Published online Nov 7, 2010. doi: 10.3748/wjg.v16.i41.5263
Revised: September 13, 2010
Accepted: September 20, 2010
Published online: November 7, 2010
Primary malignant liver mesenchymal tumor is a rare condition defined as a tumor with vascular, fibrous, adipose, and other mesenchymal tissue differentiation. We report a case of primary malignant liver mesenchymal tumor in a 51-year-old male with anemia, weight loss and hepatomegaly. Finally unconventional liver biopsy and histological manifestation led to the definitive diagnosis.
- Citation: Chen J, Du YJ, Song JT, E LN, Liu BR. Primary malignant liver mesenchymal tumor: A case report. World J Gastroenterol 2010; 16(41): 5263-5266
- URL: https://www.wjgnet.com/1007-9327/full/v16/i41/5263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i41.5263
Primary malignant liver mesenchymal tumor is a very rare tumor, accounting for less than 1% of all hepatic malignancies[1]. Hepatic angiosarcoma, leiomyosarcoma, embryonal sarcoma, schwannoma and lymphoma are the more common mesenchymal tumors. The diagnosis is dependent on histological imaging. However, some cases of multiple nodular lesions can only be diagnosed by percutaneous liver biopsy (PLB) without surgery. We present a case of malignant liver mesenchymal tumor in an adult and its diagnosis and treatment were discussed.
A 51-year-old Chinese male was referred to our hospital with a 2-mo history of mild abdominal pain, an abdominal mass in the upper quadrant, fatigue and progressive weight loss. His symptoms gradually worsened with fatigue 1 wk after onset of the disease. He had no fever, cough or skin lesions in the past 2 mo and no family history of liver and autoimmune diseases. Physical examination revealed mild pale conjunctiva and skin, and enlargement of the liver without percussion pain.
His complete blood hemoglobin was 58 g/L. Stool occult blood test was negative. Liver functional test showed that his alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ glutamyl tranzpehtidaze, total bilirubin, direct bilirubin, and albumin were 81 U/L, 86 U/L, 224 U/L, 73 U/L, 23.55 &mgr;mol/L, 11.74 &mgr;mol/L, 30.5 g/L, respectively. Series hepatitis markers (hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, Epstein-Barr virus, and cucumber mosaic virus) were negative. α-fetoprotein, carcinoembryonic antigen and CA19-9 concentrations were also normal. Human immunodeficiency virus and syphilis antibodies were negative. Coagulation profile was normal.
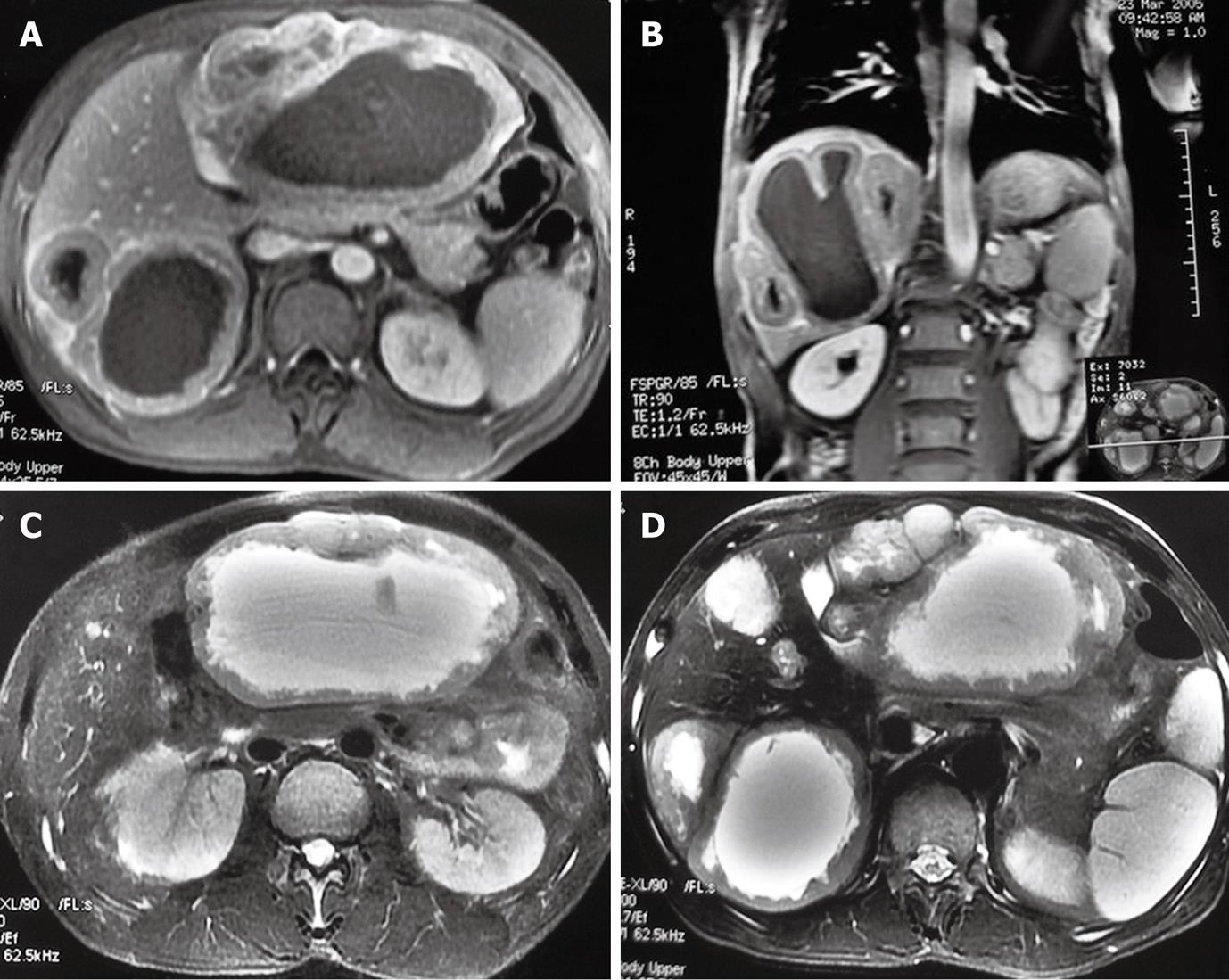
Ultrasound and magnetic resonance imaging (MRI) were used as the initial diagnostic tools. Abdominal ultrasound revealed a solid mass in the liver with a low echo, a sharp border, a rich blood supply and liquefaction necrosis. The maximum diameter of the mass was 13.9 cm. Abdominal MRI showed multiple diffuse abnormal signals suggestive of a possible malignant tumor. Axial fat-suppressed T2-weighted turbo spin echo imaging showed two larger and several smaller well-margined cyst-like lesions in the liver (Figure 1A and B). Coronal and transverse T1-weighted imaging demonstrated thickened wall of lesions with a ragged appearance and enhancement (Figure 1C and D). Endoscopy showed duodenal ulcer (S2 stage), normal colon and rectum.
PLB was performed under ultrasonography (US) guidance using a Bard biopsy gun (18-gauge cut needle) to make a final diagnosis of the lesions in liver. During biopsy, bloody liquid was observed in the lesion. Microscopic examination confirmed that the bloody liquid contained a large number of erythrocytes. However, pathology of the liver only showed a spindle cell tumor. The possible reasons are as follows. First, the liver specimen was too small because the lesion contained only fluid and was surrounded by a very thin wall. Second, the location of tumor and its hardness limited the adjustable puncture angle.
To confirm the diagnosis of this patient, another unconventional liver biopsy was performed with gastroscopic biopsy forceps as follows.
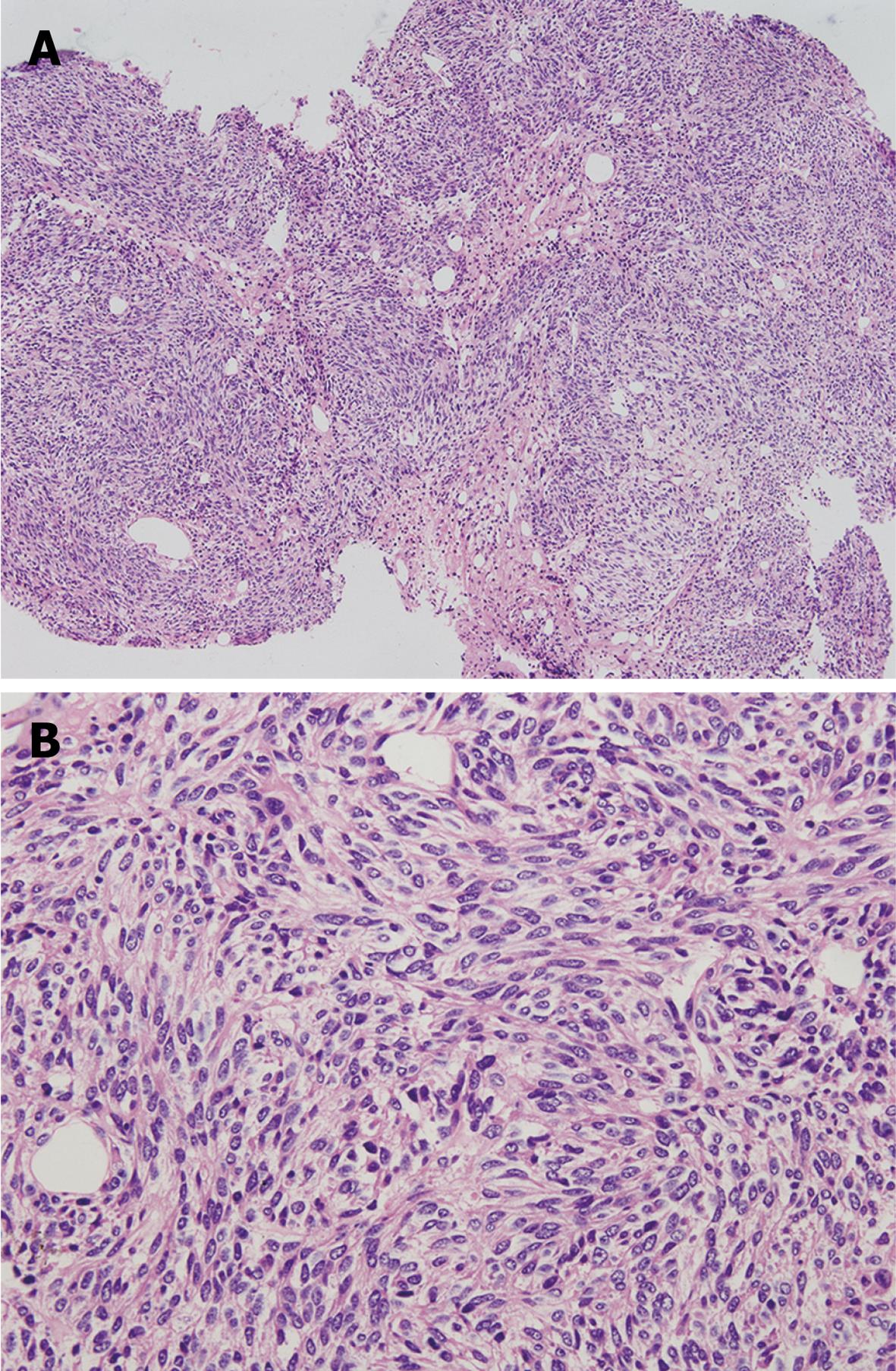
At beginning of the procedure, a local anesthetic agent (5% lidocaine) was injected subcutaneously through a 25-gauge needle. Next, a 15 cm long 18 gauge puncture needle was passed into the cyst under US guidance. After the local skin and subcutaneous tissue were dilated, a PTC exchange stainless steel guide wire with a flexible tip was introduced into the cyst cavity through the needle, and then the needle was withdrawn. A 5-French catheter was plated into the cyst using the Seldinger technique. Finally, gastroscopic biopsy forceps was passed into the cyst through the catheter to get the liver tissue sample. The histological results indicated that spindle-shaped tumor cells were well-oriented, arranged in bundles and clustered in some part of the regional tumor. Nuclei with rare mitosis were elongated in a rod-like shape with different sizes and blunt ends. The final diagnosis was established as a low grade malignant liver mesenchymal tumor (Figure 2).
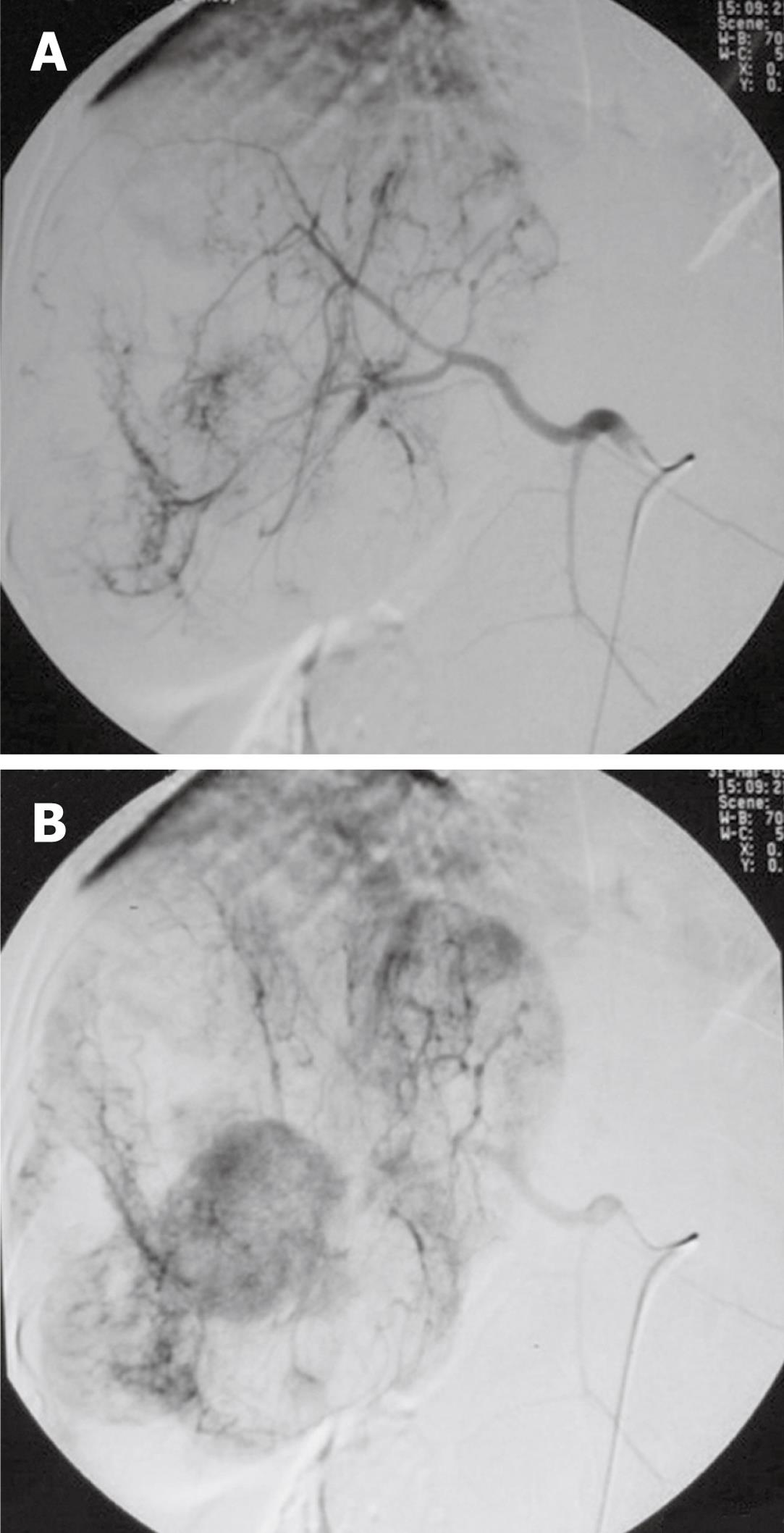
The patient was suggested to receive hepatic arterial chemoembolization. Selective digital subtraction angiography in the early and later phases showed lesions surrounded by abnormal tortuous tumor arteries (Figure 3A) and patchy enhancement (Figure 3B), respectively, in different areas. Then, 5-Fu (750 mg), mitomycin (5 mg), pyrazine imidacloprid Star (30 mg), and super-liquid iodized oil (5 mL) were infused into the tumor arteries. After treatment, liver function of the patient was improved and his hemoglobin level increased (Table 1). The patient died 2 years after treatment.
| WBC (× 109/L) | HGB (g/L) | PLT (× 1012/L) | ALT (U/L) | AST (U/L) | TBIL (&mgr;mol/L) | ALB (g/L) | |
| Diagnosis | 10.44 | 58 | 294 | 81 | 86 | 23.55 | 30.5 |
| Treatment | 6.24 | 86 | 241 | 17 | 24 | 20.74 | 34.1 |
In this paper, we presented a case of multiple nodular cystic lesions in liver. The final diagnosis was established as a low grade malignant liver mesenchymal tumor. However, HE staining could not show the source of mesenchymal cells. Further immunohistochemistry staining with Vimentin, Desmin and α-SMA was performed, which could not sill show the source of mesenchymal cells.
Primary malignant liver leiomyosarcoma and schwannoma are both rare tumors in the liver. Primary liver leiomyosarcoma is a rare malignancy involving the liver, occurring as a primary liver sarcoma in patients without any underlying disorder, and its incidence increases as a primary tumor in immunodeficiency patients[2]. Its usual clinical presentation is painful hepatomegaly or epigastric mass[3]. Malignant liver schwannoma is the most common soft tissue sarcoma in adults, but primary liver schwannoma is extremely rare. Only 12, 8, and 1 cases of benign, malignant, and semimalignant liver schwannoma are available from the literature worldwide[4-12]. Hepatic schwannoma is usually associated with neurofibromatosis. However, two cases of malignant liver schwannoma without neurofibromatosis have been reported[9,11].
Unfortunately, it is difficult to make the diagnosis of malignant liver mesenchymal tumor because both its clinical presentation and imagine are nonspecific.
The common symptoms and signs of patients with malignant liver mesenchymal tumor include abdominal pain, weight loss, weakness, loss of appetite, vomiting, enlargement of the liver, ascites, and jaundice, which lack of specificity in differential diagnosis between benign and malignant mesenchymal tumors.
Furthermore, imaging studies, such as MRI scan, computed tomography (CT) scan and angio photography are the commonly used methods to identify the characteristics of liver tumor. However, imaging findings of liver mesenchymal tumors, including leiomyosarcoma and schwannoma, are nonspecific and infrequently reported[13-19]. It is quite difficult for MRI and CT scan to differentiate primary liver mesenchymal neoplasms from other liver malignancies, although they should be included in differential diagnosis when MRI or CT scan demonstrates hepatic lesions without characteristics of hepatocellular carcinoma, especially in patients with no extrahepatic primary malignancies.
The diagnosis of malignant liver mesenchymal tumor depends on histological change in either needle or open biopsy, while metastatic status is facilitated by the presence of extrahepatic primary tumor.
Liver biopsy is an important diagnostic tool and helps make therapeutic decision for liver tumor. Open biopsy is a major surgical procedure for liver tumor. PLB under ultrasound or CT guidance is a safe and almost painless procedure for lesions with a soft tissue component or located close to vital structures[20]. Most reported cases of cystic liver mesenchymal tumor were diagnosed by open liver biopsy. In this case, uncommon PLB method was used instead of the routine PLB to make the final diagnosis, avoiding injury and complications of open biopsy. Minimally invasive treatment devices, such as gastroscopic biopsy forceps and catheters used in liver cancer intervention, make the new PLB method possible, which is safe and reliable and can thus be used in diagnosis of cystic liver lesions.
In conclusion, mesenchymal liver tumor is rare in adults and cross-sectional findings are varied, which can be diagnosed with the uncommon PLB method.
Peer reviewer: Dr. Karel van Erpecum, Department of Gastroenterology and Hepatology, University Hospital Utrecht, PO Box 855003508 GA, Utrecht, The Netherlands
S- Editor Sun H L- Editor Wang XL E- Editor Zheng XM
| 1. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [Cited in This Article: ] |
| 2. | Giuliante F, Sarno G, Ardito F, Pierconti F. Primary hepatic leiomyosarcoma in a young man after Hodgkin's disease: diagnostic pitfalls and therapeutic challenge. Tumori. 2009;95:374-377. [Cited in This Article: ] |
| 3. | Gates LK Jr, Cameron AJ, Nagorney DM, Goellner JR, Farley DR. Primary leiomyosarcoma of the liver mimicking liver abscess. Am J Gastroenterol. 1995;90:649-652. [Cited in This Article: ] |
| 4. | Tuder RM, Moraes CF. Primary semimalignant Schwannoma of the liver. Light and electron microscopic studies. Pathol Res Pract. 1984;178:345-348. [Cited in This Article: ] |
| 5. | Kim YC, Park MS. Primary hepatic schwannoma mimicking malignancy on fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography. Hepatology. 2010;51:1080-1081. [Cited in This Article: ] |
| 6. | Akin M, Bozkirli B, Leventoglu S, Unal K, Kapucu LO, Akyurek N, Sare M. Liver schwannoma incidentally discovered in a patient with breast cancer. Bratisl Lek Listy. 2009;110:298-300. [Cited in This Article: ] |
| 7. | Lee WH, Kim TH, You SS, Choi SP, Min HJ, Kim HJ, Lee OJ, Ko GH. Benign schwannoma of the liver: a case report. J Korean Med Sci. 2008;23:727-730. [Cited in This Article: ] |
| 8. | Momtahen AJ, Akduman EI, Balci NC, Fattahi R, Havlioglu N. Liver schwannoma: findings on MRI. Magn Reson Imaging. 2008;26:1442-1445. [Cited in This Article: ] |
| 9. | Kóbori L, Nagy P, Máthé Z, Hartmann E, Doros A, Paku S, Dezso K, Sápi Z. Malignant peripheral nerve sheath tumor of the liver: a case report. Pathol Oncol Res. 2008;14:329-332. [Cited in This Article: ] |
| 10. | Young SJ. Primary malignant neurilemmona (schwannoma) of the liver in a case of neurofibromatosis. J Pathol. 1975;117:151-153. [Cited in This Article: ] |
| 11. | Fiel MI, Schwartz M, Min AD, Sung MW, Thung SN. Malignant schwannoma of the liver in a patient without neurofibromatosis: a case report and review of the literature. Arch Pathol Lab Med. 1996;120:1145-1147. [Cited in This Article: ] |
| 12. | Morikawa Y, Ishihara Y, Matsuura N, Miyamoto H, Kakudo K. Malignant schwannoma of the liver. Dig Dis Sci. 1995;40:1279-1282. [Cited in This Article: ] |
| 13. | Ohtomo K, Araki T, Itai Y, Monzawa S, Ohba H, Nogata Y, Hihara T, Koizumi K, Uchiyama G. MR imaging of malignant mesenchymal tumors of the liver. Gastrointest Radiol. 1992;17:58-62. [Cited in This Article: ] |
| 14. | Danhaive O, Ninane J, Sokal E, Latinne D, Philippe M, Gosseye S, Clapuyt P, Otte JB. Hepatic localization of a fibrosarcoma in a child with a liver transplant. J Pediatr. 1992;120:434-437. [Cited in This Article: ] |
| 15. | Soyer P, Blanc F, Vissuzaine C, Marmuse JP, Menu Y. Primary leiomyosarcoma of the liver MR findings. Clin Imaging. 1996;20:273-275. [Cited in This Article: ] |
| 16. | Ferrozzi F, Bova D, Zangrandi A, Garlaschi G. Primary liver leiomyosarcoma: CT appearance. Abdom Imaging. 1996;21:157-160. [Cited in This Article: ] |
| 17. | McLeod AJ, Zornoza J, Shirkhoda A. Leiomyosarcoma: computed tomographic findings. Radiology. 1984;152:133-136. [Cited in This Article: ] |
| 18. | Noon MA, Young SW, Castellino RA. Leiomyosarcoma metastatic to the liver: CT appearance. J Comput Assist Tomogr. 1980;4:527-530. [Cited in This Article: ] |
| 19. | Soyer P, Bluemke DA, Riopel M, Hruban RH, Fishman EK. Hepatic leiomyosarcomas: CT features with pathologic correlation. Eur J Radiol. 1995;19:177-182. [Cited in This Article: ] |
| 20. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [Cited in This Article: ] |