INTRODUCTION
The liver is the largest parenchymal organ of the body and has a multitude of important and complex functions; among the most notable are the metabolism of fat, proteins, and carbohydrates, synthesis and secretion of bile, microelement recycling and detoxification of products resulting from body metabolism (bilirubin, ammonia, etc.) and from exogenous toxins (drugs, alcohol and environment)[1]. The liver is also an immune[2] and an endocrine[3,4] organ and functions as a blood capacitance reservoir[5,6]. To accomplish these many assignments, the liver accommodates a wide variety of cell types, including those that call liver “home”, namely liver progenitor cells, hepatocytes, Kupffer cells (KCs), stellate cells (SCs) and the cellular components of vasculature, and those that use the liver as a temporary and/or terminal station, such as blood cells[1,7]. Liver diseases are the 10th leading cause of death and account for significant morbidity across the entire age and gender spectrum of the US population[8,9]. The mechanisms of liver diseases are extensively researched but not fully understood. More recently, research data have emerged proposing that some pathogenic mechanisms, such as inflammation, tissue death and regeneration, and organ remodeling, are shared across a large spectrum of liver diseases of distinct etiologies[10-14], with the suggestion that common denominators of cell structure or function may be involved. Here we have focused on a common denominator of the cellular structure, the lipid rafts (LRs), as players in liver physiology and pathology.
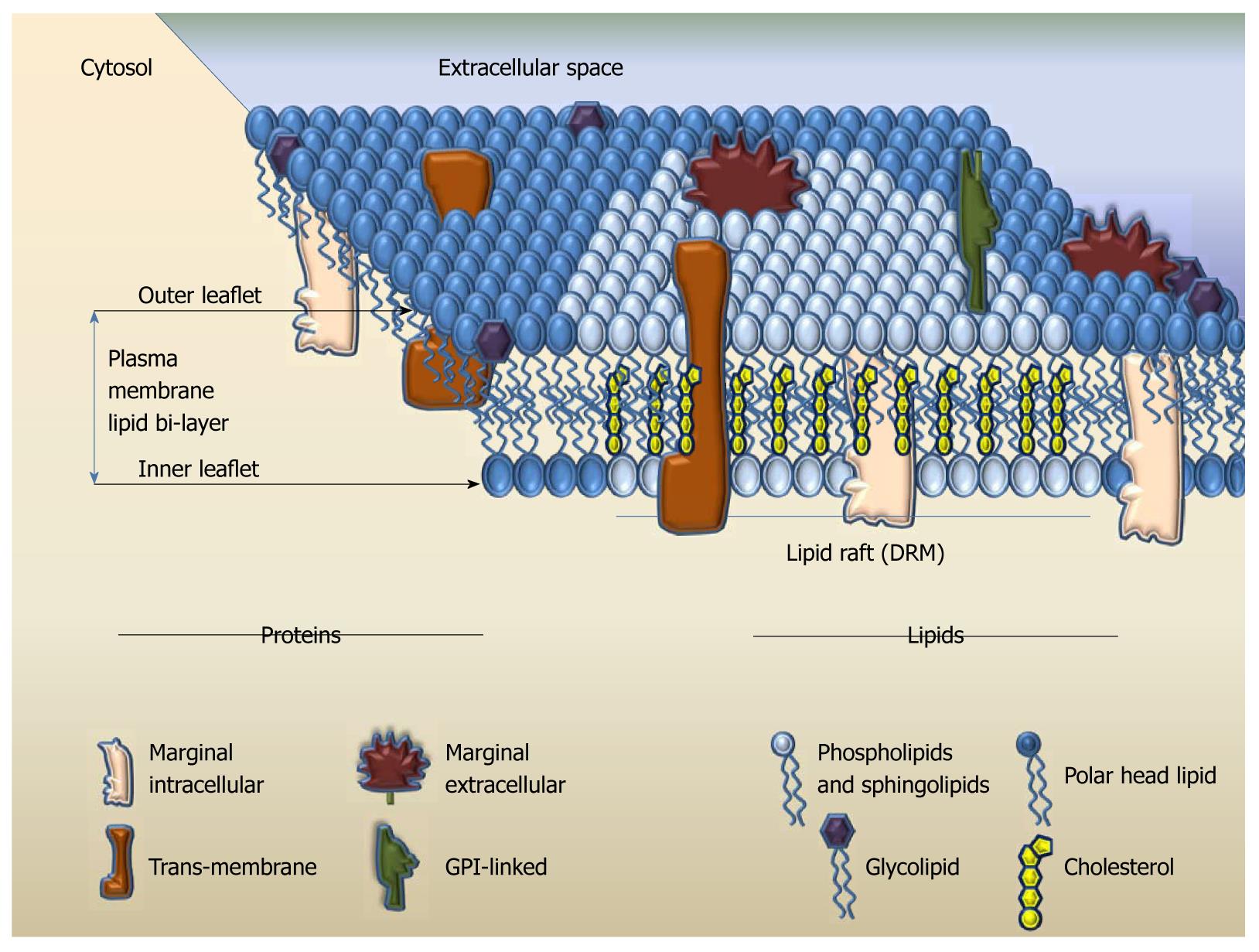
Cellular membranes separate each cell and define their entity. The main components of the cellular membrane are lipids and proteins[15]. Structurally, the membrane lipid molecules consist of a polar globular head and a straight non-polar hydrophobic backbone region. Each row of lipids aligns in a leaflet at the level of globular heads and hydrophobic areas of the leaflet attract similarly charged structures, such as the straight region of another lipid layer. Thus, the plasma membrane (PM) consists of two leaflets with the non-polar regions pointing inward and the polar heads pointing to the water rich-zones (intra- and extra-cellular spaces); architecturally this structure portrays a lipid bilayer[16] (Figure 1). Besides serving as warrant of cellular identity and integrity, the lipid bilayer assures that the membrane is flexible. At lower temperatures, the lipid bilayer forms a gel state and is tightly packed; as the temperature rises the bilayer favors tri-dimensional movement of the lipid molecules and the interior space between the two leaflets becomes more fluid. It is the gel/fluid exchange state that allows horizontal and vertical movement of lipids, and also of other components of the membrane[15-17]. Proteins are embedded into the lipid bilayer, either by spanning through, as trans-membrane integral structures that could cross in a single- or multi-pass fashion, or as attached structures; the latter exist as peripheral proteins, attached to the membrane exclusively on either the extracellular or cytosolic face, or as glycosylphosphatidylinositol (GPI)-anchored proteins on the extracellular face of the membrane[18]. At the end of the 20th century, the LR hypothesis of cellular membrane function emerged: this proposed that certain regions of the lipid bilayer modify their chemical composition to become more rigid, thus allowing some membrane proteins to physically segregate depending on their interaction with the bilayer components and with other proteins[19,20]. This theory combined the biological knowledge about cellular activation and the biochemical knowledge about protein folding with biophysical approaches to the dynamic cellular membrane structure and attempted to answer the puzzling question about how proteins cluster and what contributes to signal transduction.
Figure 1 The theoretical model of lipid bilayer and lipid rafts in cellular membranes.
GPI: Glycosylphosphatidylinositol; DRM: Detergent-resistant domain.
LRS: STRUCTURE, FUNCTIONS AND RESEARCH METHODOLOGY
LR structure and functions
LRs are areas of cellular membranes with a signature composition rich in sphingolipids and cholesterol phospholipids. Saturated fatty acids are preferentially enriched in the side chains of the membrane phospholipids, which allows closer packing and thus increased rigidity, more order, and less fluidity of the LRs compared to the surrounding membrane[15,19-21]. LRs act as a unique platform that aids co-localization of proteins involved in sensing (receptors), reacting (pores) and triggering/sustaining cell activation (intracellular signaling pathways) (Figure 1). Besides their structural signature, LRs exhibit two more unique characteristics: they are small, ranging from several to about 500 nm, and are dynamic, with a variable life span in the order of milliseconds (msec). It is not fully understood whether the LRs form and disintegrate constantly or maintain a longer lifespan when a cell is in its steady state, because a relatively constant number/composition of proteins can be identified in the LRs at this stage. However, once the cell is activated, the LRs undergo a radical change in composition by adding or eliminating certain proteins and thus accommodating the cellular needs for formation of signaling platform in a matter of msec. While they are well documented to be in the PM of the cells, it has been suggested that LRs can also reside in the intracellular structures, such as endoplasmic reticulum (ER), peroxisomes, mitochondria, and endosomes[22-26]; to date it is not clear whether such findings are truly due to LR presence in these intracellular compartments or if they are an artifact of LR isolation/investigation techniques. LRs are present throughout the entire evolutionary chain, from the viruses and bacteria[27] to mammals; the purpose of the LR in evolution is yet to be fully understood.
LR research methodology
Studying the LRs is challenging. With sizes ranging up to 500 nm in diameter, the LRs are beyond the resolution of optical microscopes. For this reason the overlay of one or more stains tagged to the proteins of interest revealing a significant co-localization with the coalesced LRs, labeled indirectly with a fluorescent choleratoxin B, is usually suggestive but never a definite indication of the LR localization of the studied proteins. In addition to being small, the rafts diffuse in msec across the cell membrane and thus require additional efforts for timely detection. The transmission electron microscope can analyze a 5-nanometer-thick cell membrane and captures short time frames; the more dynamic and short-lived rafts can only be observed with advanced imaging techniques such as atomic force microscopy (AFM)[28]. Fluorescence lifetime spectroscopy, including Förster resonance energy transfer (FRET), is becoming more accepted in the LR research field due to the fact that the capacity of energy transfer in FRET is about the size of the rafts and it occurs in the range of msec, thus covering both the size and the life span of the LR. Single point microscopic decay and fluorescence lifetime imaging microscopy are often used in combination with FRET for more comprehensive analysis of LR-related time-sensitive processes[29]. Flow cytometry analysis of LRs is emerging; however, it provides very limited resolution both for the size and for the timing of LR-localized events[30]. Fluorescence correlation and cross-correlation spectroscopy (FCS/FCCS) deliver information of fluorophore mobility and dynamics of lateral heterogeneity in the membrane and can be used in single or multiple colors[31]. Single particle tracking (SPT) can be employed to define the translational trajectory of very small particles, including quantum dots, to identify cluster size confinement based on intensity. The thinning out clusters while conserving stoichiometry of labeling (TOCCSL) technique is employed for LR analysis because of its proficiency in discriminating between clusters and monomers[32]. The additional advantage of FRET, FCS/FCCS, SPT and TOCCSL is their high spatial/temporal resolution, and thus their ability to register rafts as they form and dissociate in time. Labeling of living cells with the fluorescent probe 6-acyl-2-dimethylaminonapthalene (Laurdan), which exhibits a 50-nm red shift as membranes undergo phase transition from gel to fluid due to altered water penetration into the lipid bilayer, followed by two-photon microscopy, is often used as another indirect method of LR visualization[33].
Translocation of proteins to or from the LRs is often analyzed by sucrose-density gradient fractionation of the membranes and subsequent Western blot analysis; LRs are defined by their insolubility in diverse nonionic detergents [thus named detergent-resistant domains (DRMs)] and by floating in the low-density sucrose fractions (interface between 5% and 30%) upon ultracentrifugation[34]. Diverse detergents can be employed, yielding diverse composition of DRMs[34-39]. Furthermore, some researchers construct step sucrose gradients and further sub-divide the DRMs into light and intermediately-light fractions, which may have distinct protein composition. It is still debatable as to how closely the DRMs represent the true identity of the LRs; however, this method yields consistent results and is the only one available that allows enrichment and analysis of large protein complexes at reasonable cost and using old-fashioned technology.
The functional assessment of the LRs in vitro is usually performed using reduction and enrichment approaches. Treatment with agents leading to cholesterol sequestration, including amphotericin, filipin or nystatin, inhibition of cholesterol synthesis using HMG-CoA reductase inhibitors, or cholesterol wash-out with mβCD, are among the well-accepted approaches to disrupt putative LRs; they are largely based on cholesterol manipulation[40,41]. Another method commonly utilized for LR destabilization is treatment with fumonisin B1, which removes sphingolipids[40]. The advantage of using mβCD over fumonisin B1 is based on the fact that the former acts rapidly, while the latter requires pretreatment of cells for about 72 h, which is longer than the time some polarized cells, including hepatocytes, can remain truly polarized in isolated cell culture[42]. Cholesterol replenishment and ceramide supplementation, which displaces cholesterol, are often employed to modulate the fluidity of the LRs and thus affect their function.
In vivo LR modulation has gained recent popularity based on findings that dietary lipids can modify lipid composition of cell membranes. In this context, multiple studies have attempted to establish how dietary factors or modulation of blood lipids, including cholesterol, affect LR-based signaling in various cell types[43,44]. Comprehensive experimental-based conclusions about the effects of dietary lipids in regulating LRs in non-liver systems and targeted evaluations of the liver in this regard are still awaited. Reliable methods of in vivo visualization of LRs also await development.
The significant difficulties in analysis of LRs in primary biological membranes have led to development of model systems. Diverse artificial membranes and models have been created over time, with different ranges of spatial and temporal orders, diverse lipid and protein compositions, protein/lipid ratio or thickness of the lipid bilayer[45,46]. While helpful to elucidate the basics of the membrane function and structure, these models lack the combination of the proximity of the membrane with the cytoskeleton and regulatory/signaling proteins that are recruited in diverse ratios and in a time-dependent manner in natural biological membranes[45]; to date there is no comprehensive artificial model of LR-containing membranes.
LRS IN RESIDENT LIVER CELLS
LRs in hepatocytes
LRs aid polarized sorting and trafficking of apical proteins in both directed and trans-cytotic pathways in hepatocytes: Two structurally and functionally distinct PM domains, i.e. the apical and basolateral domains, determine the tri-dimensional architecture of the hepatocyte as true polarized cells; these domains are separated by the tight junctions. While the molecular signature of apical and basolateral domains is relatively well known, the maintenance of such identity requires sophisticated protein sorting and trafficking, the mechanisms of which are yet to be fully defined. Specific membrane-sorting signals have identified many involved proteins[47]. For example, GPI-membrane anchor and TMD target the proteins to the membrane[48]. Moreover, Tyr- and diLeu-based amino acid motifs, which are recognized by distinct molecular subunits of adaptor complexes, seem to direct the proteins to the basolateral membrane[49].
Two membrane-sorting pathways are known: direct and indirect. Unlike many polarized cells, hepatocytes employ both pathways to guide their proteins to the desired location. In “direct” pathway of membrane targeting, the newly synthesized proteins are primarily sorted in the trans-Golgi network (TGN) and then delivered directly to the membrane; this pathway serves both apical or basolateral membrane domains without advantaging either of them[50]. Membrane multispanning ATP-binding cassette (ABC) transporters, multidrug resistance protein 1 (MDR1), and P-glycoprotein, to name a few, employ the direct sorting mechanism in hepatocytes[51]. In “indirect” pathway, the new protein cargo travels from the TGN to the basolateral surface, from where it is endocytosed and subsequently transported to the opposite apical surface of the membrane, as reviewed by Nelson et al[52]. This laborious, energy-inefficient and very lengthy process is also called the trans-cytotic pathway and its purpose is not fully understood. The GPI-linked proteins and those with a single TMD preferentially employ the indirect pathway for apical trafficking[53].
Until recently, the factors that directed the proteins into direct vs indirect pathways of membrane-sorting were largely unknown. Nyasae et al[54] reported that cholesterol and glycosphingolipids are required for delivery from basolateral early endosomes to the subapical compartment. Slimane et al[55] identified that the protein structure and its lipophilic characteristics sort the differential apical vs basolateral targeting of the proteins in a sphingolipid/cholesterol-enriched LRs-dependent manner. These authors also reported that the liver apical proteins that contain multiple membrane-spanning domains are selectively incorporated into lipid microdomains in the TGN and are then transported directly to the apical membrane[55]. However, direct apical sorting is not solely driven by incorporation into LRs per se[55]. Conversely, proteins that have only one TMD or tether to the membrane using their GPI anchor are also sorted to the LRs in TNG; they travel first to the basolateral membrane, where LR-based mechanisms are again key in their delivery to the subapical compartment and further exposure on apical membrane. Slimane et al[55] additionally suggested that lipid composition of the LRs is also critical for protein sorting. Thus LRs play a key role in both direct and indirect membrane targeting of the proteins and act as sorting platform for directing the proteins via one pathway or another.
LRs in hepatocyte polarity: Hepatocytes are classical polarized cells; the adequate function of the liver as an organ is ensured by the spatial setting and by maintenance of hepatocyte polarity. The complex polarity of the hepatocyte is characterized by the existence of several basolateral and apical poles per cell. The complex mechanisms of establishment and maintenance of hepatocyte polarity are not fully understood; however, a role for calcium waves has been proposed. In hepatocytes the Ca2+ waves are polarized, thus occurring in an apical-to-basal fashion[56]. The polarity of the Ca2+ signal is largely due to the increased density of inositol 1,4,5-trisphosphate receptor (InsP3R) in the pericanalicular region; redistribution of InsP3R from the apical to the basolateral region is associated with concomitant slowing of the onset and speed of Ca2+ waves in hepatocytes[57]. The pericanalicular accumulation of InsP3Rs in not exclusive to hepatocytes, as it is also observed in other types of polarized cells such as pancreatic acinar cells, cholangiocytes, salivary acinar and duct cells[58]. The pericanalicular area has been named the “trigger zone,” because it defines the subcellular region that triggers the formation of Ca2+ waves[59,60]. The pericanalicular area is rich in LRs and it is also a preferred localization of InsP3Rs; most importantly, recruitment of InsP3Rs to this area is highly dependent upon the presence of intact LRs[57]. Similar to InsP3R redistribution, LR disruption by cholesterol depletion substantially slows Ca2+ waves in hepatocytes[57]. The expression of apical InsP3Rs is decreased or absent in cholangiocytes from patients with primary biliary cirrhosis, sclerosing cholangitis, common bile duct obstruction, and biliary atresia, suggesting that InsP3Rs deficit and/or misplacement may represent a final common pathway for the development of cholestatic disorders[61]. Given the key role of LRs in housing the InsP3Rs on the apical hepatocyte membrane, it is easy to envision a role for LRs and/or hepatocyte de-polarization in cholestatic disorders; this area remains open for investigation.
LRs as signaling platforms: The hepatocyte PM houses a wide variety of proteins that function as sensors, receptors, and/or transporters; these proteins allow the hepatocyte to react to a rapidly changing extracellular environment and accommodate the needs of homeostasis. Among the best researched hepatocyte receptors for which the intracellular signaling pathways are well defined and the ties to LRs are well established are hepatocyte growth factor (HGF) receptor (HGFR), epidermal growth factor (EGF) receptor (EGFR), angiotensin II type 1 (AT1R) and insulin receptor (IR) receptors.
The HGFR, also called c-Met, is activated by HGF and triggers hepatocyte proliferation, morphogenesis and survival. Defects of HGFR are a cause of hepatocellular carcinoma[62]. The HGFR is a heterodimer that exhibits tyrosine kinase activity and associates with a multiprotein complex to trigger downstream signal systems, including src, Grb2/SOS, PI3 kinase, Gab1 and focal adhesion kinase; the adequate function of these adaptors/signal transducers is largely dependent on LRs[63,64]. To date, it is not clear if the HGFR itself is localized in the LRs. However, HGF promotes HGFR association with CD44 and recruitment of this multi-protein receptor complex into caveolin-enriched LR microdomains[65]. The LR-dependent HGF/HGFR signal plays a role in several pathologic processes, including protection from lipopolysaccharide-induced vascular hyper-permeability[65]; its role in hepatocyte function remains to be clarified.
EGF binds to the EGFR and regulates hepatocyte growth both in vivo and in primary culture. EGFR signaling activates a group of signal transducers and activators of transcription, which increase the transcription of a characteristic set of early growth response genes thus leading to hepatic DNA replication[66]. In normal adult liver, EGFR expression is low, whereas EGF is virtually nondetectable. In contrast, in advanced cirrhosis, a continuous EGFR synthesis is accompanied by EGF expression that is localized to hepatocytes and proliferative bile epithelium[67,68]. EGF treatment is followed by rapid ubiquitination of the EGFR in hepatocytes[69] and plays a key role in trafficking of the EGFR between early and late endosomal compartments[70]. Ubiquitinated EGFR is internalized almost exclusively via a non-clathrin LR-dependent route[70]. In this context, EGFRs recruited to LRs in response to EGF derive almost exclusively from endosomes[24]. EGFRs in early endosomes are more tyrosine phosphorylated than those in late endosomes, indicating that EGFR might be partially dephosphorylated in an LR-dependent manner before accessing the late compartment; however, once localized, the EGFRs in endosomal LRs are relatively resistant to dephosphorylation[24]. Some of the kinase receptors that are internalized through caveolin-containing LRs have a high probability of being directed to the degradative pathway rather than having a function in signaling[70]. To this extent, the EGFR is no exception: the EGF-engaged EGFRs that are localized in LRs are more tyrosine phosphorylated than EGFRs in whole endosomes. Endosomal LRs recruit highly Tyr-phosphorylated EGFR along with various signaling proteins, including Grb-2, Shc, and c-Src, indicating a likely role for this compartment in signal transduction[24]. According to Teis et al[25], targeting of the MP1-ERK1/2 complex to late endosomes during EGF-mediated ERK activation depends on the adaptor protein; further, p14-MP1-ERK1/2 complex formation is needed for EGF-induced ERK activation at later stages of this process. Thus, it seems plausible that intracellular LRs target the ubiquitinated PY-EGFR/Grb2/Shc complex to the cytoplasmic face of the late endosomes for specific activation of ERK1/2. If this theory holds true, the ubiquitinated EGFRs should be later sorted into the intraluminal vesicles of late endosomes before their degradation or may be recycled back to the PM. This hypothesis was in part confirmed by data from Balbis et al[24] and by Lai et al[26], thus placing the LRs among the key players in ensuring efficient EGF/EGFR function. The possibility that this LR-dependent mechanism can be exploited is valid, because some of the widely-used drugs can be employed for this purpose. For example, heparin can suppress LR-mediated signaling and ligand-independent EGF receptor activation[71]. The detailed role of LR-mediated EGF/EGFR function in liver diseases remains to be determined.
Angiotensin II (AT) engages and initiates angiotensin AT1 receptor (AT1) signaling which is involved in cell growth and mitogenesis[72]. At least in C9 liver cells, Ang II-stimulation involves LR-mediated activation of ERK1/2[73]. Furthermore, Yin et al[74] reported that in angiotensin-stimulated C9 cells the cholesterol-rich LR domains mediate the actions of early upstream signaling molecules such as Src kinases and intracellular Ca2+ and that LR resident caveolin-1 has a scaffolding role in this process. Angiotensin causes caveolin-1 phosphorylation that is in turn regulated by intracellular Ca2+ and Src, thus indicating reciprocal interactions between LRs, caveolin-1, Src kinases, and intracellular Ca2+ through the AT1[74].
Insulin is an anabolic hormone with a role in carbohydrate and lipid metabolism and cell growth. The IR is a transmembrane tyrosine kinase that binds insulin with its two extracellular α-subunits and transmits signals via its two β-subunits that contain the tyrosine kinase domain; the receptor is activated by autophosphorylations. LRs ensure the initial steps of IR activation[24,75,76]. IR β subunits require palmitoylation for proper function[75]; however, regardless of its palmitoylation status, the unoccupied IR has low affinity to LRs[76]. In adipose or muscle tissue, IR needs to partner with caveolin-1 for function. In the liver, the autophosphorylation of the insulin engaged-IR still takes place in the LRs but can occur with[24] or without[76] caveolin participation. In the latter context, glycophospholipid clustering inhibits IR phosphorylation and IR is excluded from the LRs when the latter are more rigid[76]. These results suggest that LRs offer an additional level of control over IR function in the liver and support the hope that manipulation of LRs could modulate IR activity and thus liver metabolism.
LR role in bile formation/secretion: Bile production is one of the basic functions and an organ-specific signature of the liver. ABC transporters are located in the canalicular membrane of hepatocytes and are critical players in bile formation and detoxification. ABC is a family that includes P-glycoprotein (MDR1) for organic cations; MDR2 for phosphatidylcholine translocation; P-glycoprotein-related protein acting as bile salt export pump; and MRP2 (or cMOAT) for non-bile acid organic anions[51]. ABC transporters are located in LRs of the hepatocyte cellular membrane and mediate the transport of the majority of lipids secreted into the bile[77].
The canalicular PM is constantly exposed to bile acids, which act as detergents. Once secreted into bile canaliculi, bile salts extract phosphatidylcholine from the outer leaflet of the cellular membrane and incorporate it into mixed micelles. The bile preferentially extracts phosphatidylcholine; the latter constitutes only about 35% of canalicular phospholipids in the context of a relative abundance of sphingomyelin, thus the bile threatens the well-being of healthy cells. During evolution, a protective mechanism was developed in all bile-exposed cells to prevent self-solubilization with own bile by means of expressing a combination of distinct LRs that have unique lipid composition and protein content. Ismair et al[35] reported the existence of at least two such LR entities on the canalicular side of the hepatocyte PM. One of these is rich in caveolin-1, contains the majority of canalicular cholesterol and phospholipids, portions of the marker enzymes APN and DDPIV, and a large portion of all known ABC transporters including ABCG5, BSEP, MRP2, MDR2, and MDR1; this LR is Lubrol-soluble. In contrast, Triton-soluble LRs are associated with reggie-1/2 proteins and sphingomyelin; they contain a minor fraction of canalicular cholesterol, APN, DDPIV and MDR1/2 ABC transporters[35]. The exact purpose of such differential expression of ABC transporters in distinct LRs is not fully understood; furthermore, their distribution in LRs in steady-state vs activated state is largely unknown. However, several liver diseases are linked to ABC defects; for example, recessively inherited hepatobiliary phenotype is related to mutations in ABC transporter genes; defects in ABCB11 and MDR3 lead to familial intrahepatic cholestasis types 2 and 3, respectively; Dubin Johnson syndrome is associated with defects in MRP2 produced, and sitosterolemia with defects in ABCG5 or ABCG8[51]. It is also known that an altered secretory process of biliary cholesterol is associated with elevated hepatic cholesterol levels[78], thus suggesting the possibility that low membrane fluidity due to stabilization with cholesterol could provide an additional layer of control of ABC transporter function. It remains to be investigated whether the increased rigidity of LRs, which occurs when cholesterol content in the cell increases[15,16], may supplement the impairment of bile secretion in real-life liver diseases in addition to functional impairment of ABC transporters per se.
LRs in KCs/monocytes
KCs are active immune players and participants in a wide variety of liver diseases. The role of the LR in KCs is currently unknown, in part due to the scarcity of primary KCs and difficulties with their isolation. However, taking into account that KCs are tissue macrophages (Mf), several key findings about the role of LR in other types of tissue Mf could be extrapolated to KCs.
Inflammation occurs in a diverse array of liver diseases and is highly dependent on Mf. In Mf, the key molecules involved in pathogen recognition from exogenous danger signals either reside (CD14) or are recruited [Toll-like receptor (TLR)2, TLR4] to the LRs upon ligand engagement[79,80]; the function of pathogen recognition is thus highly dependent on LRs and suggests that LR modulation may be a candidate for managing the macrophages. Indeed, exposure to acute alcohol renders Mf temporarily insensitive to TLR4/CD14 ligands in a LR-dependent manner[81,82]. Similar to alcohol, selective modulation of fat content in LRs by macrophage-specific ABC transporter A1 also dampens inflammation by reducing MyD88-dependent TLRs trafficking to LRs[83]. Furthermore, peritoneal macrophages from dyslipidemic mice are primed for more robust TLR responses, reflecting increased LRs and increased TLR4 expression[84]. In the same dyslipidemic mice, the Mf from the lung airspace, in which cholesterol is maintained as constant during dyslipidemia, have normal responses and normal composition of LRs[84]. It is important to note that LRs also drive the Mf response to endogenous danger signals, such as those coming from dead cells. In this context, necrotic but not apoptotic cells co-localize with LRs within engulfing Mf. Interestingly, necrotic cell-induced secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-1β by Mf is susceptible to LR destruction, suggesting a role for LRs in the signaling of necrosis-driven inflammatory response[85].
Related to the role of liver in the iron cycle, the ability of Mf to capture/store iron is highly dependent on the LRs. Expression of the iron exporter ferroportin at the PM of Mf is enhanced by iron loading and is decreased by hepcidin[86]. Macrophage ferroportin is preferentially located in caveolin/flotillin 1-enriched LRs; iron overload strongly increases the presence of ferroportin in the LRs, while LR destruction decreases hepcidin activity on macrophage ferroportin[86]. Collectively, these data support the idea that LRs participate in several key Mf functions that are key for liver homeostasis.
LRs in SCs
The SCs store retinol and are the main cellular source of collagen and other extracellular matrix substances in normal as well as fibrotic livers. The LR composition, and their distribution and function in primary SCs are largely unknown, mainly due to limited numbers of SCs isolated from the normal liver and extreme technical difficulties of their isolation from the fibrotic liver. Andrade et al[87] employed the murine hepatic SC line GRX, which expresses the myofibroblast phenotype at baseline and can be induced in vitro to display the fat-storing phenotype (lipocytes), to show that total ganglioside content and GM2 synthase activity were lower in myofibroblasts compared to lipocytes. Both SC phenotypes presented similar content of gangliosides GM2, GM1, and GD1a, as well as their precursor GM3. Sphingomyelin and all the gangliosides were expressed as doublets; their ratio is increased in retinol-induced lipocytes due to increased content of long-chain fatty acids[87]. Taken together, these results indicated that myofibroblasts and lipocytes can use distinct ceramide pools for sphingolipid synthesis. Differential ganglioside expression and presence of long-chain saturated fatty acids suggested that these components could participate in the formation of LRs with specific functions in the two phenotypes of GRX-SCs[87].
Anandamide (AEA) is an amide of arachidonic acid and ethanolamine with endogenous endocannabinoid function that engages cannabinoid and other yet unknown receptors, and exerts a variety of physiological and pharmacological effects in chronic liver diseases[87]. Yang et al[88] employed the hepatic SC line T6 and reported that moderate AEA amounts inhibited hepatic SC proliferation and high-dose AEA caused hepatic SC death; cell death was necrotic rather than apoptotic and occurred independently of cannabinoid receptors. More importantly, AEA-mediated death in hepatic SCs was dependent on the cholesterol content of the membrane LRs, the fatty acid composition of the membrane, and the function of PI3K/protein kinase B signaling pathway[87]. These data gave support to speculations that AEA may be a potential antifibrogenic drug in the treatment of liver fibrosis in a LR-dependent manner.
Sonic hedgehog (Shh) is an embryonic morphogen that is key in cell proliferation, differentiation, and morphological patterning during embryogenesis; the Shh signaling pathway also promotes maintenance of adult stem cells and is involved in tumorigenesis[89]. In the hepatic SC line HSC8B, Shh physically interacts with caveolin-1 within the LRs in the Golgi apparatus to form large protein complexes that are packaged as large punctuate structures (transport vesicles) and transported to the PM in an LR-dependent manner[90]. Collectively these data suggest that LR manipulation can have a significant impact on liver SCs.
LRs in endothelial cells
Liver sinusoidal endothelial cells (ECs) isolate and protect the hepatocytes from passing blood and play an important role in hepatic microcirculation. Microvascular exchange in the liver is governed by fenestrations in sinusoidal ECs and is key to proper liver function. One of the basic properties of liver endothelium is the fact that there is no basal lamina, thus allowing free passage of macromolecules up to medium-sized chylomicrons[91]. The fenestrae are surrounded by a dense ring of actin, whereas the sieve plates are formed by microtubules; both number and size of fenestrae can be regulated by a variety of processes that will impact on hepatic function. For example, loss of fenestrae or sinusoidal capillarization occurs in alcoholic liver disease (ALD) and cirrhosis, respectively[91,92]. While the details in the liver are unknown, in other vascular systems the ECs are heavily governed by the functionality of their LRs. Disruption of LRs in ECs causes loss of cell viability and altered cell morphology, including loss of fenestration[93,94]. LRs govern the function of EGFR, multi-drug resistance P-glycoprotein, and adhesion molecules in ECs, and are critical to a sound EC-gated vascular barrier, at least at the blood/brain interface[95-98]. LRs also house the NADPH oxidase components gp91, p22phox, and p47phox; several pro-inflammatory cytokines, such as TNF-α, employ a LR-dependent mechanism to trigger oxidative stress and eNOS production in ECs[99].
LRs in cholangiocytes
Cholangiocytes outline the intrahepatic bile ducts; they are of epithelial origin, share their common hepatoblast progenitor with the hepatocytes and, similar to them, are polarized cells[100]. The cholangiocytes function as regulators of ductal bile secretion; in addition, they absorb and secrete water, organic anions, organic cations, lipids, and electrolytes[101]. Cholangiocytes interact with the immune cells and are potent cytokine producers, thus playing a role in liver immune responses[100,101]. Several research groups have recently reported that polarized primary rat cholangiocytes express LRs[102,103]. McWilliams identified that Shank2E, which is an ankyrin repeat-rich multidomain scaffolder, is localized in the LRs of the apical surface of the cholangiocytes[103]. LRs not only host but are also vital for the function of Shank proteins, which involves coordination of the targeted delivery of the proteins to the apical membrane in actin-containing cytoskeleton-dependent fashion[103]. These novel findings point to the role of LRs in cholangiocyte function.
LRs in non-resident liver immune cells (dendritic cells, natural killer cells, lymphocytes)
At any given time healthy liver accommodates a wide variety of non-resident immune cells, including dendritic cells (DCs), natural killer (NK) cells and lymphocytes; selective recruitment and retention of certain immune populations occurs during diverse liver diseases and these cells play a critical role in development and resolution of liver inflammation, remodeling and injury[2]. All immune cells have LRs as components of their cellular membranes; more importantly, LRs participate in some of the key functions of the immune cells.
DCs recognize, engulf, process and present antigens to other immune cells while producing a wide array of immune-modulating factors, including cytokines, chemokines, and metabolites, to control the immune responses. LRs provide the signaling platforms for a variety of DC receptors, including TLRs, major histocompatibility complex molecules, and co-stimulatory molecules; disruption of LRs significantly impairs the functional capacity of DCs[104-106]. More recently, Wang et al[107] reported that high-density lipoprotein (HDL) promotes reverse cholesterol transport and is protective against dyslipidemia and atherosclerosis. Further, they reported that HDL and apolipoprotein A-I promoted tolerance and inhibited immune responses by inhibiting the ability of antigen presenting cells, and DCs in particular, to stimulate T cells in a cholesterol-dependent manner[107]. HDL-induced cholesterol efflux from cells alters their LR structure, which in turn activates the TNF-α converting enzyme ADAM17-dependent processing of transmembrane substrates[108]. These findings suggest that LRs may play a role in HDL-induced DC impairment and further regulate the inflammatory processes; these authors also suggested that modulation of LRs in DCs could provide a desirable approach to inducing immune tolerance[108].
NK cells link innate and adaptive immunity via the production of cytokines and have the ability to kill/lyse infected non-malignant and tumor cells. Unlike other immune cells, NKs are activated and inhibited by separate sets of receptors which ensure their rapid initiation and prompt silencing to promote immune response without autoimmune reactions. LRs are implicated in both activating and limiting steps of NK function. Upon CD2 cross-linking or target cell binding, the NK-activating receptors aggregate in the LRs, which further leads to the formation of complexes of LAT with PI3K and PLC-γ1 which are essential for the NK lytic mechanisms[109]. Moreover, NK cell cytotoxicity was found to be closely related to total plasma cholesterol concentration in humans[110]. Further, colocalization of the IL-12 receptor and FcγRIIIa to LRs leads to activation of ERK and enhanced production of interferon-γ by NKs[111]. Engagement of inhibitory receptors by HLA class I on target cells blocks phosphorylation of 2B4 receptor which is found exclusively in LRs. CD94/NKG2A is an ITIM-containing inhibitory receptor expressed by NK cells that recognizes HLA-E; the engagement of this receptor prevents NK cell activation by disruption of the actin network and exclusion of LRs from the point of contact with its ligand. The latter structure is an inhibitory NK cell immunological synapse (iNKIS) and is key to NK function. Thus, targeted LR exclusion from the iNKIS is an active process that aims to maintain LRs outside the inhibitory synapse[112,113]. These data suggest that LRs are actively engaged in dousing the NK activity. However, the DCs and NK cells work closely together; in the reverse order of events, activation of resting NK cells by mature DCs is important at the initiation phases of immune responses; more importantly, DC/NK cross-talk is dependent on CX3CL1, intact cytoskeleton and LRs[114].
Lymphocytes are classified into T and B cells, and further differentiated in diverse subpopulations depending on their abilities to produce certain cytokines or antibodies, respectively.
In T-lymphocytes, LRs are implicated in signaling from the T-cell antigen receptor (TCR) and in localization and function of proteins recruited/activated downstream from the receptor. Statins (inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase) block cholesterol biosynthesis and reduce the pathogenesis of classical T-cell-inducing damage in experimental autoimmune encephalomyelitis by interfering with leukocyte recruitment, transmigration through vascular barriers and their adhesion, and ultimately limiting T-cell activation, as reviewed by Weber et al[115]. Polyunsaturated fatty acids (PUFAs) intercalate and thus remodel LRs; treatment of TCR-stimulated T-lymphocytes with PUFAs leads to miss-localization of LR-anchored TCR adaptor LAT, reduction in LAT tyrosine phosphorylation and low IL-2 production[116]. PUFAs inhibit the formation of the immune synapse[117]; it remains to be determined if this is due to isolated effects of PUFA on LRs of T cells, on DCs, or on both. Exposure of T cells to sialidases, which are able to hydrolyze the non-reducing terminal sialic acid linkage, led to control of LR-associated glycosphingolipid content and influenced activation in T-lymphocytes. Furthermore, sialidase inhibitor oseltamivir (Tamiflu) down-regulated the expression of LR-resident GM1 on the surface of T-cells and reduced cytokine production in activated T-cells[118,119]. These results suggest that LR-dependent modulation of T cells has translational potential.
In B lymphocytes, B-cell depleting anti-CD20 antibodies, namely rituximab, induced translocation of CD20 to the membrane LR fraction and triggered cell apoptosis; this process was prevented by LR disruption[120,121]. While the detailed mechanisms of such LR-mediated cytotoxicity of malignant B cells is not well understood, it is known that in normal B cells, trafficking of internalized Ag/B-cell receptor (BCR) complexes to intracellular Ag-processing compartments is driven by ubiquitination of the cytoplasmic domain of the BCR. The ubiquitinated Ag/BCR complexes are formed via a signaling-dependent mechanism and restricted to PM LRs[122]; the signaling from BCR is initiated only when the LR localization is accomplished. At this stage, Bright, which is a B-cell-restricted factor, forms complexes with Bruton’s tyrosine kinase (Btk) and its substrate transcription initiation factor-I (TFII-I), and acts to activate immunoglobulin heavy chain gene transcription in the nucleus[123]. In resting B cells, palmitoylated pool of Bright is diverted to LRs where it associates with signalosome components. After BCR ligation, Bright transiently interacts with sumoylation enzymes, blocks calcium flux and interferes with phosphorylation of Btk and TFII-I; Bright is then excluded from LRs as a Sumo-I-modified form. The remaining LR-associated Bright contributes to the signaling threshold of B cells: the sensitivity to BCR stimulation decreases as the levels of Bright increase[123]. Thus, LRs play a key role in governing B cell function.
IMPLICATIONS OF LRS IN LIVER DISEASES
Bacterial and viral diseases
Role of LRs in bacterial infections involving liver: Listeria monocytogenes (LM) causes severe liver disease in children, pregnant women and in immunocompromised individuals. LM proteins internalin and InIB bind E-cadherin (E-CAD) and HGFR, respectively; engagement of E-CAD and HGFR are sufficient for LM invasion into the host hepatocyte[124]. More importantly, intact host LRs are needed to complete the E-CAD/HGFR-mediated entry of LM[124,125].
Elevated intestinal permeability is implicated in the pathogenesis of a wide variety of diseases, including those of liver[126,127]. While the translocation of whole bacteria across the intact intestinal wall is unusual, the inflamed gut is permissive to bacterial-derived products, including endotoxin, which acts as TLR4 ligand and is considered a 2nd hit in the pathogenesis of several liver diseases, including non-alcoholic fatty liver disease and ALD[12,13,126,127]. In this context, it has been hypothesized that gut colonization plays a key role in the pathogenesis of liver diseases. Campylobacter species represent a risk factor for the development of inflammation in the GI tract via largely unknown mechanisms. Kalischuk et al[128] reported that Campylobacter jejuni (C. jejuni) induced translocation of commensal intestinal bacteria to the liver of infected mice. In vitro Campylobacter-induced internalization and translocation of Escherichia coli (E. coli) occurred via a transcellular pathway without increasing epithelial permeability; this process was blocked by cholesterol depletion and thus LR impairment[128]. Invasion-defective mutants and Campylobacter-conditioned cell culture medium also favored E. coli translocation, indicating that C. jejuni does not directly “shuttle” other bacteria into enterocytes. In C. jejuni-treated epithelial monolayers, translocating E. coli was associated with LRs and this phenomenon was blocked by cholesterol depletion[128]. However, translocation of commensals does not require the presence of active infection with pathogens at the intestinal epithelial border. Clark et al[129] reported that interferon (IFN)-γ influences the epithelium to mediate transcellular translocation of E. coli C25; this process required intact LRs. These data suggest that intestinal LRs are key to establishing leaky gut, which in turn plays a role in the pathogenesis of liver diseases; it remains to be identified whether liver infection with translocated commensal intestinal bacteria is mediated by the LRs in hepatocytes.
Role of LRs in viral infections of liver: (1) Hepatitis C virus (HCV). The lifecycle of HCV is dependent on the integrity of LRs. Using a subgenomic HCV replicon system, Aizaki et al[130] showed that HCV RNA synthesis occurs in LRs. HCV replication complex is highly dependent on the abundance of cholesterol and it is protected within LRs; only LRs that contain both non-structural proteins and viral RNA were capable of performing HCV RNA synthesis using the endogenous HCV RNA template, while depletion of cellular cholesterol selectively reduced HCV RNA replication[130]. However, the virus has preferences for specific types of LRs. Core protein, for example, does not colocalize with classical PM LR markers, such as caveolin-1 and the B subunit of cholera toxin, suggesting that core protein is bound to cytoplasmic raft microdomains distinct from caveolin-based LRs[131]. Furthermore, while both the structural core and NS5A protein associate with membranes, they do not always colocalize in the LR. Finally, the ability of core protein to localize to the LRs does not require other elements of the HCV polyprotein[131]. These results suggest that the LR implications in HCV lifecycle are complex and require further investigation. Nevertheless, HCV takes over LR control in the host cells and leads to modification of host LR-resident proteome upon its replication[131]. Roughly 10% of proteins residing in LRs of HUH7 cells are modified by HCV presence[132]. Interestingly, the majority of the host proteins involved in HCV recognition/internalization/sensing[133] including CD81, claudins, TLR2, LDL-R, Scavenger Receptor type B, and DC-SIGN/L-SIGN reside in LRs[134-140]. It is thus plausible to foresee that LR modulation may have a place in anti-HCV therapy; the proof of this hypothesis is awaited; (2) Hepatitis B virus (HBV). Funk et al[141] identified that duck hepatitis B virus (DHBV) attaches predominantly to detergent-soluble LR domains on the PM, but that cholesterol depletion from host membranes and thus disruption of LRs does not affect DHBV infection. In contrast, depletion of cholesterol from the envelope of both DHBV and human HBV strongly reduces virus infectivity[141]. Cholesterol depletion increases the density of viral particles and leads to changes in the ultrastructural appearance of the virus envelope; the infectivity and density of viral particles were partially restored upon cholesterol replenishment. Binding and entry of cholesterol-deficient DHBV into hepatocytes were not significantly impaired, in contrast to their release from endosomes. The authors therefore concluded that viral, but not host cholesterol is required for endosomal escape of DHBV[141]. Bremer et al[142] did not confirm a role of LRs in viral binding, but identified that LRs are indispensable for the entry process of HBV and might be important for a later step in viral uptake, such as fusion in a yet-unknown compartment.
Cholesterol accumulation/storage diseases
Niemann-Pick type C disease (NPCD) is a lysosomal storage disorder that affects the neural system and viscera, including liver[143,144]. Defective trafficking of cholesterol, sphingolipids and fatty acids leading to endosomal cholesterol sequestration coupled to impaired cholesterol transport to PM and ER were identified as key in the pathogenesis of NPCD[145]. Sequestration of cholesterol/sphingolipid-rich LRs in the late endosomal compartment was also reported in NPCD[146,147]. Vainio et al[76] reported a significant perturbation of LR composition and LR-dependent signaling in NPCD livers. They also showed that the membrane of NPCD hepatocytes is stabilized with cholesterol, exhibits higher anisotropy and resides in a more ordered phase; i.e. it is less fluid and thus has higher ratio of raft/non-raft content, compared to normal hepatocytes. More importantly, these rigid LRs lead to increased expression and enrichment of IR in the membrane of NPCD hepatocytes. Further, IR redistribution was associated with its defective function, thus aggravating the metabolic syndrome in NPCD[76].
Alcohol-induced liver disease
Alcohol exposure is associated with a significant amount of ROS production, which leads to oxidative stress[148-156]. While the generation of alcohol-induced oxidative stress is well documented, the understanding of the effects of oxidative stress on different molecular systems is poor. Marquês et al[46] employed in situ AFM to show that low ethanol concentrations lead to a marked thinning of the fluid but not of the gel domains of the model membranes, due to water/bilayer interfacial tension variation and freezing point depression, induction of acyl chain disordering (including opening and looping), tilting, and interdigitation. These results suggested that ethanol influences the bilayer properties by altering the lateral organization of the membrane. Several groups have confirmed the pivotal role of membrane fluidity in ethanol-induced oxidative stress using both in vitro and in vivo models of alcohol exposure[150-156]; however, the role of LRs per se in this process is still under evaluated.
In primary rat hepatocytes, short-term alcohol exposure increases membrane fluidity and leads to ethanol-induced ROS generation, which interferes with mitochondria, ER, cytochrome P450, cytosolic free iron, and cytosolic enzymes such as xanthine oxidase or aldehyde oxidases[153,154,157]. More recently, it was appreciated that LRs play a pivotal role in ethanol-induced oxidative stress in hepatocytes[152]. Alcohol exposure modulates the LR composition of hepatocytes, causes spontaneous clustering of LRs due to elevation in membrane fluidity, and promotes the increase of low-molecular-weight iron intracellular content via the activation of a PI-PLC-dependent mechanism. Further, ethanol oxidizes the LR proteins, as indicated by the increased LR content of malondialdehyde-acetaldehyde (MDA) adduct, which is a secondary end product of the degradation of oxidized PUFAs that can diffuse from its production site to react with free amino groups in proteins and lipids. From the therapeutic point of view, thiourea and vitamin E pretreatment inhibited the alcohol-induced MDA adduct formation in LRs[152]. The effects of chronic alcohol exposure on LR function in hepatocytes are largely unknown.
Alcohol exposure affects the Shh signaling in an in vivo model of fetal alcohol syndrome in zebrafish[90]. Various cell function alterations due to changes in cell membrane composition in alcohol-induced fetal damage have also been described[15]. Mao et al[90] identified that alcohol does not significantly interrupt translation of Shh mRNA in ER or the trafficking of Shh from the ER to the Golgi apparatus in the SC line HSC8B. However, alcohol does prevent the entry of Shh into transport vesicles from Golgi to PM and specifically decreases the amount of caveolin-1/Shh complex found in LRs, causing cytoplasmic accumulation of Shh and leading to a deficiency of Shh ligand secretion into the extracellular matrix[90]. These data indicate that SC-mediated tissue remodeling upon alcohol exposure involves LRs.
In macrophages, and presumably KCs, acute alcohol exposure dampens the inflammatory response to pathogens via TLRs in a LR-dependent manner and involves downregulation of MAPK activity, low nuclear factor κB activity and impaired TNF-α production[81,82,158]. Such profound inhibitory effects could be envisioned as a protective measure; the effects of chronic alcohol exposure on LR function in Mf are largely unknown.
RAFTS AS THERAPEUTIC TARGETS
Development of new treatment strategies using low-toxicity high-efficiency chemotherapeutic agents is a forefront priority in research. Several novel approaches to therapeutic agents are based on LRs. In drug discovery, a viable strategy to block viral entry and its replication may incorporate the use of natural dietary and plant-derived compounds that target LRs; this strategy is based on the affinity of these products to cholesterol, as proposed in a recent review by Verma regarding HIV[159]. The nanoparticle-based drug-delivery approach for delivery of lipophilic substances to the target cell PM acts via lipid mixing and subsequent intracellular trafficking through LR-dependent processes[160]; the advantage of the latter strategy is in its ability to deliver therapeutics specifically to selected cell types, thus limiting general toxicity.
Regulation of cell death is yet another area influenced by LR modulation. Cell death occurs at low rate in normal tissue and stimulates tissue regeneration as part of normal organ lifecycle; when the tissue is malignant the cell death is deliberately desired. LRs ensure the connection between extrinsic and intrinsic apoptotic pathways[161,162], thus become an attractive therapeutic target. The Fas death receptor (CD95 or APO-1) delivers apoptotic signals through binding to its cognate ligand, FasL (CD95L). However, because of severe liver toxicity due to the high presence of Fas in hepatocytes, the putative clinical antitumor action of FasL cannot be accomplished for extra-hepatic tumors; the utility of this approach for liver tumors is yet to be defined. Recent evidence for FasL-independent activation of Fas suggests that the death receptor can also be activated intracellularly, in the absence of its ligand. According to Mollinedo et al[161], Fas-mediated apoptosis involves translocation of Fas - and downstream signaling molecules - into LRs, a process that can be pharmacologically modulated. FasL-independent clustering of Fas in membrane LRs generates high local concentrations of death receptor, providing scaffolds for coupling adaptor and effector proteins involved in Fas-mediated apoptosis. Thus, LRs act as the linchpin from which a potent death signal is launched and have become a new promising anticancer target[161-164]. The utility of this therapeutic approach has been explored in B cell malignancy[120,121,163] and seems plausible for other diseases.
Sakamoto et al[165] reported that a secondary fungal metabolite, NA255, which targets LRs, also inhibits HCV replication and suggested that the inhibition of sphingolipid metabolism and thus LR modulation may provide a new therapeutic strategy for treatment of HCV infection. Hirata showed that serine palmitoyltransferase (SerPT) inhibitor had the ability to favor the transport of HCV RNA-dependent RNA polymerase NS5B to LRs, thus facilitating binding of sphingomyelin to NS5B[166]. Further, the authors identified that the anti-HCV effect of SerPT inhibitor could be translated to in vivo systems using humanized chimeric mice: SerPT inhibitor led to a rapid decline in serum HCV-RNA of about 1-2 log within 8 d. Furthermore, combination therapy of SerPT inhibitor and PEG-IFN achieved about 3 log reduction in serum HCV-RNA[166]. Several research groups have reported that lipid metabolism could be a target in anti-HCV therapy[165-167]. Amemiya et al[167] used combination treatment with myriocin, a sphingomyelin synthesis inhibitor, and IFN, a pleiotropic cytokine, or myriocin and simvastatin, an inhibitor of cholesterol biosynthesis with pleiotropic effects owed to inhibition of prenylation, to show synergistically attenuated HCV replication. They also identified impaired replication of HCV-1b replicon and of JFH-1 strain of genotype 2a infectious HCV RNA in Huh7/Rep-Feo cells, upon treatment with either myriocin-based combination[167]. While encouraging, these data do not fully translate into human systems: O'Leary et al[168] recently reported that statins alone do not exhibit antiviral activity against HCV at conventional doses.