Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3396
Revised: February 3, 2007
Accepted: February 10, 2007
Published online: June 28, 2007
Gastrointestinal stromal tumor (GIST) represents the most common kind of mesenchymal tumor that arises from the alimentary tract. GIST is currently defined as a gastrointestinal tract mesenchymal tumor showing CD117 (c-kit protein) positivity at immunohistochemistry. Throughout the whole length of the gastrointestinal tract, GIST arises most commonly from the stomach followed by the small intestine, the colorectum, and the esophagus. Only 3%-5% of GISTs occur in the duodenum, and especially, if GIST arises from the C loop of the duodenum, it can be difficult to differentiate from the pancreas head mass because of its anatomical proximity. Here, we report a case of duodenal GIST, which was assessed as a pancreatic head tumor preoperatively.
- Citation: Kwon SH, Cha HJ, Jung SW, Kim BC, Park JS, Jeong ID, Lee JH, Nah YW, Bang SJ, Shin JW, Park NH, Kim DH. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol 2007; 13(24): 3396-3399
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3396
Gastrointestinal stromal tumor (GIST) is currently defined as a gastrointestinal tract mesenchymal tumor containing spindle cells (or less commonly epithelioid cells or rarely both) and showing CD117 (c-kit protein) positivity[1-3]. Being a rare entity, GIST represents the most common subset of mesenchymal tumors that arise from the alimentary tract[1]. Primary GIST is solitary rather than multiple. GISTs are common in the stomach (60%-70% of cases), small intestine (30%), and rarely from the the rectum (5%), esophagus, colon and appendix[1-3]. There are also sporadic reports of GIST arising from the omentum, mesentery, and retroperitoneum.
Duodenal GISTs comprise less than 5% of all cases and it can be diagnosed under upper gastrointestinal endoscope due to formation of a gross ulceration in the mucosa or an intramural mass with a centrally ulcerated umblication[4]. However, if there are no specific mucosal changes in the duodenal lumen or presence of anatomical variation, it may be difficult to differentiate a duodenal GIST from a malignant lymphoma, duplication cyst, retroperitoneal tumor, or pancreatic head tumor. We report herein a rare case of a duodenal GIST masquerading as a pancreatic head tumor with history of gastric surgery due to early gastric cancer.
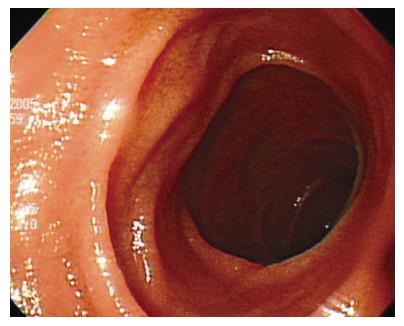
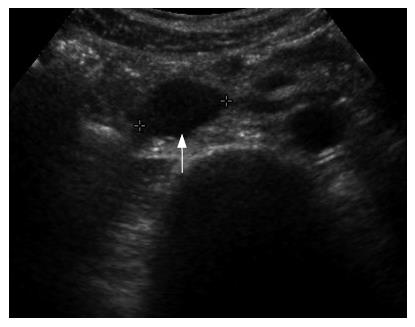
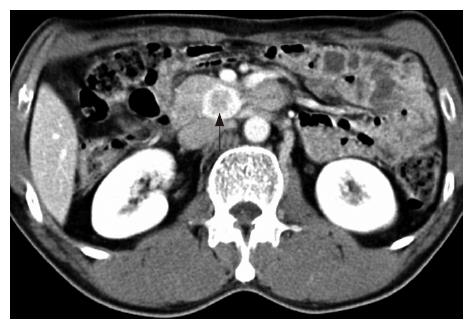
An asymptomatic 49-year-old man who underwent gastric resection was admitted to our center for the evaluation of an incidentally found pancreatic head mass. Two years ago, the patient was diagnosed with early gastric cancer and underwent subtotal gastrectomy and gastroduodenostomy. The histological examination revealed 2.5 cm × 1.5 cm sized poorly differentiated adenocarcinoma with confinement to the mucosa, free of resection margin, and free of lymph node involvement. Thereafter, the patient has been examined with endoscopic and radiologic evaluation at regular intervals. On admission, the patient appeared well-nourished, and physical examination findings were unremarkable. Laboratory examinations including peripheral blood cell count, blood chemistry, and serum tumor markers were within normal limits. Duodenal endoscopy, performed by an experienced endoscopist, neither showed mucosal nor submucosal abnormalities in the duodenum (Figure 1), nor any evidence of gastric cancer recurrence in the remnant stomach. Abdominal ultrasonography revealed 2.3 cm sized, oval-shaped hypoechoic solid nodule around the uncinate process of the pancreas (Figure 2) and the computed tomography scan showed a tumor in the uncinate portion of the pancreas, measuring 2.3 cm in diameter. The tumor was well-demarcated and strongly enhanced on contrast-enhanced CT images (Figure 3). On the basis of above findings, the presence of a tumor in the uncinate process of the pancreas, most likely a non-functioning islet cell tumor, was strongly suspected.
At laparotomy, a 2.3 cm sized soft tissue lesion was found lying between the second and third parts of the duodenum and mobilization of the duodenum revealed a tumor protruding from the duodenal wall, posterior to the head of the pancreas with a minimal adhesion. After dissection of the inferior portion of the pancreas head from the duodenal wall, segmental resection of the duodenum and duodenojejunostomy was performed.
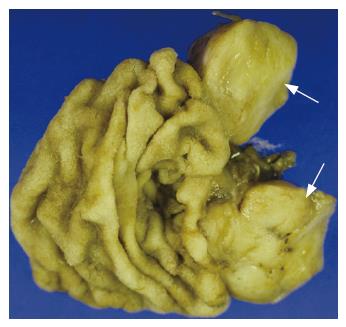
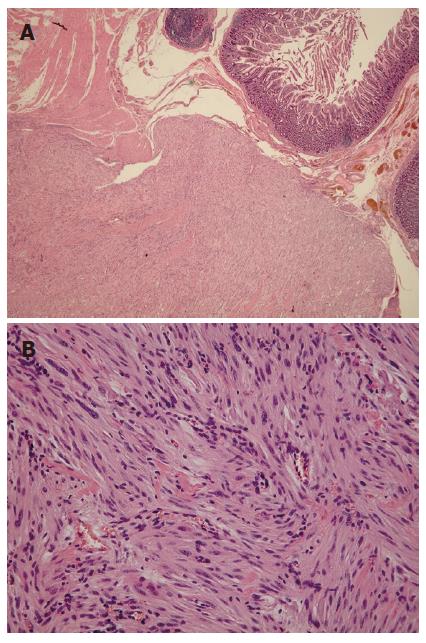
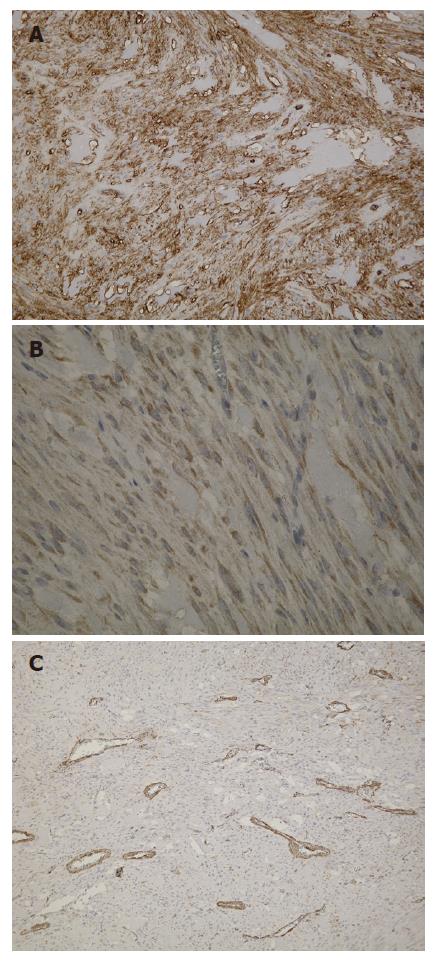
Macroscopic examination of the surgical specimen showed a well-demarcated round yellowish mass measuring 2.3 cm in maximum diameter (Figure 4). The tumor arose from the muscularis propria of the duodenum, and extended outwards to the lumen. Under microscopic examination, the tumor showed spindle cells with rare mitotic figures on the H&E stained tissue sections (Figure 5). Immunohistochemical study revealed positive staining for c-kit protein and CD34, but negative reactions to α-smooth muscle actin (Figure 6). Based on the above findings, the tumor was finally diagnosed as a GIST with low-grade malignancy originating from the duodenum.
The postoperative course proved unremarkable and further treatment was felt to be unnecessary. Clinical follow-up was on-going at the time of this report without any evidence of recurrence.
Gastrointestinal stromal tumors are low-grade malignant mesenchymal tumors of gastrointestinal tract and are believed to originate from the neoplastic transformation of the interstitial cells of Cajal from their precursors[5]. Mesenchymal tumors developing from the gastrointestinal tract had been believed to be of neuronal or muscle cell origin. However, recent immunohistochemical studies have shown that most tumors showing no typical staining for muscle type or neuronal type cells could be classified as GISTs, which are now defined as tumors showing the expression of a specific, c-kit proto-oncogene product (CD117); that is, a receptor tyrosine kinase protein (KIT)[6,7]. Approximately 70% of all GISTs are positive for the CD34 protein, which is a hematopoietic progenitor cell antigen[8], while 20%-30% are positive for SMA, 10% are positive for S-100 protein, and fewer than 5% are positive for desmin, which is the intermediate filament protein of smooth, skeletal, and cardiac muscle cells[1].
GISTs can occur throughout the gastrointestinal tract, but may also occur in the peritoneum and extragastrointestinal sites. Duodenal GISTs most frequently involve the second portion of the duodenum, followed by the third portion, fourth portion, and first portion[4]. Although many of duodenal GISTs extend from submucosal or muscularis propria to external aspects (58 tumors out of 156 duodenal GISTs), most of them comprise a gross ulceration of the mucosa with a component that bulged underneath the mucosa coincidentally, which helps in the detection of the tumor upon endoscopic examination[4]. With the present patient, the tumor showed transmural growth outwards towards the lumen, and there was no specific intraluminal mucosal changes or visible mass in the duodenum.
An extensive review of the English-language literature regarding GISTs revealed only one report of a duodenal GIST with the unusual features observed in this patient. The preceding report showed minimal epithelial changes in the intraluminal surface of the duodenum with the tumor exhibiting transmural growth into the pancreatic tissue[9]. These features are rare, and it was difficult to differentiate the organ from which the tumor originated, on the US and CT images. Thus, at first, we considered the tumor to be a nonfunctioning islet cell tumor originating in the pancreatic head indicated by its well enhanced characteristics of the tumor on contrast-enhanced CT images.
The clinical presentations of duodenal GIST are highly variable according to their size and the existence of mucosal ulceration. According to the previous report, duodenal GISTs most commonly present GI bleeding, epigastric pain, palpable mass, and intestinal obstruction[4]. Small tumors, especially which are not accompanied by mucosal ulceration, are usually incidental findings upon operation, endoscopy or imaging studies for other reasons. Endoscopy may be diagnostic in duodenal GIST. However, in this case, it was unable to detect the duodenal tumor despite adequate visualization of the duodenum. This may have been due to the relatively smaller diameter and outward growth of the tumor without forming intraluminal mass or centrally ulcerated umbilication.
CT and MRI seemed to be the best imaging modalities for assessment of the primary lesion and detection of metastases. Although their relative usefulness depends on the site of the GIST, CT is particularly useful for small bowel or omental GISTs that are not accessible by endoscopy[10]. On CT, GISTs may vary from small homogenous masses to large necrotic masses. Small tumors typically appear as sharply-marginated, smooth-walled, homogenous, soft tissue masses with moderate contrast enhancement. On the contrary, large tumors tend to have central necrosis and cavitations, as well as heterogenous enhancement[11]. Lymphadenopathy is unusual with GISTs and, if present, should raise an alternative diagnosis of lymphoma or adenocarcinoma[10]. In the present patient, although the tumor had consistent imaging features of GIST, it did not show a typical growth pattern of duodenal GISTs and the tumor was located at the C loop of the duodenum that abuts the head and uncinate process of the pancreas. With such features, it is difficult to differentiate GISTs from other types of pancreatic masses, especially pancreatic islet cell tumors, which also show a hypervascular mass on CT scan.
Optimal surgical treatment of GISTs entails complete removal of the tumor with clear surgical margins including adjacent involved organs[12,13]. Because local and regional lymph node involvement are infrequent in GISTs, smaller gastric GISTs are adequately treated utilizing wedge resection instead of formal gastrectomy[12]. Similarly, local or segmental resection should be considered in the treatment of small duodenal GISTs when technically feasible[13]. Resection of the tumor with primary closure can be performed for smaller lesions if the resulting lumen is adequate and the ampulla of Vater can be preserved. Segmental duodenectomy with duodenojejunostomy can be performed for larger tumors located in the infra-ampullary portion. When the duodenal GISTs are located at the second portion of the duodenum, major resection via a pancreaticoduodenectomy or a pancreas-sparing duodenectomy is indicated. In this patient, we performed segmental resection of the duodenum with duodenojejunostomy because the tumor was located below the second portion of the duodenum and was relatively larger in size (> 2 cm) as a duodenal GIST.
Based on Fletcher’s criteria defining the risk of malignancy[2], we assessed the degree of malignancy of our patient as a low grade malignancy because the tumor was smaller than 5 cm and had rare mitotic features. The location of the tumor, however, may be one of the important prognostic factors, especially in GISTs arising in the small intestine, which showed more malignant behavior even at low level mitotic activity than GISTs developing in the stomach[14]. Additionally, Miettinen et al[4] reported that duodenal GISTs with > 2 cm but not > 5 cm in diameter and a low mitotic rate had a low frequency of malignant behavior even ten years after excision of the primary tumor. Thus, the occurrence of relapse necessitates long-term follow-up for this patient and it is hoped that a consensus on duodenal GIST prognosis and predictive markers will be established in the near future.
Our patient also had an early gastric cancer and resulting in a subtotal gastrectomy. Thereafter, a duodenal GIST was discovered incidentally. Some reports have described the concurrence of GISTs and other malignant tumors such as renal cell carcinoma, gallbladder cancer, gastric cancer, and gastric lymphoma[15]. Furthermore, about 10% of patients with GISTs have a history of synchronous or metachronous malignant neoplasms. However, the relation of GISTs with another tumor is still unknown as to whether this phenomenon is caused by genetic abnormalities or random coincidences in patients with GISTs. Supplemental genetic studies of the relation between GISTs and other malignant neoplasms should be performed.
In summary, we have described a rare duodenal GIST with an extraluminal growth mimicking a pancreatic head tumor developed two years after the resection of early gastric cancer.
S- Editor Wang J L- Editor Li M E- Editor Lu W
| 1. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1185] [Cited by in F6Publishing: 1127] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 2. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2231] [Cited by in F6Publishing: 2087] [Article Influence: 94.9] [Reference Citation Analysis (1)] |
| 3. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 421] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] [Cited in This Article: ] |
| 6. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3215] [Cited by in F6Publishing: 3008] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 7. | Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728-734. [PubMed] [Cited in This Article: ] |
| 8. | Miettinen M, Virolainen M. Gastrointestinal stromal tumors--value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol. 1995;19:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 341] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Uchida H, Sasaki A, Iwaki K, Tominaga M, Yada K, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. An extramural gastrointestinal stromal tumor of the duodenum mimicking a pancreatic head tumor. J Hepatobiliary Pancreat Surg. 2005;12:324-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol. 2004;59:487-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304, 456; quiz 532. [PubMed] [Cited in This Article: ] |
| 12. | Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Sakamoto Y, Yamamoto J, Takahashi H, Kokudo N, Yamaguchi T, Muto T, Makuuchi M. Segmental resection of the third portion of the duodenum for a gastrointestinal stromal tumor: a case report. Jpn J Clin Oncol. 2003;33:364-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 470] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 15. | Ruka W, Rutkowski P, Nowecki Z, Nasierowska-Guttmejer A, Debiec-Rychter M. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST). Med Sci Monit. 2004;10:LE13-LE14. [PubMed] [Cited in This Article: ] |