Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2895
Revised: August 28, 2005
Accepted: October 9, 2005
Published online: May 14, 2006
AIM: To investigate the role of Beclin 1 on the susceptibility of HepG2 cells to undergo apoptosis after anti-Fas antibody or doxorubicin treatment.
METHODS: Beclin 1 silencing was achieved using RNA interference. DNA ploidy, the percentage of apoptotic cells and the mitochondrial membrane potential were assessed by flow cytometry. Levels of Beclin 1, Bcl-XL and cytochrome c, and the cleavage of poly (ADP-ribose) polymerase (PARP) were assayed by using Western blots.
RESULTS: Beclin 1 expression decreased by 75% 72 h after Beclin 1 siRNA transfection. Partial Beclin 1 silencing significantly increased the percentage of subG1 cells 24 and 40 h after treatment with doxorubicin or anti-Fas antibody, respectively, and this potentiation was abrogated by treatment with a pan-caspase inhibitor. Partial Beclin 1 silencing also increased PARP cleavage, mitochondrial membrane depolarization and cytosolic cytochrome c. The pro-apoptotic consequences of partial Beclin 1 silencing were not associated with a decline in Bcl-XL expression.
CONCLUSION: Partial Beclin 1 silencing aggravates mitochondrial permeabilization and apoptosis in HepG2 cells treated with an anti-Fas antibody or with doxorubicin.
- Citation: Daniel F, Legrand A, Pessayre D, Vadrot N, Descatoire V, Bernuau D. Partial Beclin 1 silencing aggravates doxorubicin- and Fas-induced apoptosis in HepG2 cells. World J Gastroenterol 2006; 12(18): 2895-2900
- URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2895
Beclin 1, a recently discovered tumor suppressor gene located on human chromosome 17q21, is mono-allelically deleted in 40%-70% of sporadic mammary, ovarian and prostate tumors[1]. Heterozygous beclin 1+/- transgenic mice display a high incidence of spontaneous malignancies, including hepatocellular carcinoma (HCC), although the remaining beclin 1 allele is neither lost, mutated or silenced in these tumors[2,3]. These data have led to the conclusion that beclin 1 is a haploinsufficient tumor suppressor. One mechanism whereby beclin 1 haploinsufficiency can promote cancer is impaired autophagy, and increased cell proliferation[4]. However, it is not yet clear how Beclin 1 modulates cell death in cancer cells.
Several studies have reported a role of Beclin 1 in autophagic cell death. When embryonic fibroblasts from Bax/Bak double knockout mice were treated with etoposide, cells underwent a non-apoptotic cell death characterized by the presence of a number of autophagosomes/autolysosomes and the accumulation of both Beclin 1 and the APG5-APG12 complex[5]. Cell death was largely prevented by autophagy inhibitors as well as by silencing of Beclin 1 or APG5. In another study, treatment of mouse L929 fibroblastic cells or human U937 monocytoid cells with a pan-caspase inhibitor induced autophagic cell death[6]. Silencing of Beclin 1 or ATG7, another protein required for the formation of autophagic vacuoles, reduced z-VAD-induced cell death. Beclin 1 physically interacts with the anti-apoptotic proteins, Bcl-2 and Bcl-XL[7], but the functional significance of this interaction on apoptosis is yet unclear. Thus, although beclin 1-/- mouse embryos exhibited widespread cell death and died in utero, the in vitro apoptotic rate of beclin 1-/- embryonic stem cells after UV irradiation or serum withdrawal was not significantly increased compared to wild-type cells[3]. By contrast, mice infected with a recombinant chimera of the neurotropic Sindbis virus combined with full-length human Beclin 1 exhibited increased survival, fewer apoptotic brain cells and lower brain viral titers than mice infected with chimeras encoding a Beclin 1 protein lacking the Bcl-2 binding domain or with a premature stop codon[7]. However, these results did not provide information on whether the protective effects of Beclin 1 were a consequence of anti-apoptotic effects, anti-viral effects, or a combination of both mechanisms. Lastly, a recent study reported that the over-expression of Beclin 1 in MKN28 human gastric cancer cells stimulated cis-diamminedichloroplatinum (CDD)- or doxorubicin-induced cell death, while a Beclin 1 siRNA protected the cells from apoptosis in the same experimental conditions[8].
We have previously observed a significant correlation between Beclin 1 and Bcl-XL mRNA expression in human hepatocarcinoma (unpublished results), suggesting that Beclin 1 over-expression may prevent hepatocyte apoptosis. In order to test this assumption, we investigated the effect of Beclin 1 silencing on the response of HepG2 cells to apoptotic stimuli. In the present study, we showed that partial Beclin 1 silencing aggravates the mitochondrial permeabilization and the apoptotic response induced by an agonistic anti-Fas antibody or by doxorubicin in a human HCC cell line.
HepG2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 mL/L fetal calf serum and 1% penicillin/streptomycin sulfate (Gibco, Invitrogen).
Small interfering RNA (siRNA) duplexes were purchased from Dharmacon (Lafayette, CO). We used the beclin 1 siRNA: 5’-CAGUUACAGAUGGAGCUAAtt-3’ (nt 655-673), which is located downstream from the initiation codon of the human beclin coding region, and a luciferase siRNA as a control siRNA: 5’-CUUACGCUGAGUACUUCGAtt3’. HepG2 cells (15-25 × 106 cells) were collected by trypsinization. The pellet was washed with serum-free OptiMEM (Gibco, Invitrogen) without antibiotics, and cells were resuspended in 500-600 μL of the same medium containing 12 μmol/L siRNA. After 10 min on ice, cells were electroporated at 230 V, 960 μF. Cells were resuspended in DMEM containing 100 mL/L fetal calf serum without antibiotics, and were plated in 60 mm Petri dishes (approximately 106 cells per dish). Antibiotics were added 3 h later.
In a first treatment protocol, 5 μg/mL of an agonistic anti-Fas antibody (7C11, Immunotech, Beckman Coulter, Miami, FL) was added 24 h after siRNA transfection, with or without 10 μmol/L benzyloxycarbonyl-valyl-alanyl-aspartic acid (o-methyl)-fluoro-methylketone (z-VAD-fmk) (Alexis, Coger, Paris, France), a pan-caspase inhibitor, and apoptosis was detected 40 h later, except for the assessment of cytochrome c release, which was detected at 24 h.
In a second treatment protocol, 6 μmol/L doxorubicin (Sigma, Saint-Louis, MO) was added 48 h after siRNA transfection, with or without 100 μmol/L z-VAD-fmk, and apoptosis was detected 24 h later except for the assessment of cytochrome c release, which was detected at 20 h.
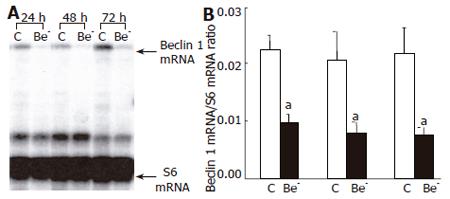
Attached HepG2 cells were washed twice with PBS and lysed in 5 mol/L guanidine thiocyanate and 0.1 mol/L EDTA (pH 7.4). RPA was carried out on cell lysates without previous RNA extraction[9]. The following DNA fragments were used to prepare riboprobes: a 200-bp fragment (nt 554-753, accession no. AF139131) from human beclin 1, and a 131-bp fragment (nt 553-683, accession no. NM00101) from human S6 used to normalize mRNA levels. The RPA and the separation of the protected mRNA fragments by polyacrylamide gel electrophoresis were performed as previously described[9]. Gel radioactivity was assessed with an Instant Imager (PerkinElmer, Courtaboeuf, France), and the ratio of the studied mRNA to the S6 mRNA was computed.
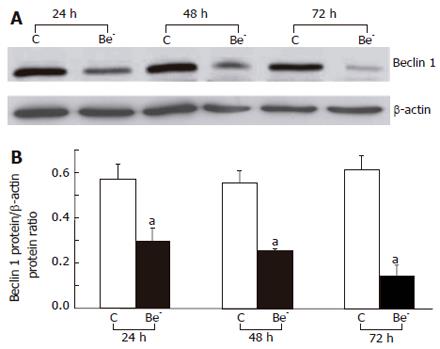
Attached HepG2 cells were washed twice with PBS, harvested in PBS, and pelleted by centrifugation. The pellet was weighed and suspended in 2 × Laemmli buffer. Proteins were separated on SDS-polyacrylamide gels (70 g/L polyacrylamide for Beclin 1 and 120 g/L for Bcl-XL) and transferred onto nitrocellulose membranes. After blocking, membranes were incubated for 1 h with either the anti-Beclin 1 antibody supplied by Dr Yoshimori[10], or an anti-Bcl-XS/L antibody (S-18) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by a horseradish peroxidase-conjugated anti-rabbit IgG antibody (Immunotech, Beckman Coulter). Specific bands were detected by an enhanced chemiluminescence system (Pierce, Rockford, IL). Membranes were subsequently probed with a monoclonal anti-β-actin antibody (Sigma), and the β-actin signal was used to normalize protein loading.
Attached and floating HepG2 cells were harvested in culture medium and pelleted by centrifugation. Nuclear proteins were extracted according to Sadowski and Gilman[11]. After protein measurement (Biorad Reagent, Pierce), nuclear extracts underwent SDS-7% polyacrylamide gel electrophoresis and blotting as described above, and were revealed with a monoclonal anti-human PARP antibody (clone C2-10, BD Pharmingen, San Diego, CA).
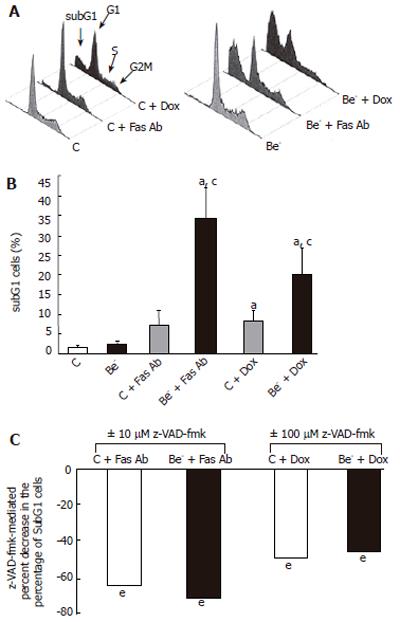
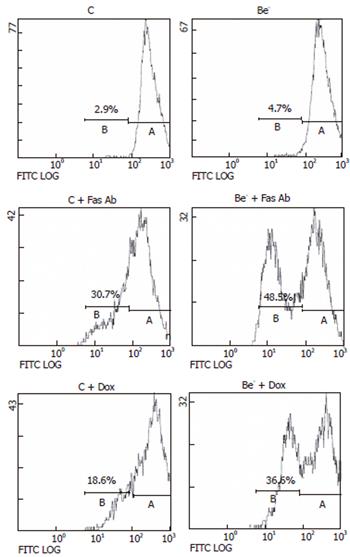
Pooled, free and adherent HepG2 cells were centrifuged, washed with PBS and fixed with 700 mL/L cold ethanol. The pellet was resuspended in 100 μL RNAse A (180 μg/mL) and incubated at room temperature for 30 min. Propidium iodide (Merck, Darmstadt, Germany; final concentration, 50 μg/mL) was added, and cells were incubated at room temperature in the dark for 15 min. The percentage of cells with a low DNA content (subG1 cells) was quantified by flow cytometry using an Epics Elite ESP coulter cytometer (Beckman coulter, Fullerton, CA). Propidium iodide was excited at 488 nm and fluorescence was analyzed at 630 nm.
To determine the mitochondrial membrane potential, we used DIOC6(3) (Molecular Probes, Eugene, OR), a lipophilic cationic dye, which accumulates into polarized mitochondria. Free and adherent HepG2 cells were collected, rinsed with PBS, and suspended in the culture medium. DIOC6(3) was added at a final concentration of 100 nmol/L, and cell suspensions were incubated at 37 °C in the dark for 30 min. Propidium iodide (1.5 µmol/L) was added before flow cytometry to exclude dead cells and to only take into account the fluorescence DIOC6(3) in living cells. DIOC6(3) fluorescence was measured by flow cytometry. DIOC6(3) was excited at 488 nm and detected at 510 nm.
Mitochondrial and cytosolic extracts were prepared using the ApoAlert® cell fractionation kit (BD Biosciences Clontech, Mountain View, CA, USA). Equal levels of protein were separated on SDS-12.5% polyacrylamide gel electrophoresis, transferred and immunoblotted with an anti-cytochrome c antibody supplied with the kit.
Data were expressed as means ± SE. An unpaired Student’s t test was used to assess statistical differences between HepG2 cells transfected with the control siRNA or the Beclin 1 siRNA. A paired Student’s t test was used to assess differences resulting from treatments in control siRNA- or in Beclin 1 siRNA-transfected HepG2 cells.
We used RNA interference to partially silence the Beclin 1 gene in HepG2 cells. The Beclin 1 mRNA was decreased by 57%, 61% and 65%, 24, 48 and 72 h after transfection of the Beclin 1 siRNA, respectively (Figure 1), and the Beclin 1 protein was decreased by 48%, 54% and 76%, respectively (Figure 2).
We then measured the percentage of cells with a decreased DNA content (subG1 cells) as a consequence of DNA fragmentation after treatment of transfected cells with doxorubicin or with an agonistic anti-Fas antibody, which both trigger apoptotic cell death in HepG2 cells[12,13]. We compared the percentage of cells with a subG1 DNA content in HepG2 cells transfected with the control siRNA (control cells) or the Beclin 1 siRNA (Be- cells) (Figure 3). The percentage of subG1 cells was not significantly increased in the untreated Be- cells compared to the untreated control cells (Figure 3B). However, after exposure to the anti-Fas antibody or doxorubicin, the increase in subG1 cells was much higher in the Be- cells compared to the control cells (Figure 3B). In both control cells and Be- cells, the pan-caspase inhibitor z-VAD-fmk markedly decreased subG1 cells after treatment with the anti-Fas antibody or doxorubicin (Figure 3C).
We also studied the apoptotic response of HepG2 cells by assessing PARP cleavage (Figure 4). The cleaved p85 fragment of PARP was not detected in the untreated control cells or untreated Be- cells. After treatment with the anti-Fas antibody, the cleaved p85 fragment was still absent in the treated control cells, but present in the treated Be- cells. After treatment with doxorubicin, the p85 fragment was more abundant, and the uncleaved p116 fragment less abundant, in the Be- cells compared to the control cells (Figure 4).
Since the apoptotic effects of Fas or doxorubicin are amplified by mitochondria[14-16], we determined whether Beclin 1 depletion increases the mitochondrial pathway of apoptosis by first assessing changes in the mitochondrial membrane potential (Figure 5). The percentage of living cells with a low mitochondrial membrane potential was not significantly increased in the untreated Be- cells compared to the untreated control cells. After treatment with the anti-Fas antibody or doxorubicin, the percentage of living cells with a low mitochondrial membrane potential was significantly higher in the Be- cells than that in the control cells (Figure 5). We next investigated whether Beclin 1 silencing increases the release of mitochondrial cytochrome c into the cytosol after treatment with the anti-Fas antibody or doxorubicin[14,16]. We firstly checked that the cytosolic fraction was not contaminated by mitochondria by assessing COX4 (data not shown). Cytosolic cytochrome c was increased by the anti-Fas antibody or the doxorubicin treatment in the control cells, and this increase was further enhanced in the Be- cells compared to the control cells, particularly after treatment with the anti-Fas antibody (Figure 6).
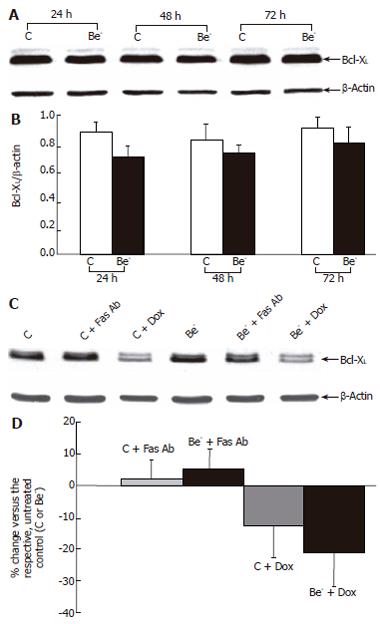
To test whether this effect could be mediated by decreased Bcl-XL expression, we assessed this protein in the untreated and treated cells (Figure 7). Bcl-XL was not significantly decreased 24, 48 or 72 h post-transfection in the cells transfected with Beclin 1 siRNA compared to the cells transfected with the control siRNA (Figures 7A-B). Treatment with the anti-Fas antibody caused no further changes (Figures 7C-D). Although treatment with doxorubicin tended to decrease Bcl-XL expression in the control cells, and even more in the Be- cells, the doxorubicin-mediated decline in Bcl-XL did not differ significantly between the control cells and Be- cells
(Figures 7C-D).
Although beclin 1 haploinsufficiency is involved in several extrahepatic human cancers[1,4], and increases the frequency of various tumors, including HCC in transgenic mice[2,3], its role in human HCC is still not clear[17], and its potential involvement in liver apoptosis has not been investigated yet. The aim of the present study was to assess the effects of partial Beclin 1 silencing on the susceptibility of HepG2 human hepatoma cells to undergo apoptosis.
In the cells transfected with a Beclin 1 siRNA (Be- cells), the Beclin 1 protein was decreased by 50% at 24 h and by 75% at 72 h compared to the cells transfected with a control siRNA (control cells). Although the spontaneous apoptotic rate of the Be- cells was not significantly increased over that of the control cells, their apoptotic response to doxorubicin or an agonistic anti-Fas antibody was markedly aggravated (Figure 3). The percentage of subG1 cells was much higher in the treated Be- cells compared to the treated control cells (Figures 3A-B) and dropped significantly when cells were pre-treated with the pan-caspase inhibitor, z-VAD-fmk (Figure 3C). In addition, PARP cleavage was more extensive in the treated Be- cells than that in the treated control cells, especially after doxorubicin poisoning (Figure 4).
Two reasons prompted us to investigate the potential effect of Beclin 1 depletion on the mitochondrial pathway. Firstly, Beclin 1 physically interacts with Bcl-2 or Bcl-XL[7,8], two anti-apoptotic proteins predominantly localized to the mitochondrial membrane that prevent loss of mitochondrial membrane potential and cytochrome c release[18-20]. Secondly, the apoptotic effects of Fas or doxorubicin are amplified by mitochondria, in particular in hepatocytes and hepatoma cell lines[14-16]. The dissipation of mitochondrial membrane potential after anti-Fas antibody or doxorubicin treatments was more severe, and the cytochrome c release was more abundant in the Be- cells than that in the control cells (Figures 5 and 6), suggesting that Beclin 1 depletion activates the mitochondrial pathway of apoptosis. In a previous study, the over-expression of full-length human Beclin 1 was associated with decreased neuronal apoptosis in mice infected with the neurotropic Sinbis virus, whereas a deleted Beclin 1 construct lacking the region interacting with Bcl-2 or Bcl-XL was not protective[7]. This previous observation may suggest that the interaction of Beclin 1 with either Bcl-2 or Bcl-XL is essential for the anti-apoptotic effects of Beclin 1[7]. Since several studies have reported that Bcl-2 is not constitutively expressed in HepG2 cells[21-23] and Bcl-2 over-expression does not protect these cells from Fas or doxorubicin treatment[22-24], Bcl-XL seems to be the major anti-apoptotic protein in the HepG2 cell line. In the present study, partial Beclin 1 silencing increased apoptosis without significantly decreasing the Bcl-XL protein (Figure 7), suggesting that normal Beclin 1 levels are required to protect HepG2 cells from apoptotic stimuli, possibly by maintaining the expression of the Bcl-XL-Beclin 1 complex. How does the interaction between Beclin 1 and Bcl-XL or other proteins act to modulate mitochondrial permeabilization and apoptosis remains to be determined.
In contrast to our results, Furuya et al[8] instead observed that Beclin 1 over-expression aggravated CDD- or doxorubicin-induced apoptosis in a gastric cancer cell line, while Beclin 1 silencing had the opposite effects[8]. However, Beclin 1 silencing did not protect another gastric cancer cell line (with lower Bcl-2 and Bcl-XL expression) against CDD-mediated apoptosis. It thus appears that Beclin 1 differently modulates apoptosis in different cell lines. Although reasons for these differences are unknown, changes in the molecular environment of Beclin 1 from one cancer cell line to another might be involved, including different expression of Bcl-2 and Bcl-XL[8], and other pro-apoptotic proteins of the Bcl-2 family interacting with these proteins, but not with Beclin 1[7,8,24,25]
In conclusion, our data demonstrate that partial Beclin 1 silencing augments the mitochondrial permeabilization and the apoptosis induced by Fas stimulation or doxorubicin treatment in hepatoma-derived HepG2 cells. As Takehara et al[21] reported that down-expression of Bcl-XL by antisense oligonucleotide aggravates apoptosis induced by staurosporine or serum deprivation in HepG2 cells, the use of both Beclin 1 and Bcl-XL antisense oligonucleotides in combination with chemotherapeutic drugs might efficiently trigger apoptosis in human hepatocarcinoma. The different effects of Beclin 1 on apoptosis in different cell lines[3,7,8] show that Beclin 1, unlike Bcl-2 or Bcl-XL, can be pro-apoptotic, anti-apoptotic or non-effective, depending on the cellular context.
The Authors are grateful to Dr. Tamotsu Yoshimori (National Institute for Basic Biology, Okazaki, Japan) for providing the anti-Beclin 1 antibody.
S- Editor Wang J L- Editor Kumar M E- Editor Bi L
| 1. | Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 582] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809-1820. [PubMed] [Cited in This Article: ] |
| 3. | Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077-15082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1534] [Cited by in F6Publishing: 1637] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 4. | Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2446] [Cited by in F6Publishing: 2546] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 5. | Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1017] [Cited by in F6Publishing: 1025] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 6. | Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 949] [Cited by in F6Publishing: 1001] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 7. | Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586-8596. [PubMed] [Cited in This Article: ] |
| 8. | Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Kaabache T, Barraud B, Feldmann G, Bernuau D, Lardeux B. Direct solution hybridization of guanidine thiocyanate-solubilized cells for quantitation of mRNAs in hepatocytes. Anal Biochem. 1995;232:225-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 672] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 11. | Sadowski HB, Gilman MZ. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993;362:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 209] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Lamboley C, Bringuier AF, Feldmann G. Apoptotic behaviour of hepatic and extra-hepatic tumor cell lines differs after Fas stimulation. Cell Mol Biol (Noisy-le-grand). 2000;46:13-28. [PubMed] [Cited in This Article: ] |
| 13. | Sadji-Ouatas Z, Lasfer M, Julien S, Feldmann G, Reyl-Desmars F. Doxorubicin and octreotide induce a 40 kDa breakdown product of p53 in human hepatoma and tumoral colon cell lines. Biochem J. 2002;364:881-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Feldmann G, Haouzi D, Moreau A, Durand-Schneider AM, Bringuier A, Berson A, Mansouri A, Fau D, Pessayre D. Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology. 2000;31:674-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Barogi S, Baracca A, Cavazzoni M, Parenti Castelli G, Lenaz G. Effect of the oxidative stress induced by adriamycin on rat hepatocyte bioenergetics during ageing. Mech Ageing Dev. 2000;113:1-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Eom YW, Kim MA, Park SS, Goo MJ, Kwon HJ, Sohn S, Kim WH, Yoon G, Choi KS. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005;24:4765-4777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Song H, Xia SL, Liao C, Li YL, Wang YF, Li TP, Zhao MJ. Genes encoding Pir51, Beclin 1, RbAp48 and aldolase b are up or down-regulated in human primary hepatocellular carcinoma. World J Gastroenterol. 2004;10:509-513. [PubMed] [Cited in This Article: ] |
| 18. | Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2197] [Cited by in F6Publishing: 2236] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 19. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2650] [Cited by in F6Publishing: 2857] [Article Influence: 84.0] [Reference Citation Analysis (1)] |
| 20. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6842] [Cited by in F6Publishing: 6716] [Article Influence: 258.3] [Reference Citation Analysis (0)] |
| 21. | Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Takahashi M, Saito H, Okuyama T, Miyashita T, Kosuga M, Sumisa F, Yamada M, Ebinuma H, Ishii H. Overexpression of Bcl-2 protects human hepatoma cells from Fas-antibody-mediated apoptosis. J Hepatol. 1999;31:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Luo D, Cheng SC, Xie H, Xie Y. Effects of Bcl-2 and Bcl-XL protein levels on chemoresistance of hepatoblastoma HepG2 cell line. Biochem Cell Biol. 2000;78:119-126. [PubMed] [Cited in This Article: ] |
| 24. | Diaz JL, Oltersdorf T, Horne W, McConnell M, Wilson G, Weeks S, Garcia T, Fritz LC. A common binding site mediates heterodimerization and homodimerization of Bcl-2 family members. J Biol Chem. 1997;272:11350-11355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Minn AJ, Kettlun CS, Liang H, Kelekar A, Vander Heiden MG, Chang BS, Fesik SW, Fill M, Thompson CB. Bcl-xL regulates apoptosis by heterodimerization-dependent and -independent mechanisms. EMBO J. 1999;18:632-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |