Published online Mar 28, 2009. doi: 10.3748/wjg.15.1512
Revised: December 16, 2008
Accepted: December 23, 2008
Published online: March 28, 2009
AIM: To repair the partial esophagus defect with a chitosan stent, a new esophageal prosthesis made of pulmonary tissue with vascular pedicle.
METHODS: Fifteen Japanese big ear white rabbits were divided into experimental group (n = 10) and control group (n = 5). Esophagus defect in rabbits of experimental group was repaired using lung tissue flap with a chitosan tube stent, gross and histological appearance was observed at week 2, 4 and 8 after operation, and barium sulphate X-ray screen was performed at week 10 after operation. Esophagus defect of rabbits in control group was repaired using lung tissue flap with no chitosan tube stent, gross and histological appearance was observed at week 2, 4 and 8 after operation, and barium sulphate X-ray screen was performed at week 10 after operation.
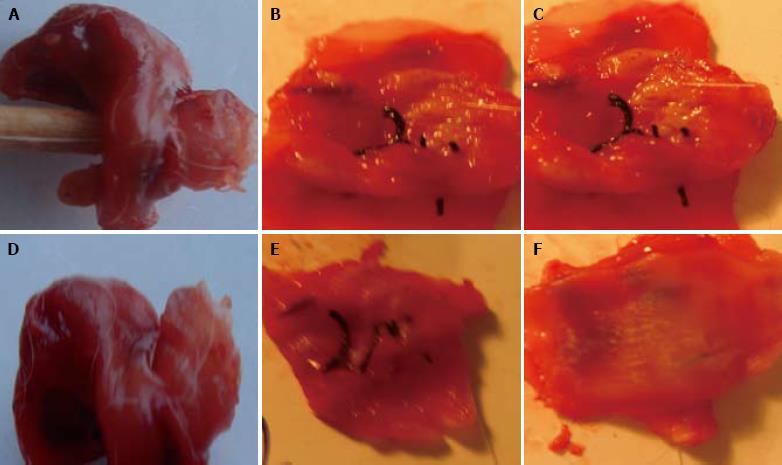
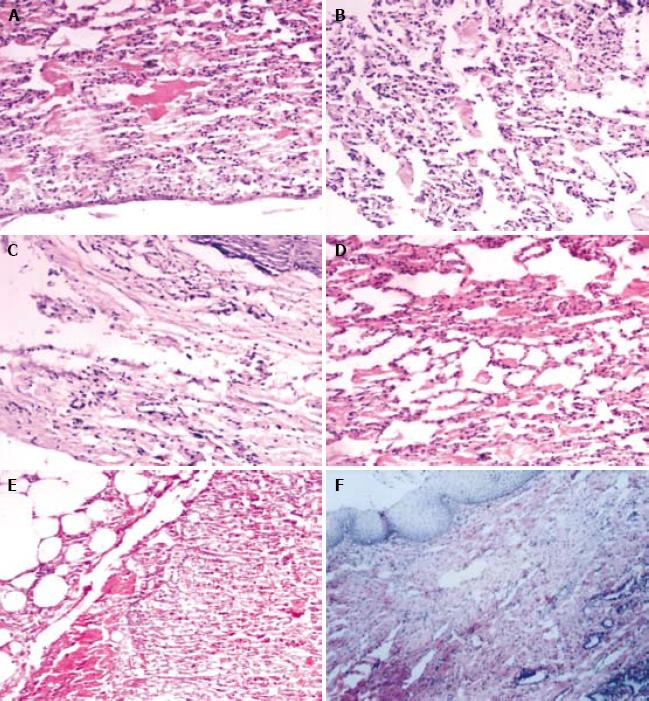
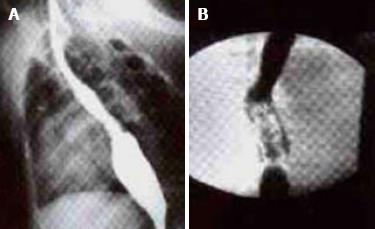
RESULTS: In the experimental group, 6 rabbits survived for over two weeks, the lung tissue flap healed esophageal defection, and squamous metaplasia occurred on the surface of lung tissue flap. At week 10 after operation, barium sulphate examination found that barium was fluent through the esophagus with no stricture or back stream, the creeping was good. In the control group, 4 rabbits survived for two weeks, the lung tissue flap healed esophageal defection with fibrous tissue hyperplasia, barium sulphate examination found that barium was fluent through the esophagus with a slight stricture or back stream, and the creeping was not good at week 10 after operation.
CONCLUSION: Esophagus defect can be repaired using lung tissue flap with an inner chitosan tube stent.
- Citation: Chen G, Shi WJ. Lung tissue flap repairs esophagus defection with an inner chitosan tube stent. World J Gastroenterol 2009; 15(12): 1512-1517
- URL: https://www.wjgnet.com/1007-9327/full/v15/i12/1512.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1512
Esophagus disease is one of the common digestive tract diseases in our country. After excision, the esophagus needs to be reconstructed to restore the digestive tract continuity. At present, the commonly used esophagus reconstruction substitutes mainly include musculo-cutaneous flap with vessel peduncle[1], platysma musculo-cutaneous flap[23], periosteum intercostal muscle flap[4] and others[5–8] from stomach, colon and jejunum. Plastic tube, metallic pipe, teflon tube, silica gel tube, etc, are also used in reconstruction of artificial esophagus. However, their effects are not really ideal. The ideal substitute should be nontoxic, absorbable, without repulsive response and carcinogenicity, and easy to gain.
At present, various esophagus reconstruction techniques are available[9–11] and each has its own particular advantages and disadvantages. Irrespective of the kind of reconstruction, reducing scar formation and preventing stricture are still the key points. Different flaps from the lung tissue petal have been successfully applied in reconstruction of trachea[12]. Application of lung tissue petal, inside lining metal and silica gel pipe stent in animals is also successful, but it is unable to overcome foreign matter response. In this study, we used inside lining metal, a new absorbable biological material-chitosan tube, to repair partial esophagus defect by preventing post-surgery stricture. Some questions concerning its application were discussed.
Fifteen healthy Japanese big ear white rabbits, weighing 3 kg, were provided by China Medical University Shengjing Hospital Animal Center. The rabbits were divided into experimental group (n = 10) and control group (n = 5). Chitosan tube stent, 15 mm long, 4 mm in inside diameter, was purchased from Shandong Province, China. Antiseptic glutaric dialdehydel were independently developed by the authors. TKR-200C micro-organism life-support machine was provided by Jiangxi Province, China. Rabbit surgery table, chest surgery instruments, infusion instruments, 20% urethane vein anesthetics, ketamine anaesthetics, 1% procaine, laryngoscope, 3.0-4.0 model trachea intubation were bought from Jiangxi Province, China. Tooth pad, medical adhesive plaster, digital camera (Sony, DSC-T10), and optical microscope (Olympics CH-20) were used in this study.
Before surgery, rabbits did not receive any medicine. Twenty percent of urethane vein anesthetics (5 mL/kg) was injected into abdominal cavity. Three minutes after anesthesia, the breath of rabbits was slow and changed to shallow and abdominal breath with corneal reflex. Ketamine (1 mg/kg) was intramuscularly injected to reduce the pain. The anaesthetized rabbits were fixed on the operation table at a supine position, and the assistant pulled in the flank with the bandage to draw in the front tooth and lower jaw. The mouth was pulled open with 1% procaine spraying. The throat was superficially anaesthetized. The operator stood in the rabbit head side, set the laryngoscope from one side of mouth, pushed away the tongue from tip to root. Epiglottis was exposed behind the tongue root resembling the white soft bone. Sometimes, vocal cord could not be found, but air bubbles could be observed. The trachea was gently pulled in intubation. The air current in the trachea pipe could be heard. When the inspiration was sent in gently, rapid vertical insertion was performed. Trachea intubation and tooth pad were fixed 13 cm away from the front teeth. When the air exhaled from the trachea intubation could be felt with hands, the life-support machine connected with oxygen was adjusted to a low current capacity.
The left side and barrier height of rabbit decubitus were exposed in the chest cavity, with the four limbs of rabbits fixed and the right flank prepared for operation of the chest. The first step was to cut open pleural membrane. When lung collapse was observed, the rabbits were given machinery ventilation, and the breath frequency was adjusted to 30 times/min, and then adjusted according to the lung inflation. A stomach tube was inserted into esophagus to support it, and the center-section was searched for its dissociation, and slung with a spun yarn cloth strip. The excision of central esophagus was an esophagus wall, 3 mm in diameter, to make a animal model of partial esophagus wall damage. In the experimental group, a chitosan tube was placed in breakage of the esophagus and fixed with a needle. The esophagus was wrapped by the nearby lung tissue petal to form an encystation in the breakage place, and the edge of esophagus breakage was sutured continuously with a 3-0 silk suture. Then, the stomach tube was withdrawn to release the stress and return the esophagus. The control group did not need any inside lining chitosan tube. No chest internal hemorrhage and lung air leakage were found. Transition to ventilation was performed several times before the last needle was inserted into the pleural membrane. At end of the inspiration, pleural cavity was closed. The rabbits received fluid diet and anti-inflammation treatment, fresh milk and normal diet a week after operation.
Observation of ordinary circumstances: Survival, feed, body weight, and complication were observed after operation.
Observation of body: If the experimental rabbits died in the observation period, prompt postmortem examination was performed to find the cause of death. The animals were killed at weeks 2, 4 and 8 after surgery, respectively. Scar formation and chitosan tube were observed.
Observation of histology: The surviving animals were executed at week 2, 4 and 8, respectively. The damaged patching lung tissue was stained with H&E. The growth of lung tissue petal was observed under optical microscope.
Barium meal: Ten weeks after operation, esophagus of survived rabbits was observed by barium meal to see whether the esophagus was unobstructed.
After operation, 6 animals in experimental group, and 4 animals in control group survived, respectively. Five animals and 1 animal died on the same day after operation, 1 animal died due to anesthesia 1 d after operation, 1 animal died of unhealed fistula on day 4 after operation, 1 animal died of infusion accident on day 7 after operation, and 1 animal died of malnutrition at week 2 after operation.
Five days after operation, animals in experimental and control groups could have oral diet. The animals were provided with a small amount of food 5 d after operation and normal diet 10 d after operation. During this period, no obvious feed barrier was observed in animals of the experimental group. Three weeks after operation, animals in the control group had a poor appetite. One week after operation, the body weight of all animals decreased about 1-2 kg. The body weight of animals in the control group was still lighter than before operation.
Two weeks after operation, the esophagus damage was observed, the esophagus breakage edge and lung tissue petal were tightly united, accompanying dropsy. The chitosan tube could be touched under the substitute lung tissue petal and was soft, and the lumen surface had membranous contamination. Four weeks after operation, the damage was completely repaired, the suture was not absorbed. In the experimental group, the internal lumen surface of esophagus substitute was smooth, the blood circulation was rich, and the chitosan tube was partly decomposed. In the control group, the damage was red in color, hyperplasia was found around the damage, accompanying dropsy. In the experimental group, eight weeks after operation, the esophagus defect was covered by the white thick membranous substance. No dropsy, necrosis and ulcer, obvious stricture, or chitosan cast were found in the pipe wall. In the control group, scar formation and hyperplasia were found on tissue petal surface (Figure 1).
Two weeks after operation, the lung structure of rabbits in experimental and control groups was observed. Pulmonary alveoli were withered and collapsed, and the denatured pulmonary alveolus cells were tumescent with acute inflammation response. Four weeks after operation, atypical pulmonary alveolus structure was observed in the experimental group. The inflammatory response was weakened accompanying a few neutral granular cells and lymphocytes, but no obvious fiber proliferation was observed. In the control group, the central pulmonary alveolus structure was atypical accompanying fiber cells and a few inflammatory cells. Eight weeks after operation, a large number of squamous epidermis cells were observed on the surface of lung tissue petal, and the chronic inflammatory response was significantly decreased in the experimental group. The control group had chronic inflammation response, accompanying obvious fiber proliferation but no superficial squamous metaplasia (Figure 2).
Ten weeks after operation, esophagus barium test was performed. In the experimental group, barium meal went smoothly through the esophagus. No obvious stricture, reverse flow, anastomotic stoma leakage and expansion were observed in the esophagus. The creeping motion was good. Mild stricture was observed in control group. The anastomotic stoma was healed with expansion, and the barium meal went through smoothly with general creeping motion (Figure 3).
Esophagus excision and reconstruction are important in esophagus surgery. In recent years, biological substitutes have been extensively studied[13–21]. Zhi et al[13] reported that biological artificial esophagus can be used to repair esophagus defect. In their study, esophagus substitution test was performed in 30 experimental dogs, showing that esophagus substitution can repair 93.3% of esophagus defects. Zhang et al[14–17] excised chest esophagus at the right chest flank of 30 Chinese hybrid dogs, and an 8 cm long biological artificial esophagus was used to reconstruct esophagus. At present, we still face the problems of fistula, stricture, and length of implants, which need further research. Based on the biological esophagus substitute, biodegradable and non-biodegradable materials have been developed for making artificial esophagus[1819]. Meanwhile, the degradation speed of biodegradable material matches that of non-degradation material, which exerts the supporting function and prolongs the supporting time of biological materials, and finally regenerates esophagus to completely substitute the artificial esophagus. The project of digestion tube has made certain progress. However, its effect is unknown. Further study is needed on implantation and production of epithelial and muscle cells, gland and nerve plexus regeneration, scar reduction, speeding up repair and regeneration of structure, etc[20].
Dr. Shi first used lung tissue petal substitution to repair trachea defect successfully[21], which is a new direction to repair esophagus defect with lung tissue petal and reconstruct esophagus. In the earlier experiment, we wrapped the damage spot using the lung tissue petal to make the artificial esophagus, in order to prevent stricture after repair of esophagus defect[2223]. Based on previous experiment results, we chose a chitosan material to make absorbable tube stent to overcome the rejection, and further explored the feasibility of this new method. The blood circulation of lung tissue petal was good, which was confirmed by pathology and electron microscopy. The compatibility of repair material was good, thus avoiding foreign matter rejection and forming reliable scars of fibers. The lung tissue petal has certain ductility, and different lobes of the lungs can be selected to make different lengths of lung tissue petal. In brief, the selection of lung tissue petal is convenient, the compatibility is good and the blood circulation is rich, with a high scar formation ability and good anti-infectiousness, and good environment for esophagus epidermis.
Chitin is the only high polymer material with widespread biodegradation[24]. Acetyl-escaped product, also known as chitosan[25], has the good biological compatibility with animal organs and cells, and degrades the low molecular oligosaccharide with no accumulation of products in vivo and no immunogenic ability. The tube we made of it is elastic and tough, slightly soft when it meets water, and can suppress inflammation response, prevent adhesion and scar formation in the breakage site. It was reported that chitosan membrane can prevent the adhesion to peritoneum and thus can be used in clinical practice[26]. It was also reported that chitosan can insert into nerve tube stent[2728]. Chitosan tube stent is degraded gradually and absorbed in vivo, with no toxicity, stimulation and rejection. It has been shown that epidermis of the esophagus has certain degree of stricture but it is not serious enough to cause feed barrier in esophagus of dogs[29]. Long-term survival and delayed chronic inflammation have been achieved using metallic pipe and silica gel tube as an inner lining support, but foreign matter rejection occurs. We used chitosan as a support to prevent scar formation and stricture of esophagus by making use of its compatibility and degradability. When stricture is formed, chitosan tube is degraded in vivo, and its product is not toxic and has no side effects. Thus, it is worthy to be further studied. The most serious complication is fistula, which occurs 5-7 d after operation and is related with infection, anastomotic techniques and blood circulation, etc. Infection is the most important factor for the occurrence of complication, because esophagus patching is a pollutant. Since we performed the surgery under strict asepsis, we solved the problem. After operation, the rabbits in experimental group were fasted for 5 d, and then received venous transfusion and anti-inflammation treatment. Because the resistance of rabbits was lower than that of dogs, the mortality rate of rabbits was 30%. Since this study was to verify the new method and explore experimental conditions, a large sample size of slightly bigger animals, like dogs, pigs, etc, should be used in the study. We used big white rabbits to establish esophagus partial damage model, and repaired esophagus wall partial damage using chitosan tube with lung tissue petal as its inside lining. Based on results of this experiment, pigs and dogs and other bigger animals, may be further tested for the replacement of chitosan tube in the entire esophagus. Our experiment did not operate chest of rabbits. Compared with big animals, rabbits are docile, convenient, inexpensive, and easy to obtain. The choice of support is the key to the maintenance of unobstructed lumen, protection of the surface granulation tissue of lung from adhesion, formation of diverticulum and false passage. The metal lattice support is widely applied in treatment of esophagus stenosis, mainly because of its good support effect. Therefore, we used the chitosan tubular support, which functions as a support, reduces inflammation response, and can be absorbed and degraded by organisms, and can be sued as a substitute of esophagus.
Further study involving smooth muscle, nerve plexus and gland regeneration is needed[30]. Creeping motion restoration, long esophagus reconstruction, etc, can be achieved in clinical practice.
At present, esophagus reconstruction techniques are available, but each of them has its advantages and disadvantages. Reducing scar formation and stricture is still the key to esophagus reconstruction.
In this study, chitosan tube was used as a support to prevent post-operation stricture.
Trachea was reconstructed using chitosan and silica gel pipe stent. A chitosan material was used to make absorbable support tubes to overcome the rejection and prevent stricture of esophagus.
Chitosan tubes provide a new surgery method for patients in whom substituting esophagus with cavity internal organs is impossible.
Lung tissue petal: A lung lobe closing the segment of trachea.
It is an interesting animal experiment about the use of chitosan stent with a lung tissue flap to repair esophageal perforation.
| 1. | Chen HC, Kuo YR, Hwang TL, Chen HH, Chang CH, Tang YB. Microvascular prefabricated free skin flaps for esophageal reconstruction in difficult patients. Ann Thorac Surg. 1999;67:911-916. [Cited in This Article: ] |
| 2. | Wang RW, Jiang YG, Gong TQ, Zhou JH, Zhao YP, Ma Z. Reconstruction of cervical esophageal stenosis with platysma myocutaneous flap. Disan Junyi Daxue Xuebao. 2007;29:749-751. [Cited in This Article: ] |
| 3. | Zhao YP, Wang RW, Jiang YG, Gong TQ, Zhou JH, Tan QY. Postoperative complication of reconstruction or repair defect of cervical esophagus using platysma myocutaneous flap. Zhongguo Xiongxinxueguan Waike Linchuang Zazhi. 2001;8:169-171. [Cited in This Article: ] |
| 4. | Zhang X, Wang XR, Li RH, Hu JG, Hou JS, Zhou SJ. Osteoperio-inercostal muscle pedicle flap: an effective material in esophagoplasty. Zhonghua Xiongxinxueguan Waike Zazhi. 2000;16:39-40. [Cited in This Article: ] |
| 5. | Uehara M, Helman JI, Lillie JH, Brooks SL. Blood supply to the platysma muscle flap: an anatomic study with clinical correlation. J Oral Maxillofac Surg. 2001;59:642-646. [Cited in This Article: ] |
| 6. | Ariyan S. The transverse platysma myocutaneous flap for head and neck reconstruction: an update. Plast Reconstr Surg. 2003;111:378-380. [Cited in This Article: ] |
| 7. | Zhou JH, Jiang YG, Wang RW, Lin YD, Gong TQ, Zhao YP, Ma Z, Tan QY. Management of corrosive esophageal burns in 149 cases. J Thorac Cardiovasc Surg. 2005;130:449-455. [Cited in This Article: ] |
| 8. | Jiang YG, Lin YD, Wang RW, Zhou JH, Gong TQ, Ma Z, Zhao YP, Tan QY. Pharyngocolonic anastomosis for esophageal reconstruction in corrosive esophageal stricture. Ann Thorac Surg. 2005;79:1890-1894. [Cited in This Article: ] |
| 9. | Schettini ST, Pinus J. Gastric-tube esophagoplasty in children. Pediatr Surg Int. 1998;14:144-150. [Cited in This Article: ] |
| 10. | ul-Haq A, Tareen F, Bader I, Burki T, Khan NU. Oesophageal replacement in children with indolent stricture of the oesophagus. Asian J Surg. 2006;29:17-21. [Cited in This Article: ] |
| 11. | Liao B. Clinical analysis of esophageal stricture in child. Zhongguo Xiongxinxueguan Waike Linchuang Zazhi. 2008;15:71-72. [Cited in This Article: ] |
| 12. | Shi WJ, Zhang SN, Yang W, Zhao JG, Zhao Y, Liu J. [Clinical application and animal experiment of thoracic tracheal reconstruction by using pulmonary tissue flap]. Zhonghua Waike Zazhi. 2003;41:218-221. [Cited in This Article: ] |
| 13. | Zhi FC, Zhang LJ, Peng XF, Wu XH, Pan DS, Wan TM, Liu SD, Zhang ZS, Zhou DY. Experimental study of esophagus reconstruction with biological artificial esophagus in dogs. Zhonghua Xiaohua Zazhi. 2003;23:3-7. [Cited in This Article: ] |
| 14. | Zhang LJ, Zhi FC, Rong TH, Peng XF, Wen DD, Yan SQ. Experimental study of the biological artificial esophagus in dogs. Zhonghua Weichang Waike Zazhi. 2001;4:157-160. [Cited in This Article: ] |
| 15. | Zhang LJ, Rong TH, Su XD, Lin P, Long H, FU JH. Experimental replacement of thoracic esophageal segment with a biomaterial artificial esophagus in dogs. J Med Coll PLA. 2008;23:1-8. [Cited in This Article: ] |
| 16. | Zhang LJ, Rong TH, Wu QL, Su XD, Long H, Zhao JM, Zhang PY, Li XD. [Histological regeneration process of "neo-esophagus"]. Ai Zheng. 2006;25:689-695. [Cited in This Article: ] |
| 17. | Zhang LJ, Rong TH, Xu GL, Su XD, Zhi FC, Guo XM, Zhang PY. [Experimental study of preventing postoperative stenosis by modifying artificial esophagus in dogs]. Zhongguo Yixue Kexueyuan Xuebao. 2006;28:325-328. [Cited in This Article: ] |
| 18. | Yang LZ, Hong ZP. Progress of the Research of Artificial Esophagus. Zhonghua Xiongxinxueguan Waike Zazhi. 2006;13:188-191. [Cited in This Article: ] |
| 19. | Watanabe M, Sekine K, Hori Y, Shiraishi Y, Maeda T, Honma D, Miyata G, Saijo Y, Yambe T. Artificial esophagus with peristaltic movement. ASAIO J. 2005;51:158-161. [Cited in This Article: ] |
| 20. | Hori Y, Nakamura T, Kimura D, Kaino K, Kurokawa Y, Satomi S, Shimizu Y. Effect of basic fibroblast growth factor on vascularization in esophagus tissue engineering. Int J Artif Organs. 2003;26:241-244. [Cited in This Article: ] |
| 21. | Chen G, Shi WJ. Clinical application of thoracic tracheal reconstruction by using pulmonary tissue flap combined with nickel-titanium alloy stent. Shandong Yiyao. 2008;48:12-13. [Cited in This Article: ] |
| 22. | Zhao JG, Shi WJ, Zhang SN, Han Y, Zhao Y, Liu J. Replacement of partial esophageal defect with pulmonary tissue with vascular pedicle. Zhonghua Xiongxinxueguan Waike Zazhi. 2003;19:166-168. [Cited in This Article: ] |
| 23. | Yang W, Shi WJ, Zhang SN, Han Y. Research of pulmonary flap substitutes for the esophagus in dogs. Zhongguo Yike Daxue Xuebao. 2002;31:7-9. [Cited in This Article: ] |
| 24. | Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24:1241-1254. [Cited in This Article: ] |
| 25. | Freier T, Montenegro R, Shan Koh H, Shoichet MS. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials. 2005;26:4624-4632. [Cited in This Article: ] |
| 26. | Ao Q, Wang AJ, Sun ZG, Zhang XF. Preparation and characterization of a chitosan conduit for neural tissue engineering. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12:47-50. [Cited in This Article: ] |
| 27. | Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111-125. [Cited in This Article: ] |
| 28. | Zhang WF, Chen XG, Li PW, He QZ, Zhou HY. Preparation and characterization of theophylline loaded chitosan/beta-cyclodextrin microspheres. J Mater Sci Mater Med. 2008;19:305-310. [Cited in This Article: ] |
| 29. | Liu J, Shi WJ, Zhang SN, Han Y, Zhao JG. Experimental research of esophagus replacement with pulmonary flap in dogs. Zhongguo Xiufu Chongjian Waike Zazhi. 2006;20:507-510. [Cited in This Article: ] |
| 30. | Qin X, Xu ZF, Zhao XW, Shi HC, Zhou JH, Sun K, Gao XY. Reconstruction of a cervical esophagus segment with an artificial prosthesis by use of a polyurethane stent covered with collagen-chitosan sponge in dogs. Zhongguo Xiufu Chongjian Waike Zazhi. 2003;17:374-377. [Cited in This Article: ] |