Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3189
Revised: March 20, 2013
Accepted: March 28, 2013
Published online: June 7, 2013
E2F family of transcription factors regulates various cellular functions related to cell cycle and apoptosis. Its individual members have traditionally been classified into activators and repressors, based on in vitro studies. However their contribution in human cancer is more complicated and difficult to predict. We review current knowledge on the expression of E2Fs in digestive system malignancies and its clinical implications for patient prognosis and treatment. E2F1, the most extensively studied member and the only one with prognostic value, exhibits a tumor-suppressing activity in esophageal, gastric and colorectal adenocarcinoma, and in hepatocellular carcinoma (HCC), whereas in pancreatic ductal adenocarcinoma and esophageal squamous cell carcinoma may function as a tumor-promoter. In the latter malignancies, E2F1 immunohistochemical expression has been correlated with higher tumor grade and worse patient survival, whereas in esophageal, gastric and colorectal adenocarcinomas is a marker of increased patient survival. E2F2 has only been studied in colorectal cancer, where its role is not considered significant. E2F4’s role in colorectal, gastric and hepatic carcinogenesis is tumor-promoting. E2F8 is strongly upregulated in human HCC, thus possibly contributing to hepatocarcinogenesis. Adenoviral transfer of E2F as gene therapy to sensitize pancreatic cancer cells for chemotherapeutic agents has been used in experimental studies. Other therapeutic strategies are yet to be developed, but it appears that targeted approaches using E2F-agonists or antagonists should take into account the tissue-dependent function of each E2F member. Further understanding of E2Fs’ contribution in cellular functions in vivo would help clarify their role in carcinogenesis.
Core tip: The E2F family of transcription factors has been in the focus of cancer research because its members regulate significant cellular functions related to cell cycle and apoptosis. E2Fs may act either as tumor-promoters or as tumor-suppressors, depending on the tissue. This review highlights the role of E2Fs in digestive system malignancies and their possible implication in diagnosis, treatment and prognosis.
- Citation: Xanthoulis A, Tiniakos DG. E2F transcription factors and digestive system malignancies: How much do we know? World J Gastroenterol 2013; 19(21): 3189-3198
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3189
The E2F family of transcription factors is involved in a vast number of cellular functions related to cell cycle and apoptosis. The study of E2F began in the mid 1980s when it was identified as a transcription activator of the adenoviral E2 gene promoter[1,2]. The role of the prototype member, E2F1, in cancer was identified in the early 1990s through its ability to bind to and regulate the retinoblastoma protein pRb[3-7] with several members to follow either through their homology to E2F1 or through their association with pRb-related proteins (pocket proteins)[8-16].
Early in vitro studies rose expectations that, through the traditional classification of the E2F family members into activators and repressors, accurate predictions about their contribution in human carcinogenesis could be possible, with oncogenic behavior expected for the former and tumor-suppressing function for the latter group. Nevertheless, in vivo studies have sunk the initial enthusiasm as their role appears to be by far more complicated[17].
E2F1 is the most thoroughly investigated member of the E2F family in human malignancies. Other members have also been studied but in lesser extent. In non-small cell lung carcinoma, increased E2F1 and E2F3 expression has been associated with worse patient prognosis[18-21]. In breast cancer, enhanced E2F1 or E2F4 expression have been proposed as poor prognostic indicators, whereas increased E2F5 expression has been reported in certain histological subtypes[22-26]. In ovarian cancer, E2F1-5, E2F7 and E2F8 expression is reportedly increased. In addition, increased E2F4 and E2F7 expression has been related to better overall or disease-free survival, respectively, whereas that of E2F8 was linked to worse overall survival[27-30]. In prostate cancer, E2F2 and E2F3 expression increases, while E2F1 is absent[25,31]. In urothelial carcinomas of the bladder, E2F3 expression is enhanced, while that of E2F1 depends on the presence of invasion[25,32-37]. Increased E2F1 expression has also been observed in thyroid cancer, small cell lung carcinoma, glioblastoma and lymph node metastases from malignant melanoma[38-42].
The aim of the current review is to summarize the collective knowledge on the role of various E2F family members in digestive system malignancies and to identify possible clinical implications for patients’ diagnosis and prognosis and for future treatment strategy design.
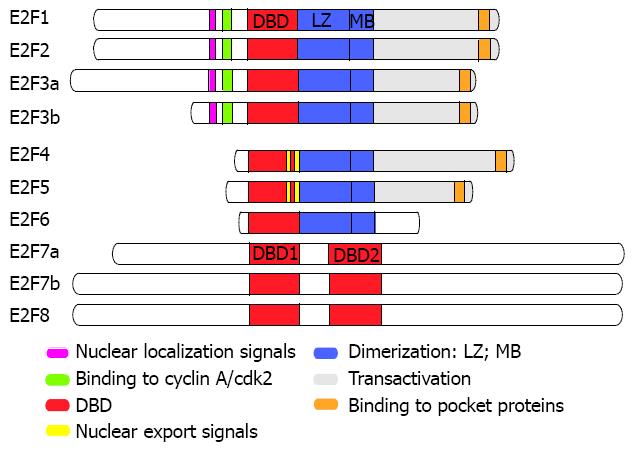
To date, eight members of the mammalian E2F family have been recognized and characterized[36,43], though the most recently identified, namely E2F7[44,45] and E2F8[46,47], bear little homology to their traditional counterparts (Figure 1). Two E2F3 proteins, E2F3a and E2F3b, have been identified, which are produced from the same gene through the use of alternate promoters[48,49]. E2F7 also has two isoforms, E2F7a and E2F7b, which are produced through by alternative splicing of the primary transcript[50].
The greatest homology among the different members of this family is observed in the DNA binding domain. The older members E2F1-6 possess N-terminal DNA binding domain, followed by leucine zipper and marked box domains that mediate heterodimerization. With the exception of E2F6, the established members of the family possess a C-terminal transactivation domain containing the pocket protein binding region[36]. E2F1-3 have nuclear localization signals adjacent to their cyclin A-binding domain. This ensures their movement to the nucleus, thereby modulating E2F activity in a cell cycle-dependent manner[51-53]. E2F1-6 require heterodimerization with one of the dimerization partner (DP) proteins, DP1, DP2 or DP3 in order to form functional transcription factors which can bind DNA with high affinity[54-59]. E2F4 and E2F5 have bipartite nuclear export signals, which mediate their export to the cytoplasmic compartment and they rely on heterodimerization with one of DP proteins for their translocation to the cell nucleus[60,61]. The proteins DP2 and DP3 are thought to be alternatively spliced products of a single gene. The role of DP subunits is not completely elucidated and it appears that the function of E2F-DP heterodimer is dictated by the E2F subunit[56,57].
The transcriptional activity of E2F1-5 is regulated through their binding with the pRb protein or the related pocket proteins p107 and p130[62-64]. E2F1-3 bind to pRb when the latter is found in its hypophosphorylated form[13], E2F4 can bind to any of the pocket proteins, whereas E2F5 associates predominantly with p130[65,66]. Generally, when a pocket protein is hypophosphorylated can associate and block the transcriptional activity of E2F-DP heterodimers by masking the transcription activation domain of E2Fs, rendering them inactive (passive repression)[43]. Furthermore, the pocket protein/E2F-DP complex is guided to E2F binding sites where it can recruit histone deacetylases that suppress transcription by remodelling the nucleosome (active repression)[67-70]. E2F6-8 are presumed to repress E2F-responsive genes independently of pocket proteins[10,11,50,71].
E2F family members have been traditionally classified as activators and repressors. E2F1-3a are often referred to as activators because they transcriptionally activate certain target genes, for example cyclin E. This E2F subclass is expressed in a cell cycle-regulated manner exhibiting highest levels in the late G1 and S phase. In other words, they induce the entrance of quiescent cells in the S phase of cell cycle and overcome arrest mediated by the p16INK4a cyclin-dependent kinase inhibitor[43,72]. E2F3b-5 comprise the repressor subclass because their main function seems to be the repression of transcription of some E2F target genes when they associate with pocket proteins. This subgroup is expressed constitutively, but transcriptional repression by these factors takes place predominantly in cells which are in quiescence and early G1 phase[43]. E2F6-8 are also considered as repressors but they do so, as previously stated, by a manner independent of pocket protein family members[10,11,16,44-46,71]. The abovementioned classification, which is based on results of in vitro studies, is probably oversimplified and does not accurately reflect the dynamics of E2F-dependent transcriptional control[17,43]. The study of E2Fs’ role in vivo has been challenging because of three main obstacles impending complete understanding of their functions. Firstly, there is a high degree of functional redundancy among activators and repressors. Secondly, there is a functional antagonism between E2F-mediated activation and repression in the regulation of normal cell proliferation. Thirdly, the members of this family have the ability to regulate each others expression, forming complex feedback loops to ensure a balance between activators and repressors in each phase of the cell cycle[17].
Amplification of E2F1 is often found in esophageal squamous cell carcinomas and the survival of patients with such aberration is significantly lower than that of patients without it[73]. Furthermore, positive E2F1 immunostaining correlates with histological grade and tumor stage with the overall survival being worse for patients with E2F1-positive tumors[74,75]. E2F1 expression is also shown to be positively associated with cell proliferation but not apoptosis[76]. Interestingly, the sequential transfer of the wild-type p53 and E2F1 genes into esophageal cancer cell lines induces tumor cell apoptosis via E2F1/ARF/MDM2/p53 pathway[77]. It is possible that reduced apoptosis in vivo can be explained by a model suggested in non-small cell lung carcinoma, whereby tumors with deregulated E2F1/pRb network cannot promote p53-dependent apoptosis under conditions of p53 mutation or MDM2 overexpression[19]. The situation is quite the opposite in esophageal adenocarcinomas arising in the background of Barrett esophagus. In such cases, E2F1 immunohistochemical expression exhibits a positive correlation with apoptosis and an inverse relationship with cell proliferation, implying that apoptosis is probably the tumor-suppressive mechanism activated by E2F1. The tumor-suppressing activity of E2F1 in adenocarcinomas of Barrett esophagus could be explained by the intestinal-type nature of the metaplastic mucosa and it may also be dictated by the embryological origin of the lower third of esophagus from the foregut[78].
E2F1 is found to be overexpressed in about 40% of gastric adenocarcinomas, whereas gene amplification of E2F1 rarely occurs[79-81]. Experimental studies have provided evidence that adenovirus-mediated E2F1 overexpression in gastric carcinoma cells induces widespread apoptosis, probably through direct or indirect upregulation of phosphatase and tensin homolog (PTEN) expression, activation of caspase-3 and -9 and decrease in nuclear factor kappa-light-chain-enhancer of activated B cells expression via PI3K/PTEN/Akt signalling pathway, especially when combined with cyclin-dependent kinase inhibitors, such as roscovitine. This points to a tumor-suppressing role of E2F1 in this type of cancer[82-86]. Interestingly, at least one study has demonstrated the opposite effect, i.e., downregulation of E2F1 significantly inhibited the infiltration and proliferation abilities of human gastric cancer cells. This inconsistency was attributed to the difference in activation of E2F1 at different points of the cell cycle to keep a dynamic balance[87]. E2F1 immunoreactivity was shown to independently predict favorable overall survival in gastric adenocarcinoma patients who received adjuvant chemoradiation therapy with 5-fluorouracil and leucovorin after gastrectomy and correlated with localized tumor, intestinal histological subtype and thymidylate synthase expression, supporting its role as a potential biological marker, predictive of clinical outcome in this particular setting[88]. Similar to colorectal adenocarcinomas, microsatellite unstable gastric adenocarcinomas frequently exhibit mutation of the adenosine-guanine-cytosine (AGC) repeat of the E2F4 gene, that probably represents an early event in this context, allowing for additional gene anomalies to accumulate during tumor progression[89-94].
There are a number of studies that investigated the role of E2F family members, especially E2F1, in the context of colorectal cancer. E2F1 expression was indeed found increased in some early studies[81,95]. Later on, increased E2F1 expression was found directly related to increased apoptotic levels and inversely related to cell proliferation, particularly when serial or semiserial sections were analyzed[25,96,97], suggesting a tumor-suppressing role. E2F1 expression is higher in lung metastasis of colon adenocarcinoma and also correlates closely with the expression of thymidylate synthase in both the primary tumor and metastases, indicating worse response to 5-fluorouracil due to resistance[98,99].
On the other hand, E2F4 has been directly associated with cell proliferation in colorectal cancer, suggesting a tumor-promoting role[97,100]. This finding is in agreement with experimental observations in cell line and animal models demonstrating increased nuclear expression of E2F4 in the replicating colon epithelium[101-104]. Of interest, the immunohistochemical expression of E2F1 was inversely correlated with that of E2F4 when studied in serial histological sections, suggesting a possible mechanistic interlink between the two family members that has yet to be identified[97]. An other interesting observation is that E2F4 contains a stretch of 13 serine residues in its trans-activation domain encoded by a microsatellite trinucleotide AGC repeat within the E2F4 gene. This repeat is often found mutated in various gastrointestinal tumors, including human colorectal cancer with microsatellite instability and it is believed to be one of the targets of DNA mismatch repair deficiency[93,105-107].
E2F2 has only recently been investigated in colorectal adenocarcinoma. Its expression at the tissue level was found to be very low without any relationship to kinetic parameters, leading to the hypothesis that E2F2 expression does not contribute in colorectal carcinogenesis but rather reflects the functional redundancy between E2F members of the same subgroup[97].
In a recent study, nuclear immunohistochemical expression for E2F1 was positively related to tumor apoptotic index in a series of human hepatocellular carcinomas, supporting a pro-apoptotic role of E2F1 in this type of cancer[108]. Interestingly, studies investigating HCC-cell lines or mouse models have provided evidence pointing towards a tumor-promoting role of E2F1 by demonstrating that its overexpression led to increased expression of upstream cell proliferation or anti-apoptotic genes[109-112]. Other investigators have demonstrated that, in a transgenic mouse model, endogenous c-myc was upregulated in the early stages of hepatocarcinogenesis, whereas p53 was overexpressed in the tumors, suggesting that both E2F1-mediated proliferation and apoptosis are operative but at different stages of hepatocarcinogenesis[113].
E2F3 and E2F4 are also shown to be upregulated in HCC[114-116]. Of note, HCCs exhibiting microsatellite instability has shown deletions in AGC triplet repeats in the coding region of the E2F4 gene in a similar manner as demonstrated for microsatellite unstable colorectal cancer[93,105-107,117]. These results suggest that both microsatellite instability and mutations of E2F4 commonly occur in HCC and may play an important role in hepatocarcinogenesis[117].
Lastly, it has been demonstrated that E2F8 is strongly upregulated in human HCC and it could thus contribute to oncogenesis and progression in this type of cancer. Mechanistic analyses indicated that E2F8 could bind to regulatory elements of cyclin D1, regulating its transcription and promoting accumulation of S-phase cells[118].
E2F1 may have a tumor-promoting role in pancreatic ductal adenocarcinoma. A direct correlation has been found between E2F1 immunohistochemical expression and cell proliferation index, as well as an inverse relationship between E2F1 immunopositivity and histological grade and disease-associated survival[119]. Interestingly, stable overexpression of E2F1 and decreased pRb expression resulting in the liberation of E2F in pancreatic cancer cell lines may be responsible for the demonstrated increase in chemotherapy-induced apoptosis[120]. Moreover, infection of pancreatic cancer cells lines by E2F1-expressing adenoviral vector has been shown to increase gemcitabine-induced apoptosis as well as etoposide- or roscovitine-induced apoptosis[121,122].
Deregulated expression of E2F family of transcription factors is a common phenomenon in human cancer. Little is known though about the magnitude and the nature of their contribution. The prevailing view is that E2F activators and repressors operate in a coordinated manner to achieve proper cell cycle progression and/or apoptosis and that disturbance of this well orchestrated interaction can contribute to carcinogenesis. According to the traditional classification of the different E2F members into activators and repressors, it would be expected that clear predictions about their function in cancer could be made. Unfortunately, this is not the case and this classical approach stemming from in vitro studies is openly challenged in practice[17,43]. To complicate things further, numerous E2F target genes have been identified reflecting the fact that E2Fs participate in cellular processes beyond the cell cycle[123-127].
Regarding digestive system malignancies, E2F1 may exhibit a tumor-suppressing role in colorectal, gastric and esophageal adenocarcinoma, as well as in hepatocellular carcinoma, probably through pro-apoptotic activity[25,78,82-86,96,97,108]. On the contrary, a tumor-promoting role has been attributed to E2F1 in the context of pancreatic ductal adenocarcinoma and esophageal squamous carcinoma[73-76,119]. It is very interesting though that in experimental models involving adenoviral transfer of E2F1 in esophageal squamous and pancreatic cancer cell lines apoptosis was evoked[77,121,122]. The possible role, correlations and prognostic significance of E2F1 expression in digestive system malignancies is summarized in Table 1.
| Tumor | Role | Clinicopathological relationships | Prognostic significance | Ref. |
| Esophageal | ||||
| Squamous cell carcinoma | Tumor-promoting | ↑ tumor stage, ↑ histological grade, ↓ overall survival | Yes | [74,75] |
| Adenocarcinoma | Tumor-suppressing | ↑ survival | Yes | [78] |
| Gastric adenocarcinoma | Tumor-suppressing | Localized disease, intestinal histological type, ↑ overall survival | Yes | [88] |
| Colorectal adenocarcinoma | Tumor-suppressing | ↑ survival | Yes | [25,96] |
| Hepatocellular carcinoma | Tumor-suppressing | No | [108] | |
| Pancreatic ductal adenocarcinoma | Tumor-promoting | ↑ histological grade, ↓ disease-free survival | Yes | [119] |
E2F4, a classical “repressor”, seems to play a tumor-promoting role in colorectal, gastric and liver carcinogenesis[90,92,93,97,117]. It is also well documented that AGC repeats in the E2F4 gene are commonly found mutated in various gastrointestinal malignant neoplasms exhibiting microsatellite instability and they are thought to represent one of the targets of DNA mismatch repair deficiency[89-94,105-107,117]. Of note, in colorectal cancer, an inverse relationship between the immunohistochemical expression of E2F1 and E2F4 has been demonstrated when examined in serial histological sections[97]. It would be interesting to investigate if such a relationship can be confirmed in other gastrointestinal cancers as well and, if so, whether there is a common “switch” connecting mechanistically these two opposing transcription factors.
E2F family of transcription factors serves key roles in cell cycle progression, apoptosis, cell differentiation and stress responses. Following their traditional classification into activators and repressors, it has been tempting to assign to them oncogenic or tumor-suppressing functions, thus predicting their role in carcinogenesis. A number of investigators have focused into showing a prognostic value of E2F expression in different kind of digestive tract malignancies[25,74-76,78,88,96,119]. Others have gone a step further and, based on their experimental work, suggest therapeutic strategies involving adenoviral transfer of E2F in a gene therapy context to sensitize cancer cells for conventional chemotherapeutic agents[82,121,122].
However, clinical and experimental studies in mice openly challenge this traditional view, which does not appear to reflect the complexity of E2F function in tumorigenesis. It appears, though, that this function is exerted in a tissue-dependent manner. It is appealing to consider the development of treatment strategies involving E2F-antagonists, that would suppress cell proliferation, and E2F-agonists, that would promote apoptosis[128]. Nevertheless, targeted therapeutic approaches against E2F family members should take into account the tissue-dependent function of each member.
It is quite obvious that, although our knowledge on this intriguing family of transcription factors is seemingly increasing, little is known about their function in vivo. The introduction and use of modern molecular techniques and experimental models have identified numerous targets for these factors, helping us unravel the mystery of their contribution in normal tissues. This can be the necessary step in order to clarify to which extent they exert pivotal roles in cancer development[17].
The authors would like to thank Mrs. Maria Chatzopoulou for her kind contribution in creating Figure 1.
P- Reviewer Lalli E S- Editor Huang XZ L- Editor A E- Editor Li JY
| 1. | Kovesdi I, Reichel R, Nevins JR. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986;45:219-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 458] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | La Thangue NB, Rigby PW. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987;49:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 126] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Bandara LR, La Thangue NB. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991;351:494-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 345] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 958] [Cited by in F6Publishing: 1018] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 5. | Fattaey AR, Helin K, Dembski MS, Dyson N, Harlow E, Vuocolo GA, Hanobik MG, Haskell KM, Oliff A, Defeo-Jones D. Characterization of the retinoblastoma binding proteins RBP1 and RBP2. Oncogene. 1993;8:3149-3156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 536] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Kaelin WG, Krek W, Sellers WR, DeCaprio JA, Ajchenbaum F, Fuchs CS, Chittenden T, Li Y, Farnham PJ, Blanar MA. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 730] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Shan B, Zhu X, Chen PL, Durfee T, Yang Y, Sharp D, Lee WH. Molecular cloning of cellular genes encoding retinoblastoma-associated proteins: identification of a gene with properties of the transcription factor E2F. Mol Cell Biol. 1992;12:5620-5631. [PubMed] [Cited in This Article: ] |
| 8. | Beijersbergen RL, Kerkhoven RM, Zhu L, Carlée L, Voorhoeve PM, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680-2690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 271] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Buck V, Allen KE, Sørensen T, Bybee A, Hijmans EM, Voorhoeve PM, Bernards R, La Thangue NB. Molecular and functional characterisation of E2F-5, a new member of the E2F family. Oncogene. 1995;11:31-38. [PubMed] [Cited in This Article: ] |
| 10. | Cartwright P, Müller H, Wagener C, Holm K, Helin K. E2F-6: a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene. 1998;17:611-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Gaubatz S, Wood JG, Livingston DM. Unusual proliferation arrest and transcriptional control properties of a newly discovered E2F family member, E2F-6. Proc Natl Acad Sci USA. 1998;95:9190-9195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Ginsberg D, Vairo G, Chittenden T, Xiao ZX, Xu G, Wydner KL, DeCaprio JA, Lawrence JB, Livingston DM. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994;8:2665-2679. [PubMed] [Cited in This Article: ] |
| 13. | Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13:7813-7825. [PubMed] [Cited in This Article: ] |
| 14. | Morkel M, Wenkel J, Bannister AJ, Kouzarides T, Hagemeier C. An E2F-like repressor of transcription. Nature. 1997;390:567-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Sardet C, Vidal M, Cobrinik D, Geng Y, Onufryk C, Chen A, Weinberg RA. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci USA. 1995;92:2403-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 254] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Trimarchi JM, Fairchild B, Verona R, Moberg K, Andon N, Lees JA. E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc Natl Acad Sci USA. 1998;95:2850-2855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 172] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 729] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 18. | Borczuk AC, Gorenstein L, Walter KL, Assaad AA, Wang L, Powell CA. Non-small-cell lung cancer molecular signatures recapitulate lung developmental pathways. Am J Pathol. 2003;163:1949-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Gorgoulis VG, Zacharatos P, Mariatos G, Kotsinas A, Bouda M, Kletsas D, Asimacopoulos PJ, Agnantis N, Kittas C, Papavassiliou AG. Transcription factor E2F-1 acts as a growth-promoting factor and is associated with adverse prognosis in non-small cell lung carcinomas. J Pathol. 2002;198:142-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Imai MA, Oda Y, Oda M, Nakanishi I, Kawahara E. Overexpression of E2F1 associated with LOH at RB locus and hyperphosphorylation of RB in non-small cell lung carcinoma. J Cancer Res Clin Oncol. 2004;130:320-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Karakaidos P, Taraviras S, Vassiliou LV, Zacharatos P, Kastrinakis NG, Kougiou D, Kouloukoussa M, Nishitani H, Papavassiliou AG, Lygerou Z. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability--evidence of E2F-1 transcriptional control over hCdt1. Am J Pathol. 2004;165:1351-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, Kim HY. E2F1 expression is related with the poor survival of lymph node-positive breast cancer patients treated with fluorouracil, doxorubicin and cyclophosphamide. Breast Cancer Res Treat. 2003;82:11-16. [PubMed] [Cited in This Article: ] |
| 23. | Polanowska J, Le Cam L, Orsetti B, Vallés H, Fabbrizio E, Fajas L, Taviaux S, Theillet C, Sardet C. Human E2F5 gene is oncogenic in primary rodent cells and is amplified in human breast tumors. Genes Chromosomes Cancer. 2000;28:126-130. [PubMed] [Cited in This Article: ] |
| 24. | Rakha EA, Pinder SE, Paish EC, Robertson JF, Ellis IO. Expression of E2F-4 in invasive breast carcinomas is associated with poor prognosis. J Pathol. 2004;203:754-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Zacharatos P, Kotsinas A, Evangelou K, Karakaidos P, Vassiliou LV, Rezaei N, Kyroudi A, Kittas C, Patsouris E, Papavassiliou AG. Distinct expression patterns of the transcription factor E2F-1 in relation to tumour growth parameters in common human carcinomas. J Pathol. 2004;203:744-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Zhang SY, Liu SC, Al-Saleem LF, Holloran D, Babb J, Guo X, Klein-Szanto AJ. E2F-1: a proliferative marker of breast neoplasia. Cancer Epidemiol Biomarkers Prev. 2000;9:395-401. [PubMed] [Cited in This Article: ] |
| 27. | De Meyer T, Bijsmans IT, Van de Vijver KK, Bekaert S, Oosting J, Van Criekinge W, van Engeland M, Sieben NL. E2Fs mediate a fundamental cell-cycle deregulation in high-grade serous ovarian carcinomas. J Pathol. 2009;217:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Reimer D, Sadr S, Wiedemair A, Goebel G, Concin N, Hofstetter G, Marth C, Zeimet AG. Expression of the E2F family of transcription factors and its clinical relevance in ovarian cancer. Ann N Y Acad Sci. 2006;1091:270-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Reimer D, Sadr S, Wiedemair A, Stadlmann S, Concin N, Hofstetter G, Müller-Holzner E, Marth C, Zeimet AG. Clinical relevance of E2F family members in ovarian cancer--an evaluation in a training set of 77 patients. Clin Cancer Res. 2007;13:144-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Lu KH, Patterson AP, Wang L, Marquez RT, Atkinson EN, Baggerly KA, Ramoth LR, Rosen DG, Liu J, Hellstrom I. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291-3300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Foster CS, Falconer A, Dodson AR, Norman AR, Dennis N, Fletcher A, Southgate C, Dowe A, Dearnaley D, Jhavar S. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871-5879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Baithun SI, Naase M, Blanes A, Diaz-Cano SJ. Molecular and kinetic features of transitional cell carcinomas of the bladder: biological and clinical implications. Virchows Arch. 2001;438:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Feber A, Clark J, Goodwin G, Dodson AR, Smith PH, Fletcher A, Edwards S, Flohr P, Falconer A, Roe T. Amplification and overexpression of E2F3 in human bladder cancer. Oncogene. 2004;23:1627-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Oeggerli M, Tomovska S, Schraml P, Calvano-Forte D, Schafroth S, Simon R, Gasser T, Mihatsch MJ, Sauter G. E2F3 amplification and overexpression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer. Oncogene. 2004;23:5616-5623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Rabbani F, Richon VM, Orlow I, Lu ML, Drobnjak M, Dudas M, Charytonowicz E, Dalbagni G, Cordon-Cardo C. Prognostic significance of transcription factor E2F-1 in bladder cancer: genotypic and phenotypic characterization. J Natl Cancer Inst. 1999;91:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Tsantoulis PK, Gorgoulis VG. Involvement of E2F transcription factor family in cancer. Eur J Cancer. 2005;41:2403-2414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Veltman JA, Fridlyand J, Pejavar S, Olshen AB, Korkola JE, DeVries S, Carroll P, Kuo WL, Pinkel D, Albertson D. Array-based comparative genomic hybridization for genome-wide screening of DNA copy number in bladder tumors. Cancer Res. 2003;63:2872-2880. [PubMed] [Cited in This Article: ] |
| 38. | Alonso MM, Fueyo J, Shay JW, Aldape KD, Jiang H, Lee OH, Johnson DG, Xu J, Kondo Y, Kanzawa T. Expression of transcription factor E2F1 and telomerase in glioblastomas: mechanistic linkage and prognostic significance. J Natl Cancer Inst. 2005;97:1589-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Eymin B, Gazzeri S, Brambilla C, Brambilla E. Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinoma. Oncogene. 2001;20:1678-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Nelson MA, Reynolds SH, Rao UN, Goulet AC, Feng Y, Beas A, Honchak B, Averill J, Lowry DT, Senft JR. Increased gene copy number of the transcription factor E2F1 in malignant melanoma. Cancer Biol Ther. 2006;5:407-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Onda M, Nagai H, Yoshida A, Miyamoto S, Asaka S, Akaishi J, Takatsu K, Nagahama M, Ito K, Shimizu K. Up-regulation of transcriptional factor E2F1 in papillary and anaplastic thyroid cancers. J Hum Genet. 2004;49:312-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Saiz AD, Olvera M, Rezk S, Florentine BA, McCourty A, Brynes RK. Immunohistochemical expression of cyclin D1, E2F-1, and Ki-67 in benign and malignant thyroid lesions. J Pathol. 2002;198:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739-748. [PubMed] [Cited in This Article: ] |
| 44. | de Bruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2003;278:42041-42049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Logan N, Delavaine L, Graham A, Reilly C, Wilson J, Brummelkamp TR, Hijmans EM, Bernards R, La Thangue NB. E2F-7: a distinctive E2F family member with an unusual organization of DNA-binding domains. Oncogene. 2004;23:5138-5150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Logan N, Graham A, Zhao X, Fisher R, Maiti B, Leone G, La Thangue NB. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene. 2005;24:5000-5004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2005;280:18211-18220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | He Y, Armanious MK, Thomas MJ, Cress WD. Identification of E2F-3B, an alternative form of E2F-3 lacking a conserved N-terminal region. Oncogene. 2000;19:3422-3433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Leone G, Nuckolls F, Ishida S, Adams M, Sears R, Jakoi L, Miron A, Nevins JR. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol Cell Biol. 2000;20:3626-3632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Di Stefano L, Jensen MR, Helin K. E2F7, a novel E2F featuring DP-independent repression of a subset of E2F-regulated genes. EMBO J. 2003;22:6289-6298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Magae J, Wu CL, Illenye S, Harlow E, Heintz NH. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J Cell Sci. 1996;109:1717-1726. [PubMed] [Cited in This Article: ] |
| 52. | Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1020] [Cited by in F6Publishing: 988] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 53. | Müller H, Moroni MC, Vigo E, Petersen BO, Bartek J, Helin K. Induction of S-phase entry by E2F transcription factors depends on their nuclear localization. Mol Cell Biol. 1997;17:5508-5520. [PubMed] [Cited in This Article: ] |
| 54. | Bandara LR, Buck VM, Zamanian M, Johnston LH, La Thangue NB. Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. EMBO J. 1993;12:4317-4324. [PubMed] [Cited in This Article: ] |
| 55. | Milton A, Luoto K, Ingram L, Munro S, Logan N, Graham AL, Brummelkamp TR, Hijmans EM, Bernards R, La Thangue NB. A functionally distinct member of the DP family of E2F subunits. Oncogene. 2006;25:3212-3218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Ormondroyd E, de la Luna S, La Thangue NB. A new member of the DP family, DP-3, with distinct protein products suggests a regulatory role for alternative splicing in the cell cycle transcription factor DRTF1/E2F. Oncogene. 1995;11:1437-1446. [PubMed] [Cited in This Article: ] |
| 57. | Rogers KT, Higgins PD, Milla MM, Phillips RS, Horowitz JM. DP-2, a heterodimeric partner of E2F: identification and characterization of DP-2 proteins expressed in vivo. Proc Natl Acad Sci USA. 1996;93:7594-7599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA. In vivo association of E2F and DP family proteins. Mol Cell Biol. 1995;15:2536-2546. [PubMed] [Cited in This Article: ] |
| 59. | Zhang Y, Chellappan SP. Cloning and characterization of human DP2, a novel dimerization partner of E2F. Oncogene. 1995;10:2085-2093. [PubMed] [Cited in This Article: ] |
| 60. | Gaubatz S, Lees JA, Lindeman GJ, Livingston DM. E2F4 is exported from the nucleus in a CRM1-dependent manner. Mol Cell Biol. 2001;21:1384-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Verona R, Moberg K, Estes S, Starz M, Vernon JP, Lees JA. E2F activity is regulated by cell cycle-dependent changes in subcellular localization. Mol Cell Biol. 1997;17:7268-7282. [PubMed] [Cited in This Article: ] |
| 62. | Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 499] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 63. | Cobrinik D, Whyte P, Peeper DS, Jacks T, Weinberg RA. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 64. | Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 425] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 65. | Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245-2262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1709] [Cited by in F6Publishing: 1704] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 66. | Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 67. | Ferreira R, Magnaghi-Jaulin L, Robin P, Harel-Bellan A, Trouche D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc Natl Acad Sci USA. 1998;95:10493-10498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Ferreira R, Naguibneva I, Mathieu M, Ait-Si-Ali S, Robin P, Pritchard LL, Harel-Bellan A. Cell cycle-dependent recruitment of HDAC-1 correlates with deacetylation of histone H4 on an Rb-E2F target promoter. EMBO Rep. 2001;2:794-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 689] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 70. | Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat Genet. 2000;25:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 679] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 71. | Christensen J, Cloos P, Toftegaard U, Klinkenberg D, Bracken AP, Trinh E, Heeran M, Di Stefano L, Helin K. Characterization of E2F8, a novel E2F-like cell-cycle regulated repressor of E2F-activated transcription. Nucleic Acids Res. 2005;33:5458-5470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Lukas J, Petersen BO, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047-1057. [PubMed] [Cited in This Article: ] |
| 73. | Fujita Y, Sakakura C, Shimomura K, Nakanishi M, Yasuoka R, Aragane H, Hagiwara A, Abe T, Inazawa J, Yamagishi H. Chromosome arm 20q gains and other genomic alterations in esophageal squamous cell carcinoma, as analyzed by comparative genomic hybridization and fluorescence in situ hybridization. Hepatogastroenterology. 2003;50:1857-1863. [PubMed] [Cited in This Article: ] |
| 74. | Ebihara Y, Miyamoto M, Shichinohe T, Kawarada Y, Cho Y, Fukunaga A, Murakami S, Uehara H, Kaneko H, Hashimoto H. Over-expression of E2F-1 in esophageal squamous cell carcinoma correlates with tumor progression. Dis Esophagus. 2004;17:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Mega S, Miyamoto M, Ebihara Y, Takahashi R, Hase R, Li L, Shichinohe T, Kawarada Y, Uehara H, Kaneko H. Cyclin D1, E2F1 expression levels are associated with characteristics and prognosis of esophageal squamous cell carcinoma. Dis Esophagus. 2005;18:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Yamazaki K, Hasegawa M, Ohoka I, Hanami K, Asoh A, Nagao T, Sugano I, Ishida Y. Increased E2F-1 expression via tumour cell proliferation and decreased apoptosis are correlated with adverse prognosis in patients with squamous cell carcinoma of the oesophagus. J Clin Pathol. 2005;58:904-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Itoshima T, Fujiwara T, Waku T, Shao J, Kataoka M, Yarbrough WG, Liu TJ, Roth JA, Tanaka N, Kodama M. Induction of apoptosis in human esophageal cancer cells by sequential transfer of the wild-type p53 and E2F-1 genes: involvement of p53 accumulation via ARF-mediated MDM2 down-regulation. Clin Cancer Res. 2000;6:2851-2859. [PubMed] [Cited in This Article: ] |
| 78. | Evangelou K, Kotsinas A, Mariolis-Sapsakos T, Giannopoulos A, Tsantoulis PK, Constantinides C, Troupis TG, Salmas M, Kyroudis A, Kittas C. E2F-1 overexpression correlates with decreased proliferation and better prognosis in adenocarcinomas of Barrett oesophagus. J Clin Pathol. 2008;61:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Yasui W, Naka K, Suzuki T, Fujimoto J, Hayashi K, Matsutani N, Yokozaki H, Tahara E. Expression of p27Kip1, cyclin E and E2F-1 in primary and metastatic tumors of gastric carcinoma. Oncol Rep. 1999;6:983-987. [PubMed] [Cited in This Article: ] |
| 80. | Yasui W, Yokozaki H, Fujimoto J, Naka K, Kuniyasu H, Tahara E. Genetic and epigenetic alterations in multistep carcinogenesis of the stomach. J Gastroenterol. 2000;35 Suppl 12:111-115. [PubMed] [Cited in This Article: ] |
| 81. | Suzuki T, Yasui W, Yokozaki H, Naka K, Ishikawa T, Tahara E. Expression of the E2F family in human gastrointestinal carcinomas. Int J Cancer. 1999;81:535-538. [PubMed] [Cited in This Article: ] |
| 82. | Atienza C, Elliott MJ, Dong YB, Yang HL, Stilwell A, Liu TJ, McMasters KM. Adenovirus-mediated E2F-1 gene transfer induces an apoptotic response in human gastric carcinoma cells that is enhanced by cyclin dependent kinase inhibitors. Int J Mol Med. 2000;6:55-63. [PubMed] [Cited in This Article: ] |
| 83. | Xiao Q, Li L, Xie Y, Tan N, Wang C, Xu J, Xia K, Gardner K, Li QQ. Transcription factor E2F-1 is upregulated in human gastric cancer tissues and its overexpression suppresses gastric tumor cell proliferation. Cell Oncol. 2007;29:335-349. [PubMed] [Cited in This Article: ] |
| 84. | Xie Y, Wang C, Li L, Ma Y, Yin Y, Xiao Q. Overexpression of E2F-1 inhibits progression of gastric cancer in vitro. Cell Biol Int. 2009;33:640-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Xie Y, Yin Y, Li L, Ma Y, Xiao Q. Short interfering RNA directed against the E2F-1 gene suppressing gastric cancer progression in vitro. Oncol Rep. 2009;21:1345-1353. [PubMed] [Cited in This Article: ] |
| 86. | Yan LH, Li L, Xie YB, Xiao Q, Wang CQ. Effects of E2F-1 overexpression on apoptosis of gastric cancer cells and expressions of apoptosis-related genes. Aizheng. 2009;28:1176-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 87. | Yin YS, Xiao Q, Xie YB, Li L, Wang CQ, Ma YL, Tang ZY. Inhibitory effects of transcription factor E2F-1 siRNA on invasion and proliferation of gastric cancer cell line MGC803. Aizheng. 2008;27:914-918. [PubMed] [Cited in This Article: ] |
| 88. | Lee J, Park CK, Park JO, Lim T, Park YS, Lim HY, Lee I, Sohn TS, Noh JH, Heo JS. Impact of E2F-1 expression on clinical outcome of gastric adenocarcinoma patients with adjuvant chemoradiation therapy. Clin Cancer Res. 2008;14:82-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Chung YJ, Kim KM, Choi JR, Choi SW, Rhyu MG. Relationship between intratumor histological heterogeneity and genetic abnormalities in gastric carcinoma with microsatellite instability. Int J Cancer. 1999;82:782-788. [PubMed] [Cited in This Article: ] |
| 90. | Kim JJ, Baek MJ, Kim L, Kim NG, Lee YC, Song SY, Noh SH, Kim H. Accumulated frameshift mutations at coding nucleotide repeats during the progression of gastric carcinoma with microsatellite instability. Lab Invest. 1999;79:1113-1120. [PubMed] [Cited in This Article: ] |
| 91. | Ogata S, Tamura G, Endoh Y, Sakata K, Ohmura K, Motoyama T. Microsatellite alterations and target gene mutations in the early stages of multiple gastric cancer. J Pathol. 2001;194:334-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | Schwemmle S, Pfeifer GP. Genomic structure and mutation screening of the E2F4 gene in human tumors. Int J Cancer. 2000;86:672-677. [PubMed] [Cited in This Article: ] |
| 93. | Souza RF, Yin J, Smolinski KN, Zou TT, Wang S, Shi YQ, Rhyu MG, Cottrell J, Abraham JM, Biden K. Frequent mutation of the E2F-4 cell cycle gene in primary human gastrointestinal tumors. Cancer Res. 1997;57:2350-2353. [PubMed] [Cited in This Article: ] |
| 94. | Woo DK, Lee WA, Kim YI, Kim WH. Microsatellite instability and alteration of E2F-4 gene in adenosquamous and squamous cell carcinomas of the stomach. Pathol Int. 2000;50:690-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Yasui W, Fujimoto J, Suzuki T, Ono S, Naka K, Yokozaki H, Tahara E. Expression of cell-cycle-regulating transcription factor E2F-1 in colorectal carcinomas. Pathobiology. 1999;67:174-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Bramis J, Zacharatos P, Papaconstantinou I, Kotsinas A, Sigala F, Korkolis DP, Nikiteas N, Pazaiti A, Kittas C, Bastounis E. E2F-1 transcription factor immunoexpression is inversely associated with tumor growth in colon adenocarcinomas. Anticancer Res. 2004;24:3041-3047. [PubMed] [Cited in This Article: ] |
| 97. | Xanthoulis A, Kotsinas A, Tiniakos D, Fiska A, Tentes AA, Kyroudi A, Kittas C, Gorgoulis V. The Relationship Between E2F Family Members and Tumor Growth in Colorectal Adenocarcinomas: A Comparative Immunohistochemical Study of 100 Cases. Appl Immunohistochem Mol Morphol. 2012;Jun 7; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Banerjee D, Gorlick R, Liefshitz A, Danenberg K, Danenberg PC, Danenberg PV, Klimstra D, Jhanwar S, Cordon-Cardo C, Fong Y. Levels of E2F-1 expression are higher in lung metastasis of colon cancer as compared with hepatic metastasis and correlate with levels of thymidylate synthase. Cancer Res. 2000;60:2365-2367. [PubMed] [Cited in This Article: ] |
| 99. | Kasahara M, Takahashi Y, Nagata T, Asai S, Eguchi T, Ishii Y, Fujii M, Ishikawa K. Thymidylate synthase expression correlates closely with E2F1 expression in colon cancer. Clin Cancer Res. 2000;6:2707-2711. [PubMed] [Cited in This Article: ] |
| 100. | Mady HH, Hasso S, Melhem MF. Expression of E2F-4 gene in colorectal adenocarcinoma and corresponding covering mucosa: an immunohistochemistry, image analysis, and immunoblot study. Appl Immunohistochem Mol Morphol. 2002;10:225-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Dagnino L, Fry CJ, Bartley SM, Farnham P, Gallie BL, Phillips RA. Expression patterns of the E2F family of transcription factors during murine epithelial development. Cell Growth Differ. 1997;8:553-563. [PubMed] [Cited in This Article: ] |
| 102. | Deschênes C, Alvarez L, Lizotte ME, Vézina A, Rivard N. The nucleocytoplasmic shuttling of E2F4 is involved in the regulation of human intestinal epithelial cell proliferation and differentiation. J Cell Physiol. 2004;199:262-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Garneau H, Alvarez L, Paquin MC, Lussier C, Rancourt C, Tremblay E, Beaulieu JF, Rivard N. Nuclear expression of E2F4 induces cell death via multiple pathways in normal human intestinal epithelial crypt cells but not in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G758-G772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Garneau H, Paquin MC, Carrier JC, Rivard N. E2F4 expression is required for cell cycle progression of normal intestinal crypt cells and colorectal cancer cells. J Cell Physiol. 2009;221:350-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Ikeda M, Orimo H, Moriyama H, Nakajima E, Matsubara N, Mibu R, Tanaka N, Shimada T, Kimura A, Shimizu K. Close correlation between mutations of E2F4 and hMSH3 genes in colorectal cancers with microsatellite instability. Cancer Res. 1998;58:594-598. [PubMed] [Cited in This Article: ] |
| 106. | Yoshitaka T, Matsubara N, Ikeda M, Tanino M, Hanafusa H, Tanaka N, Shimizu K. Mutations of E2F-4 trinucleotide repeats in colorectal cancer with microsatellite instability. Biochem Biophys Res Commun. 1996;227:553-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Zhong X, Hemmi H, Koike J, Tsujita K, Shimatake H. Various AGC repeat numbers in the coding region of the human transcription factor gene E2F-4. Hum Mutat. 2000;15:296-297. [PubMed] [Cited in This Article: ] |
| 108. | Palaiologou M, Koskinas J, Karanikolas M, Fatourou E, Tiniakos DG. E2F-1 is overexpressed and pro-apoptotic in human hepatocellular carcinoma. Virchows Arch. 2012;460:439-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Chen YL, Uen YH, Li CF, Horng KC, Chen LR, Wu WR, Tseng HY, Huang HY, Wu LC, Shiue YL. The E2F Transcription Factor 1 Transactives Stathmin 1 in Hepatocellular Carcinoma. Ann Surg Oncol. 2012;Aug 22; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 110. | Simile MM, De Miglio MR, Muroni MR, Frau M, Asara G, Serra S, Muntoni MD, Seddaiu MA, Daino L, Feo F. Down-regulation of c-myc and Cyclin D1 genes by antisense oligodeoxy nucleotides inhibits the expression of E2F1 and in vitro growth of HepG2 and Morris 5123 liver cancer cells. Carcinogenesis. 2004;25:333-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 111. | Wang C, Xiao Y, Hu Z, Chen Y, Liu N, Hu G. PEG10 directly regulated by E2Fs might have a role in the development of hepatocellular carcinoma. FEBS Lett. 2008;582:2793-2798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Pascale RM, Simile MM, De Miglio MR, Muroni MR, Calvisi DF, Asara G, Casabona D, Frau M, Seddaiu MA, Feo F. Cell cycle deregulation in liver lesions of rats with and without genetic predisposition to hepatocarcinogenesis. Hepatology. 2002;35:1341-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 113. | Conner EA, Lemmer ER, Omori M, Wirth PJ, Factor VM, Thorgeirsson SS. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene. 2000;19:5054-5062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | Liu LX, Jiang HC, Liu ZH, Zhu AL, Zhang WH, Wu LF, Zhou J, Wang XQ, Wu M. Expression of cell cycle/growth regulator genes in human hepatocellular carcinoma and adjacent normal liver tissues. Oncol Rep. 2003;10:1771-1775. [PubMed] [Cited in This Article: ] |
| 115. | Liu LX, Jiang HC, Liu ZH, Zhu AL, Zhou J, Zhang WH, Wang XQ, Wu M. Gene expression profiles of hepatoma cell line BEL-7402. Hepatogastroenterology. 2003;50:1496-1501. [PubMed] [Cited in This Article: ] |
| 116. | Liu LX, Liu ZH, Jiang HC, Zhang WH, Qi SY, Hu J, Wang XQ, Wu M. Gene expression profiles of hepatoma cell line HLE. World J Gastroenterol. 2003;9:683-687. [PubMed] [Cited in This Article: ] |
| 117. | Park YM, Choi JY, Bae SH, Byun BH, Ahn BM, Kim BS, Shin DY. Microsatellite instability and mutations of E2F-4 in hepatocellular carcinoma from Korea. Hepatol Res. 2000;17:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Deng Q, Wang Q, Zong WY, Zheng DL, Wen YX, Wang KS, Teng XM, Zhang X, Huang J, Han ZG. E2F8 contributes to human hepatocellular carcinoma via regulating cell proliferation. Cancer Res. 2010;70:782-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Yamazaki K, Yajima T, Nagao T, Shinkawa H, Kondo F, Hanami K, Asoh A, Sugano I, Ishida Y. Expression of transcription factor E2F-1 in pancreatic ductal carcinoma: an immunohistochemical study. Pathol Res Pract. 2003;199:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Plath T, Peters M, Detjen K, Welzel M, von Marschall Z, Radke C, Wiedenmann B, Rosewicz S. Overexpression of pRB in human pancreatic carcinoma cells: function in chemotherapy-induced apoptosis. J Natl Cancer Inst. 2002;94:129-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Elliott MJ, Farmer MR, Atienza C, Stilwell A, Dong YB, Yang HL, Wong SL, McMasters KM. E2F-1 gene therapy induces apoptosis and increases chemosensitivity in human pancreatic carcinoma cells. Tumour Biol. 2002;23:76-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 122. | Rödicker F, Stiewe T, Zimmermann S, Pützer BM. Therapeutic efficacy of E2F1 in pancreatic cancer correlates with TP73 induction. Cancer Res. 2001;61:7052-7055. [PubMed] [Cited in This Article: ] |
| 123. | Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684-4699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 478] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 124. | Ma Y, Croxton R, Moorer RL, Cress WD. Identification of novel E2F1-regulated genes by microarray. Arch Biochem Biophys. 2002;399:212-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Müller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 575] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 126. | Polager S, Kalma Y, Berkovich E, Ginsberg D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene. 2002;21:437-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 127. | Young AP, Nagarajan R, Longmore GD. Mechanisms of transcriptional regulation by Rb-E2F segregate by biological pathway. Oncogene. 2003;22:7209-7217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 128. | Kaelin WG. E2F1 as a target: promoter-driven suicide and small molecule modulators. Cancer Biol Ther. 2003;2:S48-S54. [PubMed] [Cited in This Article: ] |