Published online Dec 28, 2011. doi: 10.3748/wjg.v17.i48.5310
Revised: August 1, 2011
Accepted: August 27, 2011
Published online: December 28, 2011
AIM: To evaluate the clinical parameters and identify a better method of predicting pathological complete response (pCR).
METHODS: We enrolled 249 patients from a database of 544 consecutive rectal cancer patients who underwent surgical resection after preoperative chemoradiation therapy (PCRT). A retrospective review of morphological characteristics was then performed to collect data regarding rectal examination findings. A scoring model to predict pCR was then created. To validate the ability of the scoring model to predict complete regression.
RESULTS: Seventy patients (12.9%) achieved a pCR. A multivariate analysis found that pre-CRT movability (P = 0.024), post-CRT size (P = 0.018), post-CRT morphology (P = 0.023), and gross change (P = 0.009) were independent predictors of pCR. The accuracy of the scoring model was 76.8% for predicting pCR with the threshold set at 4.5. In the validation set, the accuracy was 86.7%.
CONCLUSION: Gross changes and morphological findings are important predictors of pathological response. Accordingly, PCRT response is best predicted by a combination of clinical, laboratory and metabolic information.
- Citation: Park CH, Kim HC, Cho YB, Yun SH, Lee WY, Park YS, Choi DH, Chun HK. Predicting tumor response after preoperative chemoradiation using clinical parameters in rectal cancer. World J Gastroenterol 2011; 17(48): 5310-5316
- URL: https://www.wjgnet.com/1007-9327/full/v17/i48/5310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i48.5310
The treatment strategy of rectal cancer has substantially changed in recent decades. Historically, postoperative chemoradiation was considered to be the first-line therapy for stage II and III rectal cancers[1,2]; however, preoperative chemoradiation therapy (PCRT) is now considered to be the optimal therapy regimen for locally advanced distal rectal cancer due to its improved local control, reduced toxicity, and increased rate of sphincter preservation[3-6].
Notably, most evidence suggests that patient response to PCRT is largely variable, as pathological complete response (pCR) occurs in approximately 15% to 30% of all individuals who are treated with PCRT[7,8]. As an outcome measure, the rate of pCR is significant at multiple levels: it not only represents a surrogate endpoint for comparisons of treatment regimen efficacy, but it also may affect the actual course of treatment. Furthermore, pCR has been associated with improved local control, increased recurrence-free survival rates, and better sphincter preservation[9-14]. A study by Habr-Gama et al[15] has reported excellent long-term results in the non-operative treatment of patients with clinical evidence of complete response after PCRT. Nevertheless, even though surgery is recommended after PCRT regardless of tumor response, the ability to predict an individual’s chance of achieving pCR before commencing therapy would probably enable a more tailored treatment plan[16,17].
To date, although the abilities of many techniques to predict treatment response after PCRT have been evaluated, including endorectal ultrasound (ERUS), computed tomography (CT), 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) and magnetic resonance imaging (MRI), no single modality has been proven to be efficacious, and while several molecular markers, including epidermal growth factor expression, have been associated with PCRT response[18,19], the current data are not definitive enough to support the clinical use of any of these biomarkers.
Accordingly, to identify a better method of predicting pCR, this study evaluated several clinical parameters that have previously been shown to influence pCR.
Of the 3194 consecutive patients who underwent surgical resection for rectal cancer at the Samsung Medical Center, Korea between October 1998 and May 2009, 544 underwent surgical resection after PCRT. For this study, the inclusion criteria included: (1) histologically confirmed rectal adenocarcinoma; (2) tumors located within 10 cm of the anal verge; (3) locally advanced (cT3-4 or N1) tumors; (4) curatively resected tumors; and (5) no evidence of distant metastatic disease. Additionally, individuals were excluded according to the following criteria: (1) history of any other malignancy or hereditary colon cancer syndrome; (2) history of rectal cancer requiring emergency surgery; and (3) multiple missing data points in the database or an inability to evaluate pathological tumor response.
Of the initial 544 patients with rectal cancer who underwent PCRT, 70 (12.9%) patients achieved a complete pathological response, whereas the remaining 474 did not. Of these 474 patients, 295 patients were excluded from the study for the following reasons: the presence of another malignancy (one patient); a history of hereditary colon cancer syndrome (three patients); transanal local excision including transanal endoscopic microsurgery (nine patients); a lack of sufficient clinical information in the database (14 patients); and an inability to evaluate tumor regression grade (TRG) after repeated pathological examinations (268 patients). Thus, 249 individuals with rectal cancer were included in the present study; all of whom had undergone PCRT and curative surgery.
All of the subjects underwent PCRT. Radiation therapy was administered using a three-field technique within 6-wk periods, delivering 40.4-50.4 Gy. Chemotherapy was also initiated on the first day of pelvic radiotherapy and was delivered concurrently using two chemotherapeutic regimens: (1) 5-fluorouracil (500 mg/m2 per day) for 3 d during the first and last weeks of radiotherapy; and (2) oral capecitabine (825 mg/m2) given twice daily during radiotherapy without weekend breaks. The median interval between PCRT and surgery was 55 d (range: 26-120 d).
All of the subjects also underwent radical surgery, including total mesorectal excision, high vascular ligation and en bloc resection of any adjacent involved organ after complete PCRT. The operative techniques that were employed in this study included abdominal perineal resection and low anterior resection with colorectal or coloanal anastomosis.
Before PCRT, clinical staging was determined by CT, MRI and/or endorectal ultrasound. Clinical restaging was then conducted 6 wk after the completion of PCRT via CT, MRI and 18F-FDG-PET/CT using the protocols described in our previous study[20]. Therapeutic responses with 18F-FDG-PET/CT were evaluated via the maximal standard uptake value (SUV). In 66 patients, the SUV could not be evaluated, either because 18F-FDG-PET/CT was not ordered (50 patients) or was performed at a different hospital (16 patients).
After radical surgery, the final tumor pathological staging was evaluated by experienced pathologists. Tumors were classified according to the TNM grading system, 6th edition. Responses to treatment were evaluated according to the TRG, as described by Mandard et al[21]. Specifically, the TRG classification system was defined as follows: grade 0, no regression; grade 1, dominant tumor mass with obvious fibrosis and/or vasculopathy (minimal regression); grade 2, dominant fibrotic changes with few tumor cells or groups (moderate regression); grade 3, very few tumor cells in the fibrotic tissue with or without mucous (near total regression); grade 4, no observed tumor cells with only fibrotic masses or acellular mucin pools present (complete regression).
We evaluated several variables to estimate the relationship between gross findings and tumor response. Prior to PCRT (pre-CRT) tumor location, the movability, size, morphology and involved bowel circumference were recorded. Next, after the PCRT (post-CRT), the tumor size and morphology were reassessed at a follow-up hospital visit 6 wk after completion of PCRT. The involved bowel circumference was subdivided into “encircling” and “unidirectional” categories by digital rectal examination (DRE), wherein encircling was defined as occupying more than half of the lumen. Tumor movability was also subcategorized into “movable,”“tethered,” and “fixed.” The pre-CRT tumor size was assessed using DRE in combination with any available colonoscopic or radiologic imaging results. The post-CRT tumor size was measured according to the pathological tumor or scar size. The tumor morphology was classified as being either “benign-like” or “malignant-like” in shape. By definition, benign-like shapes were observed to only consist of scar tissue, discolored lesions, erosions and shallow ulcers, whereas, in malignant-like shapes, infiltrative, fungating and ulcerofungating lesions were present. Pre-CRT tumor morphology was assessed using DRE and colonoscopic results. The resected specimens were used to classify the post-CRT morphology. Gross changes were determined to be present if malignant-like specimens had transitioned into benign-like specimens after PCRT.
All statistical analyses were performed using either the χ2 or Fisher’s exact test in addition to logistic regression modeling. Significant differences were defined as P < 0.05. To predict complete regression, we used scoring models with parameters that revealed statistical significance, wherein scoring model performance was assessed by receiver operating characteristic (ROC) curve plots for risk scoring and area under the curve (AUC) estimation. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each variable were calculated.
An independent sample set of patients with primary rectal cancer who underwent PCRT was used to evaluate the performance of the scoring model in predicting complete regression. For this validation, 15 patients were enrolled between May 2009 and July 2009. Scores from this preliminary analysis were tested for sensitivity, specificity, PPV and NPV.
The demographics of the included patients are described in Table 1. In total, 177 men (71.1%) and 72 women (22.9%) were enrolled with a median age of 55 years (range: 24-81 years). Of these individuals, 210 presented with T3 grade tumors, whereas 39 had T4 grade disease. The demographics of subjects who were enrolled in the separate validation set are also described in Table 1.
| Characteristics | Test set | Validation set |
| Total number of patients | 249 (100) | 15 (100) |
| Median age (yr, ranges) | 55, 24-81 | 56, 33-74 |
| Sex | ||
| Male | 177 (71.1) | 11 (73.3) |
| Female | 72 (28.9) | 4 (26.7) |
| Low margin from anal verge (cm) | ||
| ≤ 5 | 187 (75.1) | 12 (80) |
| > 5 | 62 (24.9) | 3 (20) |
| Histological type | ||
| Adenocarcinoma | 238 (95.6) | 14 (93.3) |
| Mucinous | 9 (3.6) | 1 (6.7) |
| Signet ring cell | 2 (0.8) | 0 (0) |
| Surgery | ||
| Abdominal-perineal resection | 37 (14.9) | 0 (0) |
| Low anterior resection | 208 (83.5) | 14 (93.3) |
| Hartmann’s operation | 4 (1.6) | 1 (6.7) |
| Chemotherapy regimen1 | ||
| FL group | 200 (80.6) | 9 (60) |
| Capecitabine group | 48 (19.4) | 6 (40) |
| Median interval to surgery (d, ranges) | 55, 26-120 | 54, 41-78 |
After PCRT, the following pathological tumor staging distribution was observed: ypT0, n = 74 (including ypTis in four patients); ypT1, n = 11; ypT2, n = 51; ypT3, n = 105; and ypT4, n = 8. At this point in time, 189 patients were found to be node-negative, whereas 60 patients were node-positive, as per the pathological N staging criteria (including ypT0N1 in three patients). TRG grading was as follows: grade 0 in two patients, grade 1 in 49, grade 2 in 86, grade 3 in 42, and grade 4 in 70.
The pre-CRT size (P = 0.001), pre-CRT movability (P < 0.001), pre-CRT carcinoembryonic antigen (CEA) (P = 0.021), ycT (P < 0.001), ycN (P < 0.001), post-CRT size (P < 0.001), post-CRT morphology (P = 0.004), gross change (P < 0.001), and post-CRT SUV (P < 0.001) were all identified to be univariate predictors of complete regression (Table 2). Multivariate predictors for complete regression included pre-CRT movability (P = 0.024), post-CRT size (P = 0.018), post-CRT morphology (P = 0.023) and gross change (P = 0.009). The data from pre-CRT CEA and post-CRT SUV were incomplete, therefore, we did not include these variables in the multivariate analysis (Table 3).
| Variable | Tumor regression grade | P value | |
| 0-3 | 4 | ||
| Sex | 0.940 | ||
| Male | 127 | 50 | |
| Female | 52 | 20 | |
| Age (yr) | 0.926 | ||
| ≤ 56 | 96 | 38 | |
| > 56 | 83 | 32 | |
| Low margin from AV (cm) | 0.641 | ||
| ≤ 5 | 133 | 54 | |
| > 5 | 46 | 16 | |
| Pre-CRT size (cm) | 0.001 | ||
| ≤ 4 | 73 | 44 | |
| > 4 | 102 | 23 | |
| cT classification | 0.054 | ||
| cT2, 3 | 146 | 64 | |
| cT4 | 33 | 6 | |
| cN classification | 0.073 | ||
| cN - | 37 | 22 | |
| cN + | 142 | 48 | |
| Pre-CRT involved bowel circumference | 0.365 | ||
| Encircling | 65 | 21 | |
| One direction | 114 | 49 | |
| Pre-CRT movability | < 0.001 | ||
| Movable | 13 | 20 | |
| Tethered/fixed | 166 | 50 | |
| Pre-CRT CEA (ng/mL)1 | 0.021 | ||
| ≤ 5 | 97 | 52 | |
| > 5 | 48 | 11 | |
| ycT classification | < 0.001 | ||
| ycT 1,2 | 42 | 38 | |
| ycT 3,4 | 137 | 32 | |
| ycN classification | < 0.001 | ||
| ycN - | 57 | 44 | |
| ycN + | 122 | 26 | |
| Post-CRT size (cm) | < 0.001 | ||
| ≤ 3 | 115 | 64 | |
| > 3 | 64 | 6 | |
| Post-CRT morphology | 0.004 | ||
| Benign-like shape | 154 | 69 | |
| Malignancy-like shape | 25 | 1 | |
| Cell type | 0.141 | ||
| High grade | 161 | 67 | |
| Low grade | 18 | 3 | |
| Gross Change | < 0.001 | ||
| Yes | 129 | 67 | |
| No | 50 | 3 | |
| Post-CRT SUV2 | < 0.001 | ||
| ≤ 5 | 63 | 29 | |
| > 5 | 86 | 5 | |
| Tumor regression grade (0-3 vs 4) | |||
| OR | 95% CI | P value | |
| Pre-CRT size | 1.265 | 0.622-2.569 | 0.516 |
| Pre-CRT movability | 2.780 | 1.141-6.777 | 0.024 |
| Post-CRT size | 3.473 | 1.235-9.767 | 0.018 |
| Post-CRT morphology | 11.100 | 1.389-88.673 | 0.023 |
| Gross Change | 5.847 | 1.519-19.814 | 0.009 |
| ycT classification | 2.073 | 0.968-4.440 | 0.061 |
| ycN classification | 1.887 | 0.917-3.883 | 0.085 |
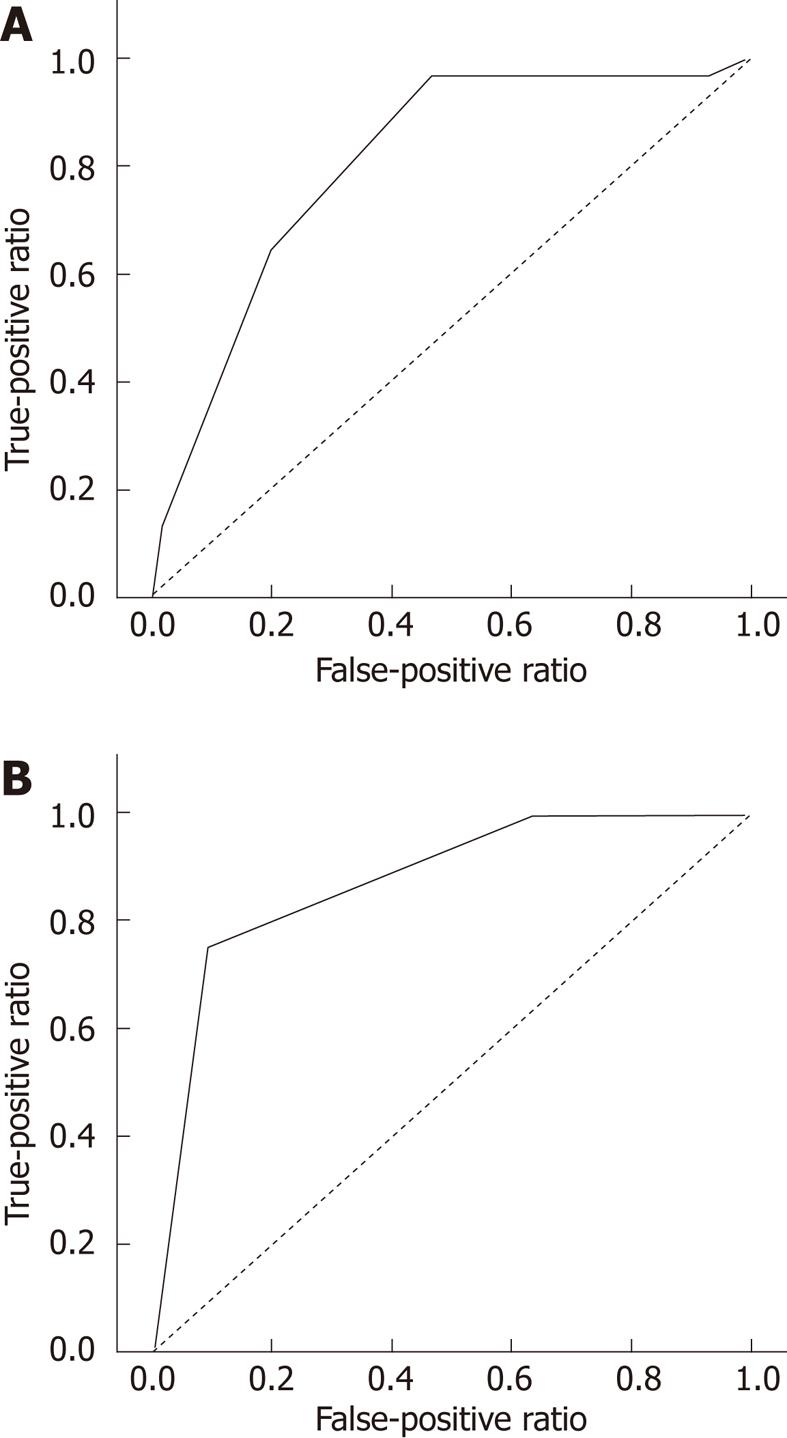
Scoring models were established to predict pCR using pre-CRT CEA and post-CRT SUV, which identified four statistically significant variables: pre-CRT movability, post-CRT tumor size, post-CRT morphology, and gross change. Each parameter was scored as either 0 or 1 according to each criterion. Although the pre-CRT CEA and post-CRT SUV were not included in the multivariate analyses, they were included in the risk score calculation, due to their assumed clinical importance. The scoring model was calculated by summing these six scores, defining the minimum scoring model as 0 and the maximum as 6 (Table 4). Next, a risk score was calculated for each subject, given that no missing data points existed for that selected risk factor. After excluding for the 98 subjects for whom 18F-FDG-PET/CT and pre-CRT CEA were not measured, scoring was performed in a total of 151 individuals. The AUC of the scoring model was 80.5%, suggesting that this was a reasonable predictor of complete regression (Figure 1). When the cutoff point was set at 4.5, the sensitivity and specificity rates for predicting complete regression were 64.5% and 80%, respectively, with a PPV of 45.5%, an NPV of 89.7% and an accuracy of 76.8% (Table 5).
| No. of points | ||
| Parameters | 0 | 1 |
| Pre-CRT movability | Tethered/fixed | Movable |
| Pre-CRT CEA (ng/mL) | > 5 | ≤ 5 |
| Post-CRT morphology | Malignancy-like shape | Benign-like shape |
| Post-CRT SUV | > 5 | ≤ 5 |
| Post-CRT size (cm) | > 3 | ≤ 3 |
| Gross change | No | Yes |
| Measure of accuracy | Cut off value, 4.5 | |
| n | % | |
| Sensitivity | 20/31 | 64.5 |
| Specificity | 96/120 | 80 |
| Positive predictive value | 30/44 | 45.5 |
| Negative predictive value | 96/107 | 89.7 |
The ROC curve for the risk score performance that was obtained from the validation set had an AUC = 0.875, which can be seen in Figure 1. These results indicate that this scoring model performed well in an independent sample. Using a cutoff point of 4.5, the prediction of complete regression had a sensitivity of 75%, specificity of 90%, PPV of 75%, NPV of 90.91%, and accuracy of 86.7%.
Even though the response to PCRT is believed to be important in predicting prognosis and treatment strategy decisions, no reliable technique has been shown to predict accurately pCR after PCRT, and only limited data exist for each modality[22]. Various imaging modalities, including ERUS, CT, MRI and PET, have been evaluated in terms of their ability to predict the response to treatment after PCRT. Recent studies have reported a limited accuracy for ERUS (48%-72%)[23,24], and the accuracy of CT and MRI in assessing the depth of tumor infiltration after PCRT has also been shown to be limited at approximately 50%[25].
Our retrospective analysis evaluated the influence of certain pretreatment parameters on pCR after PCRT, and identified an accurate method for predicting pCR. Specifically, we hypothesized that a combination of clinical, laboratory and metabolic information would best predict pCR. Particular attention was given to gross changes and morphological evaluations by surgeons or other clinicians. These data were then classified and analyzed to verify the predictive value of radiosensitivity. Previously, some reports have indicated that gross assessments by DRE had a low PPV for assessing complete response. In one retrospective review of 488 rectal cancer patients who underwent PCRT and subsequently were followed by clinical re-evaluation (DRE and sigmoidoscopic examination under anesthesia), the clinical CR rate was 19%, with only 25% of the subjects achieving pCR (10%)[26]. In another study from Guillem and colleagues, DRE was found to underestimate the response of rectal cancer to PCRT[27]. Furthermore, other evidence indicates that DRE is not a reliable technique for distinguishing between post-radiation fibrosis and residual cancer[28]. To date, the majority of studies have evaluated the efficacy of DRE as a single modality for predicting pCR through predictive value calculations. Although we concluded that DRE alone may be able to distinguish pCR, we hypothesized that the supplementation of DRE with other clinical findings would increase the accuracy of pCR predictions.
A German study has definitively shown that FDG-PET metabolic imaging is superior to CT and MRI morphological imaging in predicting response to PCRT[29]. In this particular study, the therapeutic response was evaluated through comparisons of FDG-PET imaging that was conducted pre- and post-treatment with the SUV, resulting in a sensitivity of 100% and a specificity of 60% (the PPV and NPV were 77% and 100%, respectively). Notably, another study has reported the overall accuracy of FDG-PET to be 60%, although imaging was only performed after PCRT[20]. The primary limitation of FDG-PET in predicting tumor response is the relatively low specificity, therefore, it is viewed as somewhat risky to base treatment decisions on FDG-PET exclusively. Furthermore, to achieve the best predictive rates for CR with FDG-PET, imaging must be performed twice.
To improve the accuracy of pCR predictions, we designed a scoring model using important clinical and gross parameters. The pre-CRT CEA and post-CRT SUV were identified to be significant in a univariate analysis in our study, therefore, in addition to prior reports that these variables affect pCR, we included these parameters in the scoring model[8,22,29]. In fact, the scoring model facilitated the identification of four statistically significant variables in the multivariate analysis, including pre-CRT movability, post-CRT size, post-CRT morphology and gross change, and the AUC of the scoring model was 76.5% in the test set. After the pre-CRT CEA and post-CRT SUV were added to the scoring model, the accuracy of the pCR prediction increased (AUC of the risk score was 80.5%). In addition, these parameters are objective, easily applied, and already widely used. In this respect, we included the pre-CRT CEA and post-CRT SUV in the scoring model, although these parameters were not revealed to be significant in the multivariate analysis.
After the data analysis, our scoring model was found to be significantly correlated with pCR in both the test and validation sets. At a cutoff point of 4.5, the overall accuracy of our scoring model was 76.8% for predicting pCR in the test set; however, the accuracy of the model in predicting pCR decreased to 62.85% in the test set when 18F-FDG-PET/CT (post-CRT SUV) was the only variable used. This secondary finding suggests that the predictive ability of the scoring model probably requires both 18F-FDG-PET/CT and DRE or colonoscopy morphological assessments. Furthermore, when compared with the aforementioned German study that evaluated the predictive ability of FDG-PET, our model had a superior specificity, a similar NPV, and an inferior sensitivity and PPV[29]; however, although our scoring model only used the post-CRT SUV, the German FDG-PET study required pre- and post-treatment SUV measurements. Moreover, the accuracy of our model was substantially improved in the validation set, suggesting that the ability to predict CR can be improved by combining clinical and radiological findings, including metabolic information obtained through DRE, colonoscopy and 18F-FDG-PET/CT.
Our study had some limitations that warrant discussion. First, our results may have been affected by external bias, because the investigation was not prospective. Specifically, although all data were mined from a prospective database that adopted clear definitions for gross changes, the actual classification of gross change could easily be affected by surgeons’ subjective determinations. Second, the exclusion of some individuals could have resulted in a sampling bias, although our model was vetted by a separate validation set. Despite these drawbacks, our model had significant value because it allowed clinicians to predict accurately pCR without performing any additional examinations.
In conclusion, our results indicate that gross tumor change and other associated morphological findings may represent important predictors for pathological tumor response, wherein both clinical parameters are easily estimated via physical examination. Moreover, because our scoring model includes both gross findings and clinical variables, we contend that PCRT response is best predicted by a combination of clinical, laboratory and metabolic findings.
Pathological complete response (pCR) has been associated with improved oncologic outcome. Although surgery is recommended after preoperative chemoradiation therapy (PCRT), regardless of tumor response, the ability to predict an individual’s chance of achieving pCR before commencing therapy would probably allow for a more tailored treatment.
Although it is important to predict therapeutic responses, there is no reliable technique to accurately predict pCR after PCRT. This study demonstrates that a combination of clinical, laboratory and metabolic information would best predict pCR.
Recent reports have highlighted the importance of tailored treatment in rectal cancer, including “wait and watch” after chemoradiation. In this study, the authors designed a scoring model using clinical and gross parameters for predicting pCR. The developed scoring model was found to be significantly predictive in both test and validation sets.
This study demonstrates that gross tumor changes and other associated morphological findings may represent important predictors of pathological tumor responses with both clinical parameters being easily estimated via physical examination.
The study aimed to evaluate a number of clinical parameters and identify a method of predicting pCR after CRT in patients with advanced rectal cancer. It is an interesting study, meticulously carried out and reported.
Peer reviewers: Marek Bebenek, MD, PhD, Department of Surgical Oncology, Regional Comprehensive Cancer Center, pl. Hirszfelda 12, 53-413 Wroclaw, Poland; Benjamin Perakath, Professor, Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia Hospital, Vedado, Havana City Plaza 28043, Cuba
S- Editor Tian L L- Editor Kerr C E- Editor Xiong L
| 1. | Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1610-1614. [PubMed] [Cited in This Article: ] |
| 2. | Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, Jones J, Glass A, Lerner H, Lawrence W. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80:30-36. [PubMed] [Cited in This Article: ] |
| 3. | Habr-Gama A, Perez RO, Kiss DR, Rawet V, Scanavini A, Santinho PM, Nadalin W. Preoperative chemoradiation therapy for low rectal cancer. Impact on downstaging and sphincter-saving operations. Hepatogastroenterology. 2004;51:1703-1707. [PubMed] [Cited in This Article: ] |
| 4. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3104] [Cited by in F6Publishing: 2972] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 5. | Minsky BD, Cohen AM, Enker WE, Saltz L, Guillem JG, Paty PB, Kelsen DP, Kemeny N, Ilson D, Bass J. Preoperative 5-FU, low-dose leucovorin, and radiation therapy for locally advanced and unresectable rectal cancer. Int J Radiat Oncol Biol Phys. 1997;37:289-295. [PubMed] [Cited in This Article: ] |
| 6. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4342] [Cited by in F6Publishing: 4228] [Article Influence: 211.4] [Reference Citation Analysis (1)] |
| 7. | Mehta VK, Cho C, Ford JM, Jambalos C, Poen J, Koong A, Lin A, Bastidas JA, Young H, Dunphy EP. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys. 2003;55:132-137. [PubMed] [Cited in This Article: ] |
| 8. | Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, Lim SB, Choi HS, Jeong SY, Park JG. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1167-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 11. | Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U, Silva E Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90-99; discussion 99-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Stipa F, Chessin DB, Shia J, Paty PB, Weiser M, Temple LK, Minsky BD, Wong WD, Guillem JG. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol. 2006;13:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, Khanduja KS. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895-903. [PubMed] [Cited in This Article: ] |
| 14. | Wiig JN, Larsen SG, Dueland S, Giercksky KE. Clinical outcome in patients with complete pathologic response (pT0) to preoperative irradiation/chemo-irradiation operated for locally advanced or locally recurrent rectal cancer. J Surg Oncol. 2005;92:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AH, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-717; discussion 711-717;. [PubMed] [Cited in This Article: ] |
| 16. | Huh JW, Jung EJ, Park YA, Lee KY, Sohn SK. Preoperative chemoradiation followed by transanal excision for rectal cancer. J Surg Res. 2008;148:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Lezoche E, Guerrieri M, Paganini AM, Baldarelli M, De Sanctis A, Lezoche G. Long-term results in patients with T2-3 N0 distal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg. 2005;92:1546-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majó J. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol. 2005;74:101-108. [PubMed] [Cited in This Article: ] |
| 19. | Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Cho YB, Chun HK, Kim MJ, Choi JY, Park CM, Kim BT, Lee SJ, Yun SH, Kim HC, Lee WY. Accuracy of MRI and 18F-FDG PET/CT for restaging after preoperative concurrent chemoradiotherapy for rectal cancer. World J Surg. 2009;33:2688-2694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] [Cited in This Article: ] |
| 22. | O'Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8:625-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Fleshman JW, Myerson RJ, Fry RD, Kodner IJ. Accuracy of transrectal ultrasound in predicting pathologic stage of rectal cancer before and after preoperative radiation therapy. Dis Colon Rectum. 1992;35:823-829. [PubMed] [Cited in This Article: ] |
| 24. | Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109-112. [PubMed] [Cited in This Article: ] |
| 25. | Barth C, Rau B, Hünerbein M, Schlag PM. [Comparative diagnosis of locally advanced rectal carcinoma after preoperative therapy]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1404-1407. [PubMed] [Cited in This Article: ] |
| 26. | Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-135; discussion 131-135;. [PubMed] [Cited in This Article: ] |
| 27. | Guillem JG, Chessin DB, Shia J, Moore HG, Mazumdar M, Bernard B, Paty PB, Saltz L, Minsky BD, Weiser MR. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005;23:3475-3479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Kahn H, Alexander A, Rakinic J, Nagle D, Fry R. Preoperative staging of irradiated rectal cancers using digital rectal examination, computed tomography, endorectal ultrasound, and magnetic resonance imaging does not accurately predict T0,N0 pathology. Dis Colon Rectum. 1997;40:140-144. [PubMed] [Cited in This Article: ] |
| 29. | Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, Hünerbein M, Felix R, Wust P, Amthauer H. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |