Published online Jun 28, 2013. doi: 10.3748/wjg.v19.i24.3802
Revised: April 12, 2013
Accepted: May 8, 2013
Published online: June 28, 2013
AIM: To investigate the expression and role of activin A in a mouse model of acute chemical liver injury.
METHODS: Acute liver injury in C57BL/6 male mice was induced by intraperitoneal injection with carbon tetrachloride (CCl4) (0.5 mL/kg, body weight) dissolved in olive oil (1:19 v/v). Mice were sacrificed 1, 3, 5 and 7 d after the treatment. The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were examined and pathological changes of liver observed by hematoxylin and eosin staining to evaluate the liver injury. Activin A protein levels in serum and hepatic tissue homogenate of mice were detected by enzyme-linked immunosorbent assay, and the expression pattern of activin A protein in livers of mice was examined by immunohistochemistry. Activin type IIA receptor (ActRIIA) and Smad3 expressions in the liver were analyzed by real-time quantitative reverse transcription-polymerase chain reaction. In order to further investigate the role of activin A, we also utilized activin A blocking experiment by anti-activin A antibody (500 μg/kg, body weight) injection into mouse tail vein.
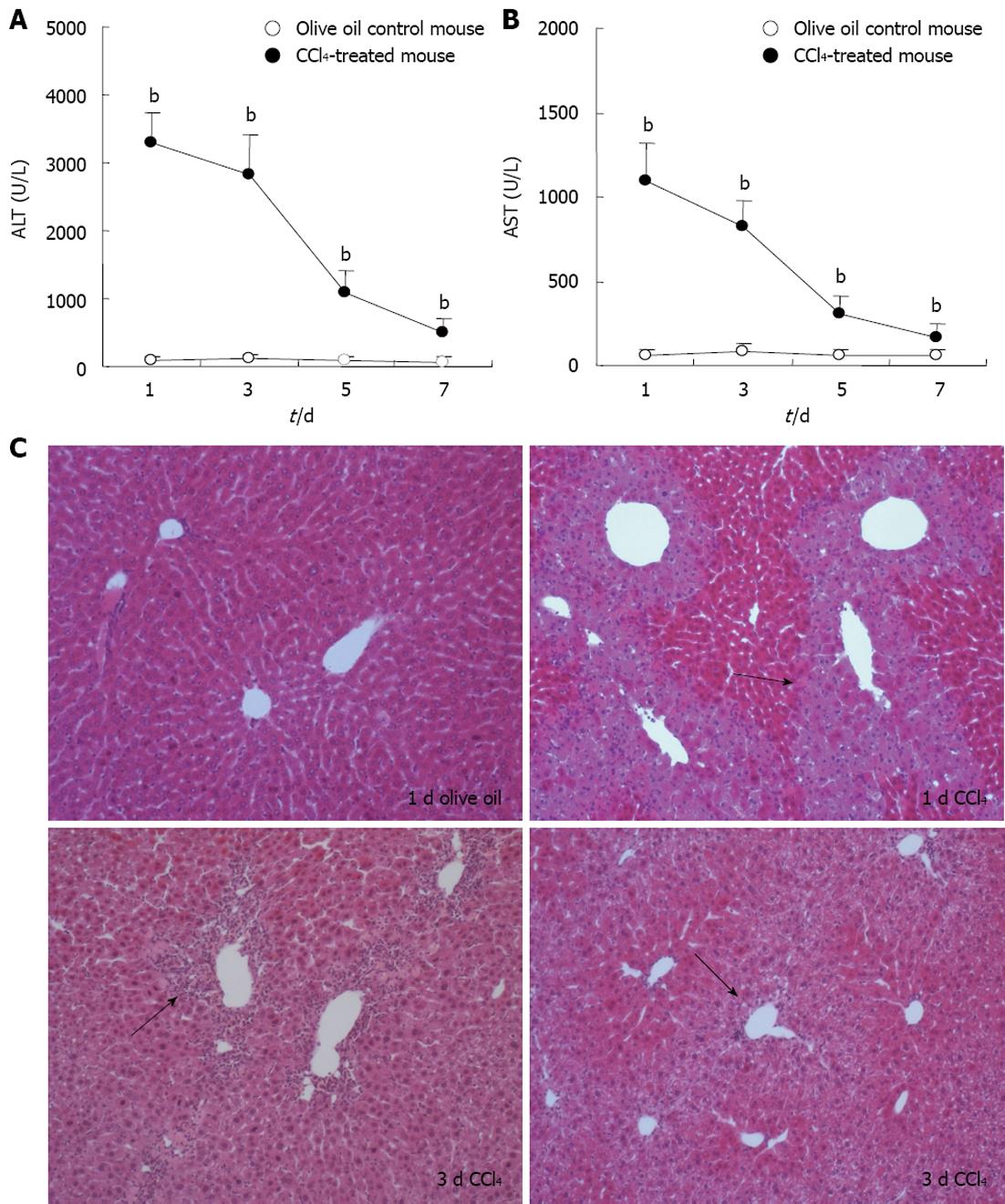
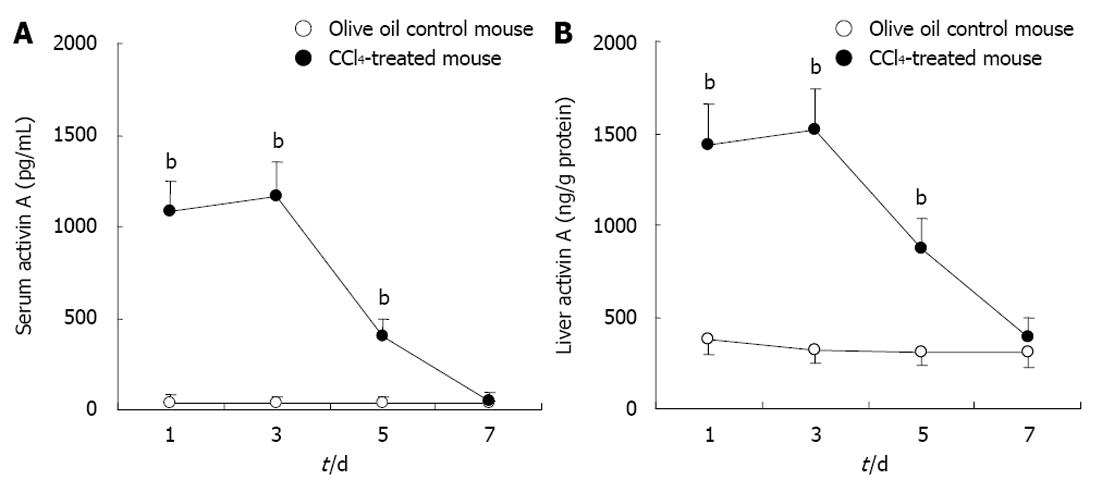
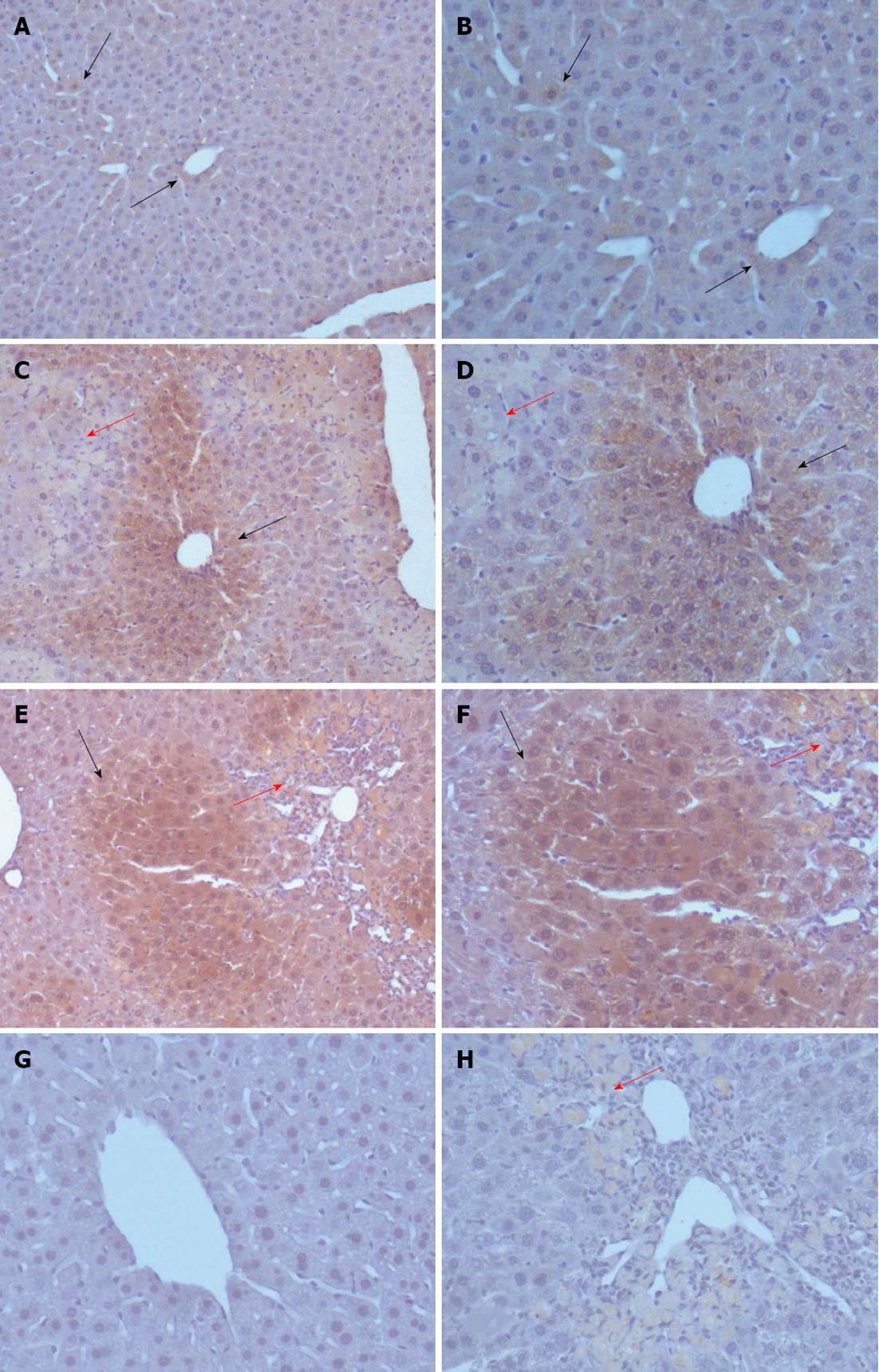
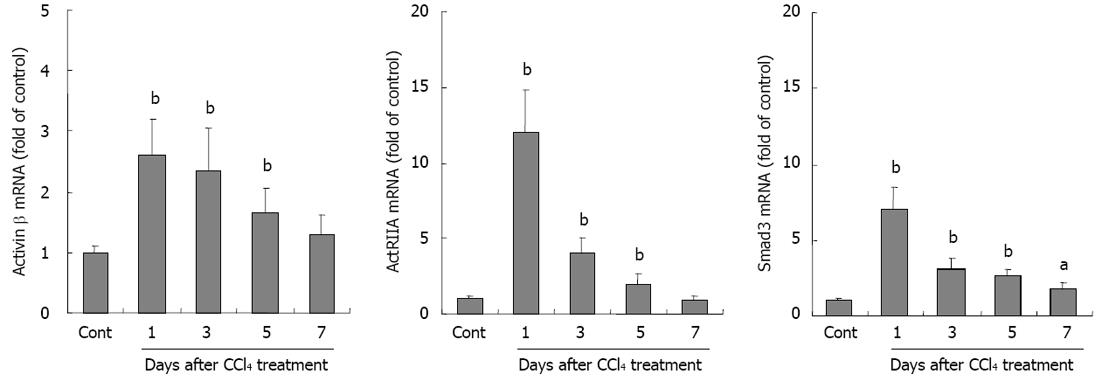
RESULTS: In CCl4-treated mice, serum ALT and AST levels were significantly increased, compared with that in control mice (P < 0.01). Furthermore, the serious necrosis was observed around hepatic portal areas in CCl4-treated mice. Simultaneously, activin A levels in serum and hepatic tissue homogenate of mice treated with CCl4 for 1, 3 and 5 d increased significantly, compared with that in control mice (P < 0.01). Activin A protein expression in hepatocytes not within the necrotic area was also upregulated in mice following CCl4 treatment. Not only activin A, but also ActRIIA and activin signaling molecule Smad3 mRNA expressions in injury liver induced by CCl4 were significantly higher than that in control liver. In addition, levels of serum ALT and AST in CCl4-treated mice were significantly decreased by injection of anti-activin A antibody to block endogenous activin A action, compared with that in CCl4-treated mice by injection of immunoglobulin G instead of anti-activin A antibody (P < 0.01), and the severity of liver injury was also reduced remarkably.
CONCLUSION: These data show that activin A is involved in CCl4-induced acute liver injury. Blocking activin A actions may be a therapeutic approach for acute liver injury.
Core tip: The objective of this study was to investigate the expression and role of activin A in acute liver injury. A carbon tetrachloride (CCl4)-induced acute liver injury mouse model was used. Liver injury effects were examined by measuring alanine aminotransferase and aspartate aminotransferase levels in serum and liver pathological changes. Activin A protein expression levels were detected by enzyme-linked immunosorbent assay and immunohistochemistry. Activin type IIA receptor and Smad3 expressions in liver were analyzed by real-time quantitative reverse transcription-polymerase chain reaction. We found that activin A is involved in CCl4-induced acute liver injury, suggesting activin A could be a potential therapeutic option for acute liver injury diseases.
- Citation: Wang DH, Wang YN, Ge JY, Liu HY, Zhang HJ, Qi Y, Liu ZH, Cui XL. Role of activin A in carbon tetrachloride-induced acute liver injury. World J Gastroenterol 2013; 19(24): 3802-3809
- URL: https://www.wjgnet.com/1007-9327/full/v19/i24/3802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i24.3802
Activin A, a member of the transforming growth factor-β (TGF-β) superfamily, has a wide range of biological roles[1-3]. It is distributed widely in various tissues and produced by numerous cells including macrophages, T-helper 2 cells and hepatocytes[4-6]. It plays important roles in regulation of pituitary hormone release, neuron survival, hematopoiesis and the early development of embryos[7-10]. The expression of activin A is closely related to liver diseases, and abnormal expression of activin A and its signal proteins are found during the development of virus hepatitis, hepatic fibrosis, liver cancer and other diseases[11-14]. Activin A affects the function of hepatocytes by inhibiting DNA synthesis while stimulating the synthesis of the extracellular matrix of hepatic stellate cells, which can result in the hepatic fibrosis[2,11,12].
The liver is an important metabolic organ in the body, and can be injured by various factors, which include viral infection, trauma, and chemical reagents. Carbon tetrachloride (CCl4)-induced liver injury is a classic model of chemical liver injury in mice[15]. Activin A expression increased significantly in CCl4-induced chronic liver injury in mice, marking it as an important factor in liver fibrosis development[16]. However, it is still unclear whether activin A is involved in the process of CCl4-induced acute chemical liver injury.
In the present study, the expression and role of activin A were investigated in CCl4-induced acute chemical liver injury in mice. Further, the role of activin A in the process of acute liver injury was confirmed by in vivo blockade of activin A action.
CCl4 was purchased from Beijing Chemical Factory (batch number 20050106). Olive oil was obtained from Beijing KeLipei Tsui olive oil Development Centers. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assay kit was provided by NJBI (Nanjing, China). Anti-activin A antibody was obtained from Sigma Company. Trizol reagent was provided by Invitrogen Corporation. SYBR fluorescence quantitative reverse transcription-polymerase chain reaction (PCR) kit was purchased from Takara Company.
C57BL/6 male mice were provided by Animal Center of Jilin University (Changchun, China). All animal experiments were performed following an institutionally approved protocol in accordance with the Jilin University Guide for the care and use of laboratory animals.
C57BL/6 mice were randomly divided into the olive oil control group and the CCl4 group (n = 24). In the control group, mice were treated with olive oil (10 mL/kg) by intraperitoneal injection; in the CCl4 group, mice were injected intraperitoneally with CCl4 (0.5 mL/kg) + olive oil (9.5 mL/kg) (1:19 v/v). Mice were executed 1, 3, 5 and 7 d after the treatment, serum was collected for the determination of transaminases and activin A levels, and hepatic tissues were obtained for pathological examination and immunohistochemical staining.
The serum transaminases ALT and AST levels were detected by assay kit according to the manufacturer’s protocol (NJBI, Nanjing, China). Briefly, 25 μL AST or ALT substrates and 5 μL serum were added into one well of polystyrene microtiter plates at 37 °C for 30 min. And then 25 μL of 2,4-dinitrophenylhydrazine was added in to all wells at 37 °C for 30 min. Finally, 250 μL of 0.4 mol/L sodium hydroxide was added to stop the reactions at room temperature for 15 min, and the absorbance at 510 nm in each well was measured with an enzyme-linked immunosorbent assay (ELISA) reader (BioRad Laboratories, Hercules, CA, United States). The levels of AST or ALT are expressed in U/L.
The right lobe of each mouse liver was collected, fixed with 40 g/L paraformaldehyde for 24 h, embedded in paraffin, and sliced into a thickness of 3-4 μm. The sections were deparaffinized and pathological liver change were examined by hematoxylin and eosin (HE) staining.
To prepare the mouse hepatic tissue homogenate, 50 mg mouse liver was added to 1 mL lysis buffer (1% Triton X-100, pH 7.5, 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L sodium fluoride, 4 μg/mL leupeptin, 1 μg/mL aprotinin). The lysate was centrifuged for 30 min at 12000 rpm at 4 °C and the supernatant was harvested. The levels of activin A in serum and hepatic tissue homogenates of mice were determined by ELISA according to the manufacturer’s protocol (R and D).
Total hepatic tissue RNA was extracted using the Trizol reagent according to the manufacturer’s protocol (Invitrogen), and then activin βA, Activin type IIA receptor (ActRIIA) and Smad3 mRNA expressions were examined by SYBR real-time-PCR kit using a ABI PRISM 7700 sequence detection system (Perkin-Elmer Applied Biosystems) in a two-stage, single-tube reaction. The following reaction conditions were used: stage one, 95 °C, 10 s for 1 cycle; stage two, 95 °C, 5 s and 60 °C, 31 s for 40 cycles, collecting fluorescence in this phase; stage three, 95 °C, 15 s, 60 °C 1 min, 95 °C 15 s for 1 cycle. Primers were synthesized by BECL (Shanghai, China), and the sequences are shown in Table 1. The reverse transcription (RT)-PCR products were quantitatively analyzed according to a standard cDNA calibration curve[17].
| Genes | Primers | Sequences | Product size (bp) |
| GAPDH | Sense | 5’-GATTGTTGCCATCAACGACC-3’ | 371 |
| Antisense | 5’-GTGCAGGATGCATTGCTGAC-3’ | ||
| Activin A | Sense | 5’-GAGAGGAGTGAACTGTTGCT-3’ | 514 |
| Antisense | 5’-ATGACTGTTGAGTGGAAGG-3’ | ||
| ActRIIA | Sense | 5’-ATTGGCCAGCATCCATCTCTTG-3’ | 296 |
| Antisense | 5’-TGCCACCATCATAGACTAGATTC-3’ | ||
| Antisense | 5’-ACTCTGTGGCTCAATTCCTGCT-3’ | ||
| Smad3 | Sense | 5’-CGGTCAAGAGCCTGGTCAAGA-3’ | 241 |
| Antisense | 5’-TTGAAGGCGAACTCACACAGC-3’ |
The right lobe of the liver was fixed by 40 g/L paraformaldehyde for 24 h, embedded in paraffin, and then sliced into a thickness of 3-4 μm. The sections were deparaffinized, and 3% hydrogen peroxide (H2O2)-methanol was used to block endogenous peroxidase at room temperature for 30 min. Two percent of bovine serum albumin in 0.01 mol/L phosphate-buffered saline (PBS) was used to block nonspecific reactivity by a preincubation of section for 30 min. The sections were then incubated in anti-activin A antibody (1:400) at 4 °C overnight. The sections were washed with PBS, bound antibodies were detected with SP1 kit (ZSJQ, Beijing, China) and immunoreactive products were visualized in 0.05% diaminobenzidine and 0.03% H2O2. The sections were counterstained with hematoxylin, dehydrated, cleared, counted and observed under an Olympus microscope (BX51). In control staining, the sections were incubated with normal mouse immunoglobulin G (IgG) instead of anti-activin A antibody[18].
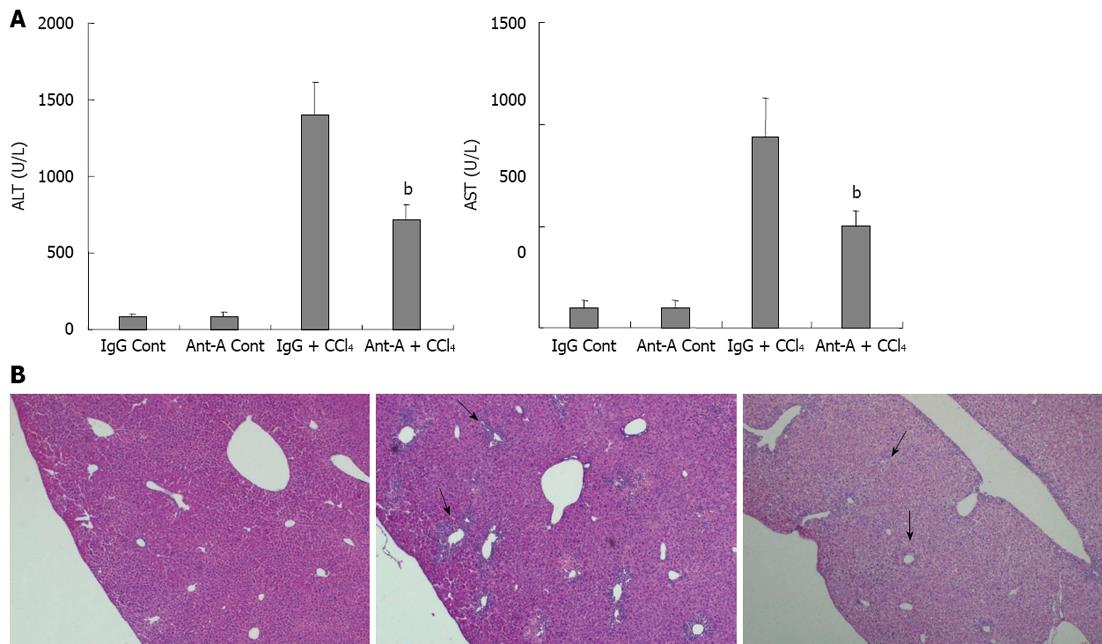
C57BL/6 mice adaptive feeding for 7 d were randomly divided into four groups (n = 12). The IgG control group (IgG Cont), in which each mouse was injected intraperitoneally with olive oil (10 mL/kg), 2 h later, injected by tail vein with normal IgG (500 μg/kg); the antibody control group (Anti-A Cont), in which each mouse was injected intraperitoneally with olive oil (10 mL/kg), 2 h later, injected by tail vein with anti-activin A antibody (500 μg/kg); the CCl4 group (IgG + CCl4), in which each mouse was injected intraperitoneally with CCl4 (0.5 mL/kg) + olive oil (9.5 mL/kg), 2 h later, injected by tail vein with IgG (500 μg/kg); the antibody plus CCl4 group (Anti-A + CCl4), in which each mouse was injected intraperitoneally with CCl4 (0.5 mL/kg) + olive oil (9.5 mL/kg), 2 h later, injected by tail vein with anti-activin A antibody (500 μg/kg). Sera and livers of these mice were collected 3 d after CCl4 injection for determination of serum ALT and AST levels and pathological examination of livers.
The statistical software SPSS 10.0 was used to analyze all data. P < 0.05 was considered statistically significant.
Change of serum transaminases ALT and AST levels is an important indicator of liver injury. Our results revealed that serum ALT and AST levels were significantly elevated on days 1, 3, 5 and 7 after the administration of CCl4, compared with that in control group, P < 0.01 (Figure 1A). Furthermore, lobular architecture in the control mouse liver was clear and the hepatic cells arranged in neat rows by HE staining. In mouse liver 1 d following CCl4 treatment, there were round necrotic lesions around the hepatic lobule portal area, and 3 d after CCl4 treatment, there was inflammatory cell infiltration in the hepatic lobule portal area (Figure 1B).
Activin A levels in serum and hepatic homogenates of experimental mice were determined by ELISA. The results showed that activin A levels increased significantly in serum and hepatic homogenate from CCl4-induced acute liver injury mice for 1, 3 and 5 d, compared with that in control group, P < 0.01 (Figure 2). These levels peaked on days 1 and 3, and returned to normal levels by day 7.
To explore the reason for increased activin A levels in the serum of CCl4-treated experimental mice and the expression pattern of activin A in acute liver injury, the expression of activin A protein was examined by immunohistochemical staining. The results revealed that activin A protein expression was detectable in the livers of olive oil-treated mice and in injured livers from CCl4-treated mice. In addition, activin A protein expression in hepatocytes with non-necrotic area was up-regulated on days 1 and 3 after CCl4 treatment (Figure 3).
Activin A is a dimeric glycoprotein formed by two βA subunits. To further investigate activin and its signal molecule expression in liver injury, activin βA, ActRIIA and Smad3 mRNA expressions were examined by real-time quantitative RT-PCR. The results showed that not only activin βA mRNA, but also ActRIIA and intracellular signal protein Smad3 mRNA expressions were significantly increased in livers from CCl4-treated mice when compared with that in control group, P < 0.01 (Figure 4).
In order to further confirm that activin A was involved in acute liver injury, pathological changes of livers in CCl4-treated mice were examined after injection of an anti-activin A antibody to block endogenous activin action. The results revealed that serum ALT and AST levels in mice treated with anti-activin A + CCl4 were significantly lower than that in IgG + CCl4 control mice (Figure 5A, P < 0.01). Furthermore, the results showed that the necrotic severity around the hepatic lobule portal area in mice treated with anti-activin A + CCl4 was remarkably reduced compared with that in IgG + CCl4 control mice (Figure 5B).
Activin A as a multifunctional factor plays an important role in acute phase immune response, and its expression levels are increased in several inflammatory diseases such as septicemia, inflammatory bowel disease, and rheumatoid arthritis[19-22]. Recent studies have reported that activin A is closely related to the liver diseases hepatitis and hepatic fibrosis[3,14,16]. Activin receptors belong to serine/threonine kinase receptors of the TGF-β superfamily, are divided into ActRI and ActRII. Activin binds directly to the type II receptor on the cell membrane, and then recruits the type I receptor[23,24]. Further, the activated type I receptor propagates the signal through a cascade reaction elicited by downstream Smad proteins into the nucleus to activate target genes[25,26].
CCl4 is a chemical reagent that induces mouse liver injury, a classic chemical model for studying liver injury. The principle of injury is that in the function of cytochrome P450, CCl4 generates chloromethyl free radicals (-CCl3), which enable the peroxidation of the membrane lipids on the liver cell membrane or subcellular structure causing the rise of membrane permeability that results in the release of large number of ALT in the cytoplasm into blood. Another possibility is covalent binding of -CCl3 and hepatic microsomal lipids and proteins that damage the integrity of the structure and function of hepatic cell membranes[27-29]. Additionally, various factors can further aggravate CCl4-induced liver injury in mice, such as the increase of activin A expression in chronic liver injury[16].
Abnormal expression of activin A is related to a variety of liver diseases, and activin A can directly induce hepatocyte apoptosis and inhibit hepatocyte proliferation and DNA synthesis[2,12,30]. To investigate whether activin A is involved in acute chemical liver injury, C57BL/6 mice were injected with CCl4 through peritoneal cavity to establish acute liver injury model. The results showed that serum ALT and AST increased, and serious necrosis of hepatic tissue was observed by HE staining in CCl4-treated mice. Simultaneously, activin A levels increased significantly in serum and hepatic tissue homogenate of mice treated with CCl4. By immunohistochemical staining, the elevated expression of activin A protein was also observed in the injured livers of CCl4-treated mice. Since CCl4 induced necrotic death of hepatocytes in large area, which necrotic hepatocytes could not release cytokines, but the decomposition product of necrotic tissues as inflammatory stimulator could induce hepatocytes activation to secrete activin A. Thus, activin A protein was expressed in hepatocytes around the necrotic area and the activated hepatocytes have lager clear nuclei. In addition, the real-time quantitative RT-PCR results revealed that in CCl4-treated mice, activin A, ActRIIA and intracellular activin signal protein Smad3 mRNA expressions significantly increased. In order to further confirm the role of activin A in CCl4-induced liver injury, pathological changes of livers in CCl4-treated mice were examined after injection with anti-activin A antibody to block endogenous activin action. These results revealed that activin A might participate in the process of CCl4-induced liver injury via inhibiting proliferation and DNA synthesis of hepatocytes to aggravate the CCl4-induced liver injury, and blockade of activin A actions by anti-activin A antibody could significantly reduce the levels of serum transaminases and the necrosis severity of hepatic tissue in CCl4-treated mice.
In summary, these data suggested that the activin A-ActRIIA-Smad signal transduction cascade was induced in CCl4-treated mice. Further, activin A was involved in the process of CCl4-induced acute chemical liver injury of mice in an autocrine/paracrine manner. Activin A may be a potential therapeutic target for acute liver injury disease.
Activin A is an important mediator of liver cells, not only in proliferation of hepatocytes, but also in activation of hepatic stellate cells, secretion of liver extracellular matrix, as well as the formation and development of liver injury. Carbon tetrachloride (CCl4)-induced liver injury is a classical model of chemical liver injury in mice. Previous studies reported that the expression of activin A increased significantly in CCl4-induced chronic liver injury in mice, and it was an important factor leading to liver fibrosis. The effect of activin A on the process of CCl4-induced acute chemical liver injury has not been reported.
Previous studies have demonstrated the effect of activin A on CCl4-induced chronic liver injury in mice, however, the effect and mechanism of activin A on CCl4-induced acute liver injury remains undefined.
New therapies that aim to block biological role of activin A in liver injury diseases are being actively explored and evaluated. Authors believe this study to be the first to explore the effect of activin A on acute liver injury.
The findings that block the biological action of activin A may be clinically relevant to potential liver injury therapeutics.
Activin is a multifunctional factor of transforming growth factor-β superfamily. There are three types of activins formed by homo- or hetero-dimerization of two inhibin subunits (βA and βB), activin A (βAβA), activin B (βBβB) and activin AB (βAβB). Activin A is an important mediator in CCl4-induced acute liver injury in mice, and blockade of biological action of activin A may be a potential therapeutics for acute liver injury diseases.
This is a good descriptive study in which authors analyze the role of activin A in carbon tetrachloride-induced acute liver injury in mouse. The results are interesting and suggest that activin A is involved in the process of CCl4-induced acute liver injury in an autocrine/paracrine manner. Further, the authors show that an antibody-mediated blockade of activin A biological action may provide insights into potential therapeutics.
P- Reviewers Ding WX, Ohkohchi N, Shimizu Y, Tarantino G S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133-146. [PubMed] [Cited in This Article: ] |
| 2. | Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood). 2002;227:75-87. [PubMed] [Cited in This Article: ] |
| 3. | Phillips DJ, de Kretser DM, Hedger MP. Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev. 2009;20:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Wang SY, Tai GX, Zhang PY, Mu DP, Zhang XJ, Liu ZH. Inhibitory effect of activin A on activation of lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Cytokine. 2008;42:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol. 2006;177:6787-6794. [PubMed] [Cited in This Article: ] |
| 6. | Gold EJ, Zhang X, Wheatley AM, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Fleming JS. betaA- and betaC-activin, follistatin, activin receptor mRNA and betaC-activin peptide expression during rat liver regeneration. J Mol Endocrinol. 2005;34:505-515. [PubMed] [Cited in This Article: ] |
| 7. | Mendis SH, Meachem SJ, Sarraj MA, Loveland KL. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod. 2011;84:379-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton DA. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485-493. [PubMed] [Cited in This Article: ] |
| 9. | Fang L, Wang YN, Cui XL, Fang SY, Ge JY, Sun Y, Liu ZH. The role and mechanism of action of activin A in neurite outgrowth of chicken embryonic dorsal root ganglia. J Cell Sci. 2012;125:1500-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Liu ZH, Shintani Y, Sakamoto Y, Harada K, Zhang CY, Fujinaka Y, Abe M, Goto T, Saito S. Effects of LHRH, FSH and activin A on follistatin secretion from cultured rat anterior pituitary cells. Endocr J. 1996;43:321-327. [PubMed] [Cited in This Article: ] |
| 11. | Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 2006;17:157-171. [PubMed] [Cited in This Article: ] |
| 12. | Date M, Matsuzaki K, Matsushita M, Tahashi Y, Sakitani K, Inoue K. Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury. J Hepatol. 2000;32:251-260. [PubMed] [Cited in This Article: ] |
| 13. | Zhang HJ, Tai GX, Zhou J, Ma D, Liu ZH. Regulation of activin receptor-interacting protein 2 expression in mouse hepatoma Hepa1-6 cells and its relationship with collagen type IV. World J Gastroenterol. 2007;13:5501-5505. [PubMed] [Cited in This Article: ] |
| 14. | Deli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, Schulte-Hermann R, Rodgarkia-Dara C, Grusch M. Activins and activin antagonists in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1699-1709. [PubMed] [Cited in This Article: ] |
| 15. | Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, Tanabe KK. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Gold EJ, Francis RJ, Zimmermann A, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Wheatley AM, Fleming JS. Changes in activin and activin receptor subunit expression in rat liver during the development of CCl4-induced cirrhosis. Mol Cell Endocrinol. 2003;201:143-153. [PubMed] [Cited in This Article: ] |
| 17. | Liu HY, Wang YN, Ge JY, Li N, Cui XL, Liu ZH. Localisation and role of activin receptor-interacting protein 1 in mouse brain. J Neuroendocrinol. 2013;25:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Liu HY, Chen FF, Ge JY, Wang YN, Zhang CH, Cui XL, Yu F, Tai GX, Liu ZH. Expression and localization of activin receptor-interacting protein 2 in mouse tissues. Gen Comp Endocrinol. 2009;161:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Cui X, Tai G, Ge J, Li N, Chen F, Yu F, Liu Z. A critical role of activin A in maturation of mouse peritoneal macrophages in vitro and in vivo. Cell Mol Immunol. 2009;6:387-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | El-Gendi SS, Moniem AE, Tawfik NM, Ashmawy MM, Mohammed OA, Mostafa AK, Zakhari MM, Herdan OM. Value of serum and synovial fluid activin A and inhibin A in some rheumatic diseases. Int J Rheum Dis. 2010;13:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Zhang YQ, Resta S, Jung B, Barrett KE, Sarvetnick N. Upregulation of activin signaling in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G768-G780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Michel U, Ebert S, Phillips D, Nau R. Serum concentrations of activin and follistatin are elevated and run in parallel in patients with septicemia. Eur J Endocrinol. 2003;148:559-564. [PubMed] [Cited in This Article: ] |
| 23. | Thompson TB, Woodruff TK, Jardetzky TS. Structures of an ActRIIB: activin A complex reveal a novel binding mode for TGF-beta ligand: receptor interactions. EMBO J. 2003;22:1555-1566. [PubMed] [Cited in This Article: ] |
| 24. | Allendorph GP, Vale WW, Choe S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc Natl Acad Sci U S A. 2006;103:7643-7648. [PubMed] [Cited in This Article: ] |
| 25. | Tsuchida K, Nakatani M, Matsuzaki T, Yamakawa N, Liu Z, Bao Y, Arai KY, Murakami T, Takehara Y, Kurisaki A. Novel factors in regulation of activin signaling. Mol Cell Endocrinol. 2004;225:1-8. [PubMed] [Cited in This Article: ] |
| 26. | Liu ZH, Tsuchida K, Matsuzaki T, Bao YL, Kurisaki A, Sugino H. Characterization of isoforms of activin receptor-interacting protein 2 that augment activin signaling. J Endocrinol. 2006;189:409-421. [PubMed] [Cited in This Article: ] |
| 27. | Kim HY, Kim JK, Choi JH, Jung JY, Oh WY, Kim DC, Lee HS, Kim YS, Kang SS, Lee SH. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J Pharmacol Sci. 2010;112:105-112. [PubMed] [Cited in This Article: ] |
| 28. | Allman M, Gaskin L, Rivera CA. CCl4-induced hepatic injury in mice fed a Western diet is associated with blunted healing. J Gastroenterol Hepatol. 2010;25:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Ma X, Xu L, Wang S, Chen H, Xu J, Li X, Ning G. Loss of steroid receptor co-activator-3 attenuates carbon tetrachloride-induced murine hepatic injury and fibrosis. Lab Invest. 2009;89:903-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 30. | Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137-G144. [PubMed] [Cited in This Article: ] |