Published online Jul 21, 2008. doi: 10.3748/wjg.14.4377
Revised: June 16, 2008
Accepted: June 23, 2008
Published online: July 21, 2008
AIM: To investigate the effects of mirtazapine and fluoxetine, representatives of the noradrenergic and specific serotonergic antidepressant (NaSSA) and selective serotonin reuptake inhibitor (SSRI) antidepressant respectively, on body weight, ingestive behavior, locomotor activity and tumor growth of human pancreatic carcinoma xenografts in nude mice.
METHODS: A subcutaneous xenograft model of human pancreatic cancer cell line SW1990 was established in nude mice. The tumor-bearing mice were randomly divided into mirtazapine group [10 mg/(kg·d)], fluoxetine group [10 mg/(kg·d)] and control group (an equivalent normal saline solution) (7 mice in each group). Doses of all drugs were administered orally, once a day for 42 d. Tumor volume and body weight were measured biweekly. Food intake was recorded once a week. Locomotor activity was detected weekly using an open field test (OFT).
RESULTS: Compared to the fluoxetine, mirtazapine significantly increased food intake from d 14 to 42 and attenuated the rate of weight loss from d 28 to 42 (t = 4.38, P < 0.05). Compared to the control group, food intake was significantly suppressed from d 21 to 42 and weight loss was promoted from d 35 to 42 in the fluoxetine group (t = 2.52, P < 0.05). There was a significant difference in body weight of the mice after removal of tumors among the three groups. The body weight of mice was the heaviest (13.66 ± 1.55 g) in the mirtazapine group and the lightest (11.39 ± 1.45 g) in the fluoxetine group (F(2,12) = 11.43, P < 0.01). The behavioral test on d 7 showed that the horizontal and vertical activities were significantly increased in the mirtazapine group compared with the fluoxetine and control groups (F(2,18) = 10.89, P < 0.01). These effects disappeared in the mirtazapine and fluoxetine groups during 2-6 wk. The grooming activity was higher in the mirtazapine group than in the fluoxetine group (10.1 ± 2.1 vs 7.1 ± 1.9 ) (t = 2.40, P < 0.05) in the second week. There was no significant difference in tumor volume and tumor weight of the three groups.
CONCLUSION: Mirtazapine and fluoxetine have no effect on the growth of pancreatic tumor. However, mirtazapine can significantly increase food intake and improve nutrition compared with fluoxetine in a pancreatic cancer mouse model.
- Citation: Jia L, Shang YY, Li YY. Effect of antidepressants on body weight, ethology and tumor growth of human pancreatic carcinoma xenografts in nude mice. World J Gastroenterol 2008; 14(27): 4377-4382
- URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4377.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4377
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States[1]. However, its incidence has increased steadily in China in recent years. At the time of diagnosis, 80% of patients present with either locally advanced or metastatic disease. In recent years, although gene therapy and biological targeting therapy can significantly inhibit the growth of pancreatic cancer in animal experiments, there is no satisfactory therapy for pancreatic cancer patients to extend their median survival time and improve their quality of life. The overall five-year survival rate is less than 5%[2].
The stress associated with the diagnosis and treatment of pancreatic cancer can cause significant psychiatric morbidity. It was reported that pancreatic cancer patients have the highest rate of major depression compared with other cancer patients[3]. Depression occurs in 47%-71% of patients with pancreatic cancer[45]. Unfortunately, depression adversely affects many clinical oncology outcomes. It can prolong hospital stay, augment the complication of therapy, decrease the ability to care oneself, reduce the compliance with medical treatment, lead to a poorer quality of life, and even shorten survival time[6–8]. Antidepressant medications not only improve depressive symptoms of patients with cancer but also reverse these adverse impacts[9].
Clearly, antidepressant treatment constitutes one of the new strategies of cancer adjuvant therapy. However, data on treatment of depression with antidepressants in cancer patients are relatively scarce. The effect of different agents on distressing symptoms of cancer patients is still a subject for discussion. At present, selective serotonin reuptake inhibitor (SSRI) antidepressants are recommended as the first-line therapy for major depressive patients. Furthermore, mirtazapine is a new noradrenergic and specific serotonergic antidepressant (NaSSA), which stimulates 5-HT1 receptors, but blocks serotonin 5-HT2 and 5-HT3 receptors and histamine H1 receptors[10], which may be associated with increasing appetite and weight gain. Recent studies have shown that serotonin has been extensively implicated in the regulation of ingestive behavior[1112].
Therefore, we performed experiments using fluoxetine and mirtazapine as representatives of SSRI and NaSSA, respectively. The aim of the study was to study the effects of oral mirtazapine and fluoxetine on body weight, food intake, locomotion and tumor growth in a subcutaneous pancreatic tumor model.
Mirtazapine was kindly provided by Organon, Oss, the Netherlands. Fluoxetine was purchased from Eli Lilly & Co (Indianapolis, IN). RPMI1640 and fetal bovine serum were purchased from Gibco (Grand Island, NY).
BALB/c nu/nu male and female mice (5 wk old, weighing 17-20 g) of SPF class were purchased from the Experimental Animal Center, Guangzhou University of Chinese Medicine. The mice were housed under pathogen-free conditions in the Animal Center of Sun Yat-Sen University (4-5 mice per cage at 22 ± 1°C room temperature) with free access to water and standard rat chow, in a 12 h light-dark cycle.
Human pancreatic cancer cell line SW1990 was a kind gift from the Second Affiliated Hospital of Sun Yat-Sen University. The cell line was maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Monolayer cultures were maintained on a culture flask and incubated in a mixture of 50 mL/L CO2 and 950 mL/L oxygen at 37°C. Trypsinization was terminated with a medium containing 10% FBS and the cells were washed once with a serum-free medium and resuspended in Hank’s balanced salt solution. Only single-cell suspensions displaying greater than 90% viability were used for injection.
To produce SW1990 donor tumors, 3 × 106 cells per animal in a total volume of 0.2 mL were inoculated subcutaneously into the right flank of a nude mouse. Tumor size was measured via callipering. When the subcutaneous solid tumor reached approximately 1 cm in diameter and was aseptically removed from the donor animals. Macroscopically necrotic tissues and the remaining healthy tumor tissues were cut with scissors and minced into approximately 1 mm3 pieces in Hanks’ balanced salt solution containing 100 units/mL penicillin and 100 &mgr;g/mL streptomycin. A small incision was then made through the right dorsal flank and a tumor tissue piece was implanted subcutaneously beneath the dorsal flank skin of a nude mouse. We established a subcutaneous pancreatic cancer model as previously described[13] with certain modifications.
After tumor transplantation, the mice were randomly assigned into three groups (7 mice each group). Treatment was initiated one day after tumor transplantation as the first day experiment. The first group received an equivalent normal saline solution as control. The second and third groups received 10 mg/(kg.d) mirtazapine and 10 mg/(kg.d) fluoxetine, respectively[14], once a day for 42 d. Oral application was chosen as it is the standard application of antidepressants. For the study, the treated mice were closely monitored for any side effects and sacrificed on d 43. The transplanted tumor sizes were measured with a caliper, twice a week, and the tumor volume was calculated using the formula[15]: V = W2× L/2, where W is the width and L is the length of the tumor. Body was weighed biweekly and food intake was expressed as daily consumption in gram per animal weekly.
OFT is a widely used test to evaluate the emotion and locomotor activity in rodents. As a test of spontaneous (unconditioned) behavior, it allows the animal to exhibit a wide range of behaviors and is therefore highly suitable for the study of complex phenomena such as anxiety or depression[16]. The open field apparatus used is a rectangular chamber (35 cm × 35 cm × 20 cm) made of plexigal, which was built from black walls and white floor. The floor of the open field was divided into 25 identical squares by 4 × 4 black lines[16]). A video camera was placed 1.5 m above the apparatus. After each trial, the apparatus was cleaned with water containing 0.1% acetic acid. The behavioral parameters registered during the first 5 min exposure to the open field apparatus were horizontal activity (the number of squares an animal entered), rearing known as vertical activity (the number of times an animal was standing on its hind legs with forelegs in the air or against the wall), grooming activity (the number of paws or tongues used to clean or scratch the body), which could reflect a stable individual trait “nonspecific excitability level”. The OFT was performed weekly between 13:00 and 15:30. Any abrupt loud noise could markedly inhibit locomotion and even induce prolonged immobility of the mice. Therefore, the testing room was comprised merely of the background noise.
Statistical analysis was performed with the SPSS 13.0 for Windows. Data were expressed as mean ± SD. Pancreatic tumor weight, tumor volume and behavioral parameters were compared using one-way ANOVA. Significant differences in body weight and food intake were determined by two-way ANOVA and the Student-Newman-Keuls test for multiple comparisons between groups. P < 0.05 was considered statistically significant based on a two-tailed test.
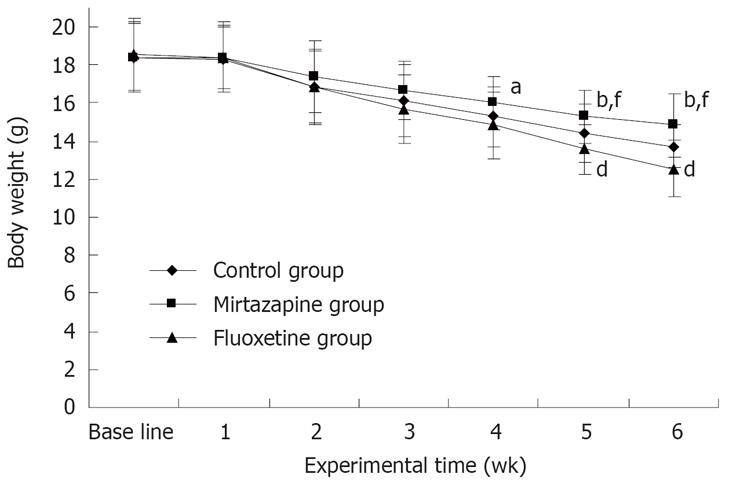
The change in body weight during the treatment is shown in Figure 1. The mice had a progressive weight loss. The body weight of mice in the three groups was very close in the first week. In the first 3 wk of treatment, the body weight of mice in the mirtazapine group was greater than that of mice in the other groups. However, no significant difference was observed. In wk 4, the body weight of mice was significantly greater in the mirtazapine group (16.00 ± 1.41 g) than in the fluoxetine group (14.86 ± 1.77 g) (F(2,12) = 4.2, P < 0.05). The effect of mirtazapine lasted until the end of experiment. Nevertheless, the body weight was significantly decreased in the fluoxetine group in week 5-6 compared with the control group (P < 0.01, Figure 1). The body weight of mice after removal of the tumor was also significantly increased in the mirtazapine group (13.66 ± 1.55 g) but decreased in the fluoxetine group (11.39 ± 1.45 g) compared with the control group (12.56 ± 1.29 g) (F(2, 12) = 11.43, P < 0.01).
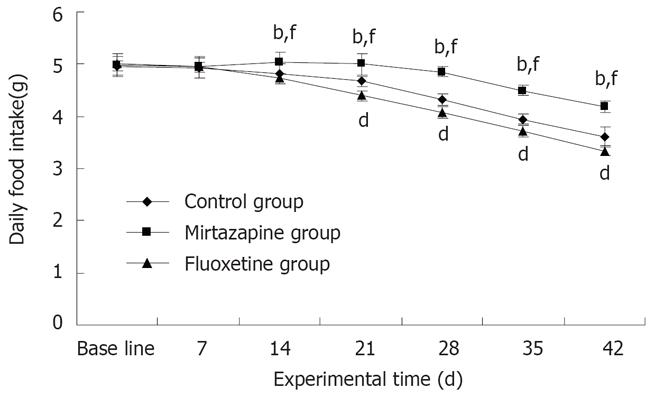
Daily food intake of the tumor-bearing mice was gradually reduced over the whole treatment period (Figure 2). At initiation of the study, no difference was observed in ingestive behavior of mice in different groups. On day 14, food consumption of mice was significantly increased in the mirtazapine group (5.03 ± 0.16 g) compared with the fluoxetine (4.73 ± 0.11 g) and control groups (4.79 ± 0.16 g) (F(2,12) = 23.31, P < 0.01). Mirtazapine exerted its effect to the end of experiment. However, fluoxetine treatment significantly decreased food consumption of mice compared with the control group from day 21 to 42 (P < 0.01, Figure 2).
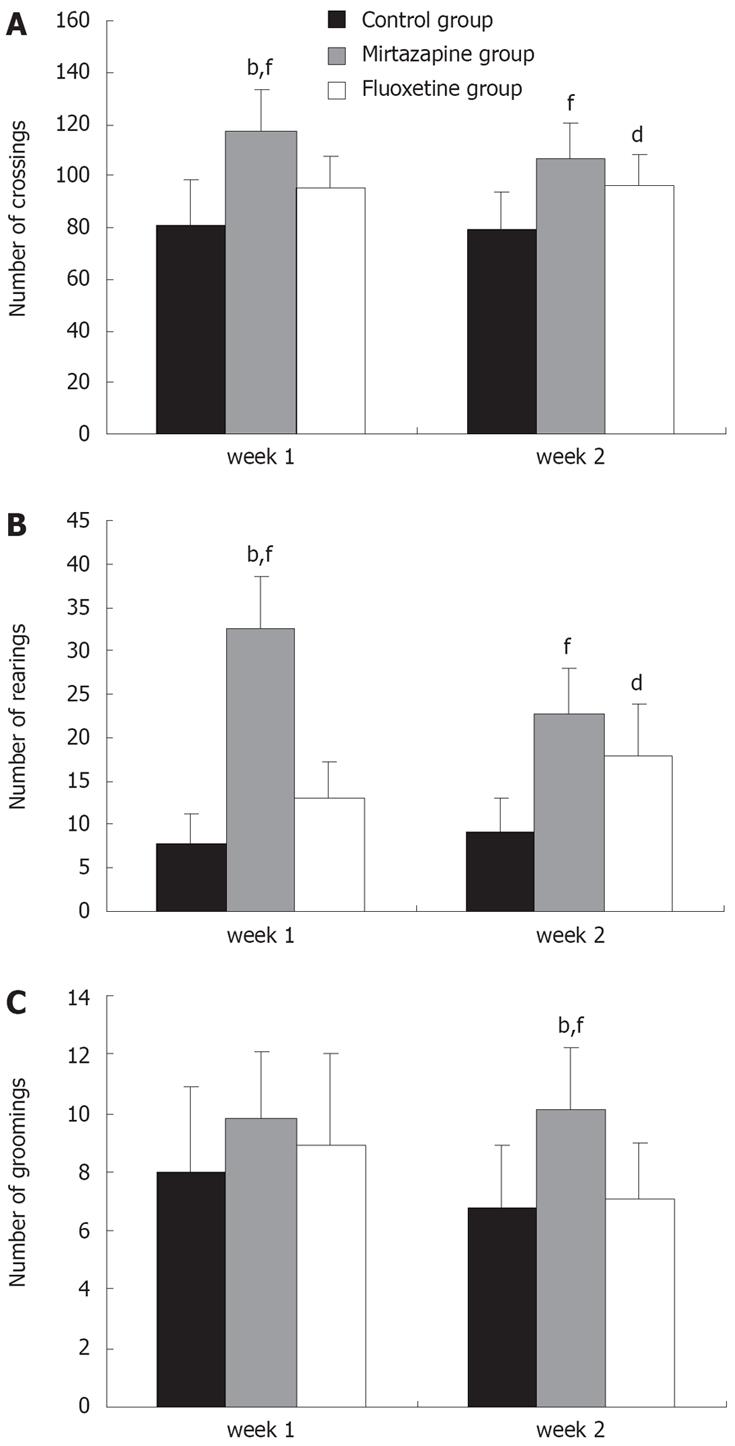
Mirtazapine and fluoxetine significantly increased the locomotor activity of mice in the OFT. In the first week of behavioral test, the horizontal activity and vertical activity were significantly increased in the mirtazapine group (117.3 ± 16.4, 95.3 ± 13.6) compared with the fluoxetine group (95.3 ± 13.6, 13.0 ± 4.2) and control group (80.6 ± 18.0, 7.9 ± 3.4) (F(2,18) = 10.89, F(2,18) = 97.09, P < 0.01, Figure 3A and B). There was no difference in grooming activity among the three groups. However, the grooming activity was significantly higher in the mirtazapine group (10.1 ± 2.1) than in the fluoxetine group (7.1 ± 1.9) (F(2,18) = 4.90, P < 0.01, Figure 3C) in the second week. Meanwhile, the horizontal and vertical activities were significantly increased in the mirtazapine and fluoxetine groups compared with the control group (P < 0.01, Figure 3A and B). Nevertheless, these parameters obtained from the mice treated with mirtazapine did not differ from those treated with fluoxetine during week 3-6.
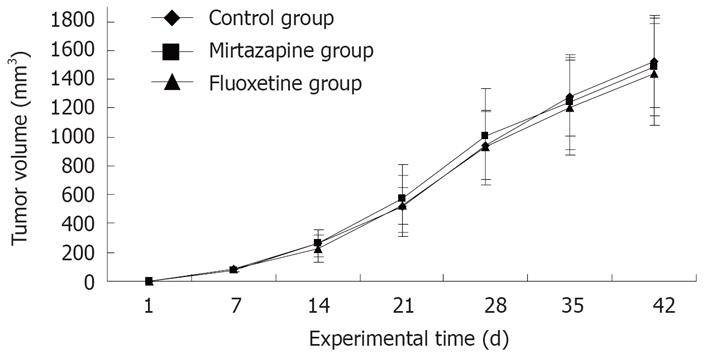
As shown in Figure 4, the tumor xenograft grew very rapidly with the prolongation of experiment. Nevertheless, no significant difference was observed in tumor volume of each group at any time point during the whole experiment. After 6-wk treatment, the animals were killed when the tumors were removed and weighed. However, no significant difference in tumor weight was detected in the mirtazapine group (1.18 ± 0.20 g), fluoxetine group (1.20 ± 0.28 g) and control group (1.23 ± 0.34 g) (F(2,18) = 0.06, P > 0.05).
The results of the present study show that daily oral mirtazapine (10 mg/kg) could significantly increase food consumption and attenuated the rate of weight loss. However, treatment with fluoxetine (10 mg/kg) significantly suppressed food intake and promoted weight loss. Mirtazapine and fluoxetine showed their effects on the regulation of food intake and body weight to the end of experiment, suggesting that there is an extensive implication between serotonin and food intake. One explanation for the effects may be that the plasma half life of the two drugs is very long. It was reported that pharmacological agents that increase the levels of 5-HT in the central nervous system (CNS) suppress food intake, whereas drugs that antagonize the actions of 5-HT increase food intake[17]. Mirtazapine is a potent antagonist at postsynaptic 5HT2 and 5HT3 receptors which may potentially increase the appetite and body weight[18–20]. However, fluoxetine augments serotonergic activity by selectively inhibiting the reuptake of neurotransmitter, and reduces food intake and body weight in both animals[2122] and human beings[23], and is thus used in the treatment of obesity[24].
We examined the behavioral effects of mirtazapine and fluoxetine in the OFT. The horizontal activity fully reflected the animal activity, rearing the degree of curiosity to the novel surroundings, and grooming the level of alert against the novel environment. Mirtazapine and fluoxetine significantly increased the locomotor activity of pancreatic tumor-bearing mice compared with the control group. In the initial behavioral test, the horizontal activity was significantly increased in the mirtazapine group compared with the fluoxetine and control groups. Rearings were also significantly increased in the mirtazapine group compared with the fluoxetine and control groups (Figure 3A and B). Grooming activities increased earlier in the mirtazapine group than in the fluoxetine group. The results of the present study are consistent with the reported data[2526], showing that antidepressants increase locomotor activity in a depressed model of normal rats. Nevertheless, mirtazapine adapted faster to the new environment and elevated earlier the alert of tumor-bearing mice against the novel environment. These findings indicate that mirtazapine is better than fluoxetine to tolerate the stress associated with the diagnosis and treatment of pancreatic cancer.
To our knowledge, the effect of mirtazapine and fluoxetine on the growth of pancreatic cancer in nude mice has not been reported. In the present study, the tumor volume at any time points and tumor weight were not significantly different in the three groups. Interestingly, a previous study demonstrated that fluoxetine is neither a complete carcinogen nor a tumor promoter[27], which is in agreement with our results obtained from pancreatic tumor-bearing mice. Moreover, Abdul et al[28] reported that the growth of subcutaneous PC-3 xenografts in athymic nude mice is significantly inhibited by antidepressants. Mirtazapine and fluoxetine also did not exhibit any toxicity throughout the whole treatment, suggesting that mirtazapine and fluoxetine can be safely used in the treatment of depression in pancreatic cancer patients.
Pancreatic cancer patients have not only distressing symptoms such as appetite loss, nausea, vomitting, weight loss, sleep disturbances and pain, but also psychiatric comorbidities such as adjustment disorder, depression frequently accompanying the disease process. It was reported that patients with pancreatic cancer have a weight loss of 83%-87% and approximately 30% of the patients have a weight loss of over 10%[29].
In summary, mirtazapine as an adjuvant therapy is beneficial to the pancreatic cancer patients with depression[30]. Mirtazapine as the first-line therapy for depressed patients with advanced pancreatic cancer has a bright future. Nevertheless, further investigation and evaluation of mirtazapine are needed before it is widely used in clinical practice.
The treatment of pancreatic cancer remains a great challenge. The majority of patients with pancreatic cancer develop major depression. Depression adversely affects many clinical outcomes. Antidepressant treatment has been accepted as one of the new strategies in cancer adjuvant therapy. However, systemic studies on the treatment of depression in patients with cancer have not been well documented. The effect of different antidepressants on distressing symptoms of cancer patients is a subject for further evaluation.
At present, fluoxetine is one of the selective serotonin reuptake inhibitor (SSRI) antidepressants which are recommended as the first-line therapy for depression. Mirtazapine belongs to a new family of noradrenergic and specific serotonergic antidepressants (NaSSA) used in the treatment of major depression.
On the basis of previous data, this was the first study examining the effects of mirtazapine and fluoxetine on the growth of pancreatic cancer in nude mice. The results of the present study show that mirtazapine could significantly increase food intake and attenuate the rate of weight loss in experimental mice. However, fluoxetine could significantly suppress food intake and promote weight loss in tumor-bearing mice.
To summarize the actual application values, mirtazapine neither inhibits nor promotes pancreatic tumor growth according to the findings from this study. The results support the hypothesis that mirtazapine as an adjuvant therapy is superior to fluoxetine for pancreatic cancer patients with depression.
The title accurately reflects the major contents of the article. On the basis of previous researches, this paper is an original research article on the effect of mirtazapine and fluoxetine on the growth of human pancreatic carcinoma in nude mice. The findings are of great interest and provide a foundation for their application in clinical practice. The conclusions are reliable and valuable.
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [Cited in This Article: ] |
| 2. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [Cited in This Article: ] |
| 3. | McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB. Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry. 1995;52:89-99. [Cited in This Article: ] |
| 4. | Carney CP, Jones L, Woolson RF, Noyes R Jr, Doebbeling BN. Relationship between depression and pancreatic cancer in the general population. Psychosom Med. 2003;65:884-888. [Cited in This Article: ] |
| 5. | Fazal S, Saif MW. Supportive and palliative care of pancreatic cancer. JOP. 2007;8:240-253. [Cited in This Article: ] |
| 6. | Okano Y, Okamura H, Watanabe T, Narabayashi M, Katsumata N, Ando M, Adachi I, Kazuma K, Akechi T, Uchitomi Y. Mental adjustment to first recurrence and correlated factors in patients with breast cancer. Breast Cancer Res Treat. 2001;67:255-262. [Cited in This Article: ] |
| 7. | Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002;53:859-863. [Cited in This Article: ] |
| 8. | Thompson DS. Mirtazapine for the treatment of depression and nausea in breast and gynecological oncology. Psychosomatics. 2000;41:356-359. [Cited in This Article: ] |
| 9. | Unutzer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836-2845. [Cited in This Article: ] |
| 10. | Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. [Cited in This Article: ] |
| 11. | Vickers SP, Easton N, Webster LJ, Wyatt A, Bickerdike MJ, Dourish CT, Kennett GA. Oral administration of the 5-HT2Creceptor agonist, mCPP, reduces body weight gain in rats over 28 days as a result of maintained hypophagia. Psychopharmacology (Berl). 2003;167:274-280. [Cited in This Article: ] |
| 12. | Bechtholt AJ, Hill TE, Lucki I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology (Berl). 2007;190:531-540. [Cited in This Article: ] |
| 13. | Jia L, Zhang MH, Yuan SZ, Huang WG. Antiangiogenic therapy for human pancreatic carcinoma xenografts in nude mice. World J Gastroenterol. 2005;11:447-450. [Cited in This Article: ] |
| 14. | Weber CC, Eckert GP, Muller WE. Effects of antidepressants on the brain/plasma distribution of corticosterone. Neuropsychopharmacology. 2006;31:2443-2448. [Cited in This Article: ] |
| 15. | Hwang RF, Yokoi K, Bucana CD, Tsan R, Killion JJ, Evans DB, Fidler IJ. Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clin Cancer Res. 2003;9:6534-6544. [Cited in This Article: ] |
| 16. | Palumbo ML, Fosser NS, Rios H, Zorrilla Zubilete MA, Guelman LR, Cremaschi GA, Genaro AM. Loss of hippocampal neuronal nitric oxide synthase contributes to the stress-related deficit in learning and memory. J Neurochem. 2007;102:261-274. [Cited in This Article: ] |
| 17. | Garattini S, Bizzi A, Codegoni AM, Caccia S, Mennini T. Progress report on the anorexia induced by drugs believed to mimic some of the effects of serotonin on the central nervous system. Am J Clin Nutr. 1992;55:160S-166S. [Cited in This Article: ] |
| 18. | Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. [Cited in This Article: ] |
| 19. | Kraus T, Haack M, Schuld A, Hinze-Selch D, Koethe D, Pollmacher T. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry. 2002;35:220-225. [Cited in This Article: ] |
| 20. | Gerrits M, Bakker PL, Koch T, Ter Horst GJ. Stress-induced sensitization of the limbic system in ovariectomized rats is partly restored by cyclic 17beta-estradiol administration. Eur J Neurosci. 2006;23:1747-1756. [Cited in This Article: ] |
| 21. | Heisler LK, Kanarek RB, Gerstein A. Fluoxetine decreases fat and protein intakes but not carbohydrate intake in male rats. Pharmacol Biochem Behav. 1997;58:767-773. [Cited in This Article: ] |
| 22. | Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav. 1999;63:377-385. [Cited in This Article: ] |
| 23. | Foltin RW, Haney M, Comer SD, Fischman MW. Effect of fluoxetine on food intake of humans living in a residential laboratory. Appetite. 1996;27:165-181. [Cited in This Article: ] |
| 24. | Ward AS, Comer SD, Haney M, Fischman MW, Foltin RW. Fluoxetine-maintained obese humans: effect on food intake and body weight. Physiol Behav. 1999;66:815-821. [Cited in This Article: ] |
| 25. | Huang YL, Yu JP, Wang GH, Chen ZH, Wang Q, Xiao L. Effect of fluoxetine on depression-induced changes in the expression of vasoactive intestinal polypeptide and corticotrophin releasing factor in rat duodenum. World J Gastroenterol. 2007;13:6060-6065. [Cited in This Article: ] |
| 26. | Reneric JP, Bouvard M, Stinus L. In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressed. Eur Neuropsychopharmacol. 2002;12:159-171. [Cited in This Article: ] |
| 27. | Bendele RA, Adams ER, Hoffman WP, Gries CL, Morton DM. Carcinogenicity studies of fluoxetine hydrochloride in rats and mice. Cancer Res. 1992;52:6931-6935. [Cited in This Article: ] |
| 28. | Abdul M, Logothetis CJ, Hoosein NM. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol. 1995;154:247-250. [Cited in This Article: ] |
| 29. | Mattox TW. Treatment of unintentional weight loss in patients with cancer. Nutr Clin Pract. 2005;20:400-410. [Cited in This Article: ] |
| 30. | Theobald DE, Kirsh KL, Holtsclaw E, Donaghy K, Passik SD. An open-label, crossover trial of mirtazapine (15 and 30 mg) in cancer patients with pain and other distressing symptoms. J Pain Symptom Manage. 2002;23:442-447. [Cited in This Article: ] |