p inhibitor group, and miR-9a-5p regulates HSC proliferation and migration by targeting Sirt1. Interestingly, when miR-9a-5p was upregulated, the expression of Sirt1 was significantly decreased in rats with liver fibrosis compared with controls.
Overall, these systematic studies indicate that miR-9a-5p is a novel regulator of HSCs in the setting of increased pressure and hepatic fibrosis in rats, and that overexpression of miR-9a-5p following HSC activation perpetuates the fibrotic response. MiR-9a-5p could negatively regulate Sirt1 expression and plays an important role in the regulation of HSC proliferation and migration, which provides a potential therapeutic target for portal hypertension.
We thank Liebing Company for miRNA next-generation sequencing.
Portal hypertension is the consequence of an increase in both intrahepatic vascular resistance and portal blood flow. Clinical manifestations include esophageal gastric varices, ascites, splenomegaly and with gastrointestinal bleeding, hepatic encephalopathy and hepatorenal syndrome. In this study, the authors used hepatic stellate cells (HSCs) pressure-loading apparatus to screen the pressurization induced miRNA expression profile in HSCs by next-generation sequencing (Choose 14 d fully activated HSCs). In addition, miR-9a-5p was verified to be importantly upregulated during HSC pressure-loading by real-time quantitative polymerase chain reaction.
HSC activation is the key factor of promoting the development of cirrhotic portal hypertension. Portal hypertension with liver sinus pressure also promoted the sustained activation of HSCs. Therefore, there is a vicious cycle between sustained activation of HSCs and cirrhotic portal hypertension. MicroRNAs (miRNAs) play an important role in regulating the proliferation, migration and activation of HSCs.
The pressure-induced miRNA expression profile was detected by next-generation sequencing. MiR-9a-5p, which targets Sirt1, regulated proliferation, migration and activation of hepatic stellate cells. In vivo, the expression level of miR-9a-5p was up-regulated and the expression level of Sirt1 was down-regulated.
The study results suggest that high expression of miR-9a-5p directly and indirectly results in activation of HSCs due to pressure. miR-9a-5p is involved in cirrhotic portal hypertension, and may be regarded as a potential molecular and a therapeutic target in this disorder.
Portal hypertension is a syndrome as the main characteristics of the portal system hemodynamics change. MiR-9a-5p is a recently found miRNA. MiRNAs are small noncoding RNAs that regulate gene expression by binding to the 3′ untranslated region of mRNAs to cause their degradation or translational repression. Hepatic stellate cell is a kind of liver cell, accounting for about 15% of the whole liver cells, located in the clearance between hepatic sinus endothelial cells and liver plate.
In this manuscript authors analyzed miRNA profiling in HSCs. They obtained evidence that miR-9a-5p was upregulated during HSC pressure overload, and Sirt1 is one of its functional targets. Their observation is interesting that miR-9a-5p, as well as Sirt1, might influence the activation, proliferation and migration of HSCs.
P- Reviewer: Ma L, Seren O, Yang J S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Ma S
| 1. | de Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis. 2004;36:787-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Török NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008;43:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Soon RK, Yee HF. Stellate cell contraction: role, regulation, and potential therapeutic target. Clin Liver Dis. 2008;12:791-803, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571-581. [PubMed] [Cited in This Article: ] |
| 5. | van Leeuwen DJ, Howe SC, Scheuer PJ, Sherlock S. Portal hypertension in chronic hepatitis: relationship to morphological changes. Gut. 1990;31:339-343. [PubMed] [Cited in This Article: ] |
| 6. | Blendis LM. Hepatocyte swelling and portal hypertension. J Hepatol. 1992;15:4-5. [PubMed] [Cited in This Article: ] |
| 7. | Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980-G987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1545] [Cited by in F6Publishing: 1568] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 9. | Chitwood DH, Timmermans MC. Target mimics modulate miRNAs. Nat Genet. 2007;39:935-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Lakner AM, Bonkovsky HL, Schrum LW. microRNAs: fad or future of liver disease. World J Gastroenterol. 2011;17:2536-2542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 68] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Chen C, Wu CQ, Zhang ZQ, Yao DK, Zhu L. Loss of expression of miR-335 is implicated in hepatic stellate cell migration and activation. Exp Cell Res. 2011;317:1714-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Lakner AM, Steuerwald NM, Walling TL, Ghosh S, Li T, McKillop IH, Russo MW, Bonkovsky HL, Schrum LW. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Watanabe S, Nagashio Y, Asaumi H, Nomiyama Y, Taguchi M, Tashiro M, Kihara Y, Nakamura H, Otsuki M. Pressure activates rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1175-G1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 289] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Okada Y, Tsuzuki Y, Hokari R, Miyazaki J, Matsuzaki K, Mataki N, Komoto S, Watanabe C, Kawaguchi A, Nagao S. Pressure loading and ethanol exposure differentially modulate rat hepatic stellate cell activation. J Cell Physiol. 2008;215:472-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wang W, Meng M, Zhang Y, Wei C, Xie Y, Jiang L, Wang C, Yang F, Tang W, Jin X. Global transcriptome-wide analysis of CIK cells identify distinct roles of IL-2 and IL-15 in acquisition of cytotoxic capacity against tumor. BMC Med Genomics. 2014;7:49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12015] [Cited by in F6Publishing: 10698] [Article Influence: 764.1] [Reference Citation Analysis (0)] |
| 19. | George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:319-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011;278:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1993] [Cited by in F6Publishing: 1929] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 23. | Shan Y, Zheng J, Lambrecht RW, Bonkovsky HL. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 727] [Cited by in F6Publishing: 709] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 25. | Jansen C, Eischeid H, Goertzen J, Schierwagen R, Anadol E, Strassburg CP, Sauerbruch T, Odenthal M, Trebicka J. The role of miRNA-34a as a prognostic biomarker for cirrhotic patients with portal hypertension receiving TIPS. PLoS One. 2014;9:e103779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2010;107:3240-3244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Noetel A, Elfimova N, Altmüller J, Becker C, Becker D, Lahr W, Nürnberg P, Wasmuth H, Teufel A, Büttner R. Next generation sequencing of the Ago2 interacting transcriptome identified chemokine family members as novel targets of neuronal microRNAs in hepatic stellate cells. J Hepatol. 2013;58:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q, Li J, Zhang Q, Gao Y, Gao L. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J Nutr Biochem. 2014;25:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Han JY, Park SH, Yang JH, Kim MG, Cho SS, Yoon G, Cheon SH, Ki SH. Licochalcone Suppresses LXRα-Induced Hepatic Lipogenic Gene Expression through AMPK/Sirt1 Pathway Activation. Toxicol Res. 2014;30:19-25. [PubMed] [Cited in This Article: ] |