Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.15965
Revised: June 23, 2014
Accepted: August 13, 2014
Published online: November 21, 2014
Pegylated interferon (IFN) α-2a or 2b in combination with ribavirin for children aged 3 years and older is the standard treatment for paediatric chronic hepatitis C. This treatment regimen was developed firstly in adults. In recent years, a number of direct-acting antiviral agents (DAAs) are under development for treatment of chronic hepatitis C virus (HCV) infection. These agents block viral replication inhibiting directly one of the several steps of HCV lifecycle. DAAs are classified into several categories based on their molecular target: HCV NS3/4A protease inhibitors, HCV NS5B polymerase inhibitors and HCV NS5A inhibitors. Other promising compounds are cyclophilin A inhibitors, mi-RNA122 and IFN-λ. Several new drugs associations will be developed in the near future starting from the actual standard of care. IFN-based and IFN-free regimens are being studied in adults. In this constantly evolving scenario new drug regimens targeted and suitable for children would be possible in the next future. Especially for children, it is crucial to identify the right combination of drugs with the highest potency, barrier to resistance and the best safety profile.
Core tip: The discovery of new drugs and new therapeutic regimens is changing radically the approach to chronic hepatitis C virus infection. The efficacy and safety of pegylated interferons (IFNs) and ribavirin are well known in children. The introduction of direct antiviral agents has already changed the standard of care in adults. Other new drugs and IFN free regimens have been proposed with promising results. This scenario could pone new perspectives in treatment of children with chronic hepatitis C.
- Citation: Serranti D, Indolfi G, Resti M. New treatments for chronic hepatitis C: An overview for paediatricians. World J Gastroenterol 2014; 20(43): 15965-15974
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/15965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.15965
Hepatitis C virus (HCV) chronically infects an estimated 170 million people worldwide[1,2]. HCV infections have been significantly reduced by the implementation of blood-donor screening and the leading source of HCV infection in children is transmission from the mother to the child[3]. Approximately 20% of perinatally infected children undergo spontaneous clearance of HCV RNA in the first 5 years of life, with subsequent normalization of aminotransferase levels[4]. The cumulative probability of chronic progression is approximately 80%[4], although children with chronic hepatitis C are often asymptomatic. Children with chronic infection are evaluated usually on a six-montly basis. Serum aminotransferases, bilirubin (total and direct/conjugated), albumin, HCV RNA levels, complete blood count and prothrombin time/international normalized ratio are considered appropriate monitoring tests[5]. Liver biopsy is generally unnecessary[5] but it is appropriate in children with clinical and biochemical signs suggestive of advanced liver disease[6].
Pegylated (PEG)-interferon (IFN) α-2a or 2b in combination with ribavirin for children aged 3 years and older is the standard treatment for children with chronic hepatitis C. This treatment was previously approved in adults[7]. Recently, different direct-acting antiviral agents (DAAs) are under development for treatment of chronic HCV infection in adults[8,9]. These agents block viral replication by directly inhibiting one of several steps of the HCV lifecycle[10]. In daily clinical practice the current therapeutic approach to children with chronic hepatitis C should be influenced and driven by the knowledge of the ongoing and rapidly progressing development of new drugs for HCV infection. The aim of the present review is to summarize and to provide paediatrician with an up-to-date summary of the rationale underlying new treatment regimens for HCV infection and to discuss critically the suitability of the different regimens for children.
PEG-IFN is a compound synthesized by covalently linking a polyethylene glycol moiety to IFN. This addition confers an extended serum half-life, compared with the non- PEG IFN, allowing once-weekly dosing. Ribavirin is a synthetic guanosine analogue with activity against several RNA and DNA viruses, including Flaviviridae. It acts by interfering with the synthesis of guanosine triphosphate, inhibiting the viral capping of mRNA and inhibiting viral RNA polymerase. The combination of PEG-IFN with ribavirin is the standard of care for adults with chronic infection by HCV other than genotype 1. PEG-IFN with ribavirin associated to telaprevir or boceprevir is the standard of care for adults with chronic infection by HCV genotype 1. The FDA and EMA approved PEG-IFN α-2b in combination with ribavirin for children aged 3 years and older in 2008 and 2009, respectively. The use of PEG-IFN α-2a for children aged 5 years and older was approved by the FDA in 2011 and by the EMA in 2013. These approvals were based on open-label single arm clinical trials in children and adolescents[11,12] and supported by evidences coming from randomized controlled trials in adult populations. Only one randomized clinical trial had been conducted in children comparing and demonstrating the superiority of the combination therapy of PEG-IFN and ribavirin on monotherapy with PEG-IFN[13].
PEG-IFN standard doses are 1.5 μg/kg per week for α-2b and 100 μg/m2 per week for α-2a[14]. Ribavirin standard dose is 15 mg/kg per day[14]. Genotypes 1 and 4 should be treated for 48 wk. Patients with genotypes 2 and 3 should be treated for 24 wk[15].
A recent meta-analysis collected results from 8 trials evaluating the efficacy of administration of PEG-IFN plus ribavirin in children infected by HCV and conclude that PEG-IFN plus ribavirin is an effective therapy in the majority of children and adolescents with HCV[16]. By this treatment regimen, sustained viral response (SVR) is achieved in 58% of children affected and the rate of relapse is low (7%)[16]. The discontinuation rate due to virologic breakthrough was very low (4%). However, 15% of the children treated did not show virologic response to drugs[16].
The efficacy of PEG-IFN plus ribavirin regimen was found to be different according to viral genotypes. Early viral response (EVR) and SVR rates (see Table 1 for definitions) were high for genotypes 2 and 3 by this treatment (87% and 89%, respectively)[16]. Instead genotypes 1 and 4 presented a lower rate of EVR (61%) and SVR (52%)[16].
| RVR | Undetectable serum hepatitis C virus (HCV) RNA after 4 wk of treatment |
| EVR | Undetectable or at least a 2 log decrease in serum HCV RNA from baseline level after 12 wk of treatment |
| End-of-treatment response | Undetectable serum HCV RNA at the conclusion of treatment |
| SVR | Undetectable serum HCV RNA 24 wk after the end of treatment |
| Non-response | Detectable serum HCV RNA at 24 wk of treatment without any significant decrease in serum HCV RNA level |
| Relapse | Detection of serum HCV RNA after SVR had been achieved |
Rapid viral response (RVR) was identified as the best predictor of SVR in adults receiving PEG-IFN plus ribavirin. Eighty nine percent of genotype 1 infected patients who attained RVR were described to achieve SVR[12]. RVR and EVR could be considered important tools to decide for treatment withdrawn in patients who are unlikely to attain SVR. Viral load under 600000 IU/mL has been defined as a positive predictor of SVR among patients infected by genotype 1[12]. Single-nucleotide polymorphisms (SNP) around the gene for interleukin 28B (IL-28B) have been associated with different results of treatment in adults[17-20]. Preliminary studies are confirming the predictive role of SNPs of IL-28B also in children[21,22]. Others predictors of SVR identified in adults, such as younger age, baseline aminotransferases levels, sex, previous treatments and liver histology, were not confirmed in children[12].
Children treated with PEG-IFN plus ribavirin generally experienced at least one adverse event, however, discontinuation of treatment due to adverse events was low, reported approximately in 4% of the children treated[16]. Appropriate counselling with parents about the impact of therapy adverse effects is crucial before starting the treatment. Constitutional symptoms such as fever, fatigue, myalgias, arthralgias, headaches and nausea are frequently reported[23] and attributed to IFN[24]. Generally these symptoms are well tolerated and disappear after the first weeks of therapy[12]. Weight loss and inhibited growth are common while receiving PEG-IFN plus ribavirin. However, most patients experience compensatory weight and height gain after completion of treatment[12]. Approximately one third of children treated experienced bone marrow suppression due to IFN administration[13]. Neutropenia is frequent but it is not associated to increased infection susceptibility and generally resolves after the end of therapy[25]. IFN usually induces asymptomatic thrombocytopenia due to suppression of megakaryopoiesis, to platelets sequestration in capillaries[26] or to an immune-mediated mechanism[23]. Ribavirin is responsible of hemolytic anemia[27]. Its metabolites seem to induce oxidative damage of red blood cells[28]. Drug dose reduction generally improves the red blood cells count without decreasing the SVR rate[5]. Neuropsychiatric complications are frequently associated to PEG-IFN therapy[29]. Irritability and depression are the most frequent symptoms in children. Suicidal ideations are rare[13]. Thyroid abnormalities are very common during PEG-IFN plus ribavirin treatment[30,31] and the production of antithyroid peroxidase or antithyroglobulin autoantibodies may be responsible of the thyroid function decrease[32]. The administration of PEG-IFN is responsible of a wide variety of ophthalmological complications in adults. Ocular manifestations are rare in children[33], but ophtalmological follow up during therapy is warranted. Skin rashes, dry skin, pruritus and alopecia were commonly observed during therapy with PEG-IFN plus ribavirin and the injection site reactions are common when PEG-IFN is administered[34,35].
Recently, viral enzymes responsible for the crucial steps of the life cycle of HCV have become the target small inhibiting molecules called DAAs. HCV genome is a positive-strand 9.6-kb RNA[36,37]. The HCV genome encodes a single precursor polyprotein that is processed by host signal peptidases and HCV proteases into structural (core, envelope E1, and E2/p7), and non-structural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins[36,38]. The virus replicates with the help of a polymerase. When the virus enters liver cells, it releases its RNA and is translated into the precursor polyprotein. The polyprotein is processed by the proteases into several polypeptides with different functional roles in the virus life cycle[38,39]. DAAs are small molecules interfering at different steps of viral life cycle. They are classified into several categories, based on their molecular target: HCV NS3/4A protease inhibitors, HCV NS5B polymerase inhibitors, HCV NS5A inhibitors and others.
NS3-4A inhibitors target the shallow enzymatic groove of the HCV protease and thereby inhibit HCV polyprotein procession[40]. NS3-4A protease inhibitors can be divided in two molecular classes: macrocyclic inhibitors and linear tetra-peptide α-ketoamide derivatives[40]. They are characterized by a remarkable antiviral activity, but they present some limitations at the same time. First approved NS3-4A protease inhibitors were telaprevir and boceprevir (linear ketoamide derivates)[41,42]. Telaprevir or boceprevir monotherapy resulted in a sharp initial decline in plasma HCV-RNA concentration during the first days of dosing, but also in a rapid selection of resistant variants and viral breakthrough[43,44]. The association of telaprevir or boceprevir with PEG-IFN plus ribavirin reduces the risk of resistance development and results in SVR rates of approximately 70%-80% in treatment-naïve HCV genotype 1 patients[45,46]. The association of telaprevir or boceprevir with PEG-IFN plus ribavirin is the actual standard of care in adults with chronic genotype 1 HCV infection.
The effectiveness of triple therapy with telaprevir or boceprevir is clear, but the low barrier to resistance remains an important limitation[47-49]. Although newer NS3-4A protease inhibitors are being developed, the resistance profiles of linear tetrapeptide and macrocyclic inhibitors are overlapping. Amino acid position R155 in NS3 constitutes the central position for resistance development and different mutations at this amino acid site confer resistance to all protease inhibitors that are currently in advanced clinical development[50-53]. The overlapping resistance avoid the possibility of combination with other NS3-4A protease inhibitors.
Another important limitation of NS3-4A protease inhibitors is the occurrence of severe side effects[45,46,54]. The most important side-effects associated with boceprevir are anemia, neutropenia and dysgeusia. Telaprevir is responsible for skin rashes and anorectal symptoms[55,56]. Drug-to-drug interactions limit the use of these two compounds. Telaprevir and boceprevir are both substrates and inhibitors of CYP3A. CYP3A metabolized approximately 60% of medications. Drug-to-drug interactions could modify the concentrations of telaprevir and boceprevir reducing their efficacy or increasing their side effects[57,58]. Furthermore, telaprevir and boceprevir should be administered with fatty foods and three times per day. These factors make telaprevir and boceprevir two drugs that could be very difficult to be given to children especially in association with PEG-IFN plus ribavirin.
NS5A is an RNA binding protein that interacts with other HCV non-structural proteins[8]. It is capable of altering NS5B polymerase activity in vitro. NS5A role is not completely clear[59]. It is involved in modulation of multiple aspects of the cellular environment and is also required for viral assembly[60]. Daclatasvir is the first NS5A inhibitor discovered and newer drugs of this class are under development[61,62]. NS5A inhibitors show broad coverage of HCV genotypes in vitro[63]. Their activity in vivo have been only tested for genotype 1 patients. Daclatasvir monotherapy resulted in an rapid HCV RNA decline[64]. Similarly to NS3-4A protease inhibitors, the decline was as fast as the development of resistant variants[64-66]. Substitutions at positions L31 and Y93 had the greatest ability to confer resistance to daclatasvir[67]. Resistance acquired for daclatasvir was overlapping with other compounds of NS5A inhibitor class[67,68]. However, HCV variants resistant to NS5A inhibitors were not resistant to other class of DAAs, so NS5A inhibitors remain a good chance for combination therapy thanks to their potential broad genotypic coverage. Diarrhea and mild headache were the most common adverse events observed in phase II clinical studies[69]. Considering its efficacy, the possibility of single daily dosing and the relative safety profile, daclatasvir should be considered a suitable drug for combination therapy also in children with chronic HCV infection.
NS5B inhibitors interfere with viral replication by binding to the NS5B RNA-dependent RNA polymerase[70-72]. They can be divided into two distinct categories: nucleoside analogue inhibitors and non-nucleoside inhibitors[73]. Nucleoside analogue inhibitors mimic the natural substrates of the polymerase and are incorporated into the growing RNA chain, thus causing direct chain termination by tackling the active site of NS5B[74].
Nucleoside analogue inhibitors present a broad genotypes activity and a high barrier to resistance. NS5B is a highly conserved region among HCV genotypes and mutation in this site can lead to loss of function or impaired viral replication. Sofosbuvir is the most promising drug of this class of DAAs[75]. It is a once-daily drug, which can be taken with or without food. A recent clinical trial showed that 100% of previously untreated genotype 2 and 3 patients achieved SVR with sofosbuvir plus ribavirin regimen[76]. In other studies, response for genotype 3 was lower than 2. In the FISSION trial, 56% of treatment-naïve genotype 3 patients achieved SVR after 12 wk of treatment with sofosbuvir plus ribavirin[77]. In the VALENCE trial, 24 wk of treatment in genotype 3 patients showed SVR rates of 90% in treatment-naïve patients, 87% in treatment-experienced patients and 60% in treatment-experienced patients with cirrhosis, suggesting that a longer duration of therapy is better for genotype 3[78].
For previously untreated genotype 1 patients, sofosbuvir plus ribavirin resulted in 84% SVR rate[76]. The same regimen resulted in very low SVR rate (10%), when administered to genotype 1 patients who had no response to previous therapy[76]. Sofosbuvir was generally well tolerated when given alone, in combination with ribavirin or in combination with PEG-IFN and ribavirin. Headache, fatigue, insomnia, nausea, rash, and anaemia were adverse events observed with sofosbuvir-based therapies[76]. This drug presented high antiviral activities and, in addition, its high barrier to resistance development suggests that it is an optimal candidate for IFN-free combination therapies in adults with chronic hepatitis C[79]. These features, together with the possibility of single daily dosing, make sofosbuvir a suitable drug also for children.
Non-nucleoside NS5B inhibitors act on various allosteric sites to induce conformational changes in the HCV polymerase[80]. NS5B protein structure offers different biding sites, so many different non-nucleoside NS5B inhibitors are under development[81]. Difference with nucleoside inhibitors consists in more distant binding sites from the active site of NS5B. Studies evaluating the efficacy of non-nucleoside NS5B inhibitors demonstrated a good efficacy in reduction of HCV RNA levels and mild well tolerated side effects[82]. However, non-nucleoside NS5B inhibitors presented low barrier to resistance and high frequency of viral breakthrough when administered in monotherapy[82,83]. Another limitation of non-nucleoside NS5B inhibitors is the absence of broad genotype activity[84], in contrast with nucleoside inhibitors.
An increasing number of host factors, such as cyclophilin A or microRNA-122 (miR-122), which are required for HCV replication have been identified as promising candidates for host-targeting antiviral agents.
Cyclophilin A is a cellular enzyme that enhances HCV replication[85-87]. Its cellular functions are not completely understood. Cyclophilin A interacts with NS5A and, as consequence, also modulates the polymerase activity of NS5B[85,88].
Alisporivir is the first cyclophilin A inhibitor and studies on its activity are currently ongoing[89]. Alisporivir acts blocking cyclophilin A-NS5A interactions in a dose-dependent manner. Alisporivir had a potent activity and an additive effect on HCV replication in genotype 1 and 4 patients, when combined with PEG-IFN[90]. Its efficacy was demonstrated also in patients with genotype 2 or 3 infections[91]. The efficacy of alisporivir against all HCV genotypes is associated with a very high barrier to resistance[91]. Mutations conferring viral resistance can emerge in the NS5A protein, but occur less frequently than with DAAs[92]. Alisporivir is orally administered one time a day. This drug is a promising antiviral agent that can be possibly used in different drug regimens. Despite these data, it is currently on hold due to rare cases of severe pancreatitis during combination therapy with alisporivir and PEG-IFN.
MiR-122 is a liver-expressed microRNA which binds the 5’ non-coding region of the HCV genome, resulting in an upregulation of viral RNA levels[93]. MiR-122 plays a key role in HCV replication[94-96]. Miravirsen acts preventing the binding of miR-122 to the HCV genome[97]. It is the first micro-RNA targeting drug evaluated in clinical studies. First results demonstrate that the use of miravirsen in patients with chronic HCV genotype 1 infection was associated with prolonged dose-dependent reductions in HCV RNA levels without evidence of viral resistance[98].
The combination of the PEG-IFN type α with ribavirin is associated with significant side effects, resulting in high rates of noncompliance, and demonstrates variable efficacy against numerous HCV genotypes. In addition, completion of treatment often suffers because of poor adherence by patients due to drug-related adverse events, including psychiatric disorders, flu-like symptoms, and/or haematological abnormalities, such as haemolytic anaemia and neutropenia[99]. Human IFN-λ is a recently described human type III IFN[100,101]. Its biological characteristics are comparable to those of type I IFNs, such as IFN-α and IFN-β[100,102]. Similarly, IFN-λ exerts its antiviral activities by inducing the expression of IFN-stimulated genes through activation of the Janus kinases Jak1 and Tyk2[103]. The receptor complex for IFN-λ is more restricted than that of the type I IFN receptor and is highly expressed in liver cells and minimally on hematopoietic cells[104].
These characteristics have made IFN-λ an ideal candidate for antiviral therapy against HCV, with the potential of having reduced adverse events normally associated with IFN-α. Moreover, the lack of cross-resistance between IFN-λ and DAAs may be crucial for future treatment strategies. Results from a phase 2 trial investigating IFN-λ as a new therapeutic agent against chronic infection with HCV were recently reported[105]. Compared with IFN-α, the IFN-λ based regimen showed similar to slightly better SVR rates in adult patients infected with genotypes 2 or 3[105]. Furthermore, the treatment was associated with fewer adverse events[105] and preliminary results showed a more robust decline at week 12 of treatment in adult patients infected with genotypes 1 and 4[105]. These results encourage the possible future applications of IFN-λ in chronic hepatitis C therapy in adults as well as in children. In children adverse events related to IFN-α are less frequently observed than adults. However, a further reduction would be desirable.
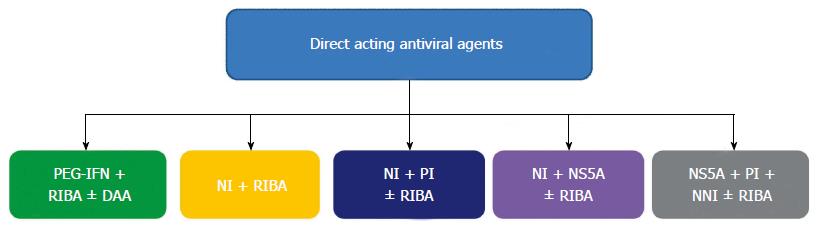
The present standard of care in children is PEG-IFN plus ribavirin. The efficacy of this association is higher in children than in adults, though the low SVR rate for genotype 1 and 4 is a major problem also in children. The new standard of care in adults, recently approved only for genotype 1, is the triple therapy with PEG-IFN, ribavirin and telaprevir/boceprevir. Triple therapy is not yet approved in children, but its limitations seem to be relevant in terms of adherence to therapy, especially in paediatric patients. The new drugs discovered open new possibilities of therapy in adults and, in the next future, in children too. New all-oral drug associations will be possible. New promising drug regimens could be targeted on children, considering the specific needs of these patients. Different drugs regimens are being tested (Figure 1) and we can distinguish IFN-based and IFN-free regimens.
As the addiction of telaprevir/boceprevir to PEG-IFN plus ribavirin, other newer NS3-4A inhibitors have been proposed for triple therapy in treatment-naïve HCV genotype 1 patients. Simeprevir has been tested in phase III clinical trials. It needs once daily dosing (in contrast with triple daily dosing of telaprevir and boceprevir) and showed a SVR rate of approximately 80%. Triple therapy by the association of nucleoside inhibitor sofosbuvir and PEG-IFN plus ribavirin has been tested in phase III clinical trial. After only 12 wk of treatment, 90% of SVR rate was obtained in treatment-naïve patients with HCV genotype 1, 4, 5 and 6[106]. Triple therapy with the NS5A inhibitor daclatasvir led to SVR in 59%-100% of treatment-naïve HCV genotype 1 and 4 patients, according to daclatasvir dosage and HCV genotype[107]. Data on triple therapies demonstrates their efficacy. However, considering that the success of triple therapies differs significantly according to infection with specific HCV subtypes and emerging viral resistance, a more futuristic approach could be quadruple therapy, including two DAAs of different classes in combination with PEG-IFN-a and ribavirin[108].
The introduction of DAAs in HCV treatment emphasized the possibility of exclusion of PEG-IFN from therapy[109]. The PEG formulation only partially solved the impact of therapy on the quality of life of the patients. However, the elimination of injections could be considered a progress in chronic hepatitis C treatment, in order to provide an all-oral regimen with a reduced impairment of life quality, most of all in children. In this contest a large number of possible IFN-free regimens, with or without ribavirin are object of study right now. Combining drugs that have different targets of action could results in an additive or synergistic antiviral effect while lessening the chance of antiviral resistance. Associations that have been tested are NS5B inhibitors with or without ribavirin, NS5A inhibitors combined with NS5B inhibitors, NS3-4A inhibitor with or without ribavirin and, finally, the combinations of multiple DAAs with or without ribavirin[77].
Progresses achieved during the last five years are rapidly and radically changing the approach to chronic HCV infection. Recently, the continuous emerging of new compounds dramatically increased the possibilities of a successful treatment. Numerous new drugs targeting various aspects of the HCV life cycle and host-targeting antiviral agents are in development. Combinations of DAAs have synergistic effects, decrease the risk of resistance, improve antiviral efficacy, are effective on different genotypes and could have favourable safety profiles. Different new drug regimens are still based on the association of DAAs with PEG-IFN however all-oral therapies are extremely promising. The possible emerging of viral resistance is the most relevant problem related to IFN-free regimens, though the discovery of host targeting antiviral agents could supply this limit of DAAs. IFN-free treatment regimens would be more suitable in children, than triple or quadruple IFN-based therapies. It is mandatory to identify the right combination of drugs with the highest potency and barrier to resistance and the best safety profile. In this constantly evolving scenario new drug regimen targeted on paediatric population would be possible in the next future.
P- Reviewer: Abbas Z, Abenavoli L, Chen J, Rendina M, Zamir DL S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Brownell J, Polyak SJ. Molecular pathways: hepatitis C virus, CXCL10, and the inflammatory road to liver cancer. Clin Cancer Res. 2013;19:1347-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 1020] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 3. | Indolfi G, Resti M. Perinatal transmission of hepatitis C virus infection. J Med Virol. 2009;81:836-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Resti M, Jara P, Hierro L, Azzari C, Giacchino R, Zuin G, Zancan L, Pedditzi S, Bortolotti F. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70:373-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, Rosenthal P, Schwarz KB. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012;54:838-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Guido M, Bortolotti F. Chronic viral hepatitis in children: any role for the pathologist? Gut. 2008;57:873-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Tsubota A, Fujise K, Namiki Y, Tada N. Peginterferon and ribavirin treatment for hepatitis C virus infection. World J Gastroenterol. 2011;17:419-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Kohler JJ, Nettles JH, Amblard F, Hurwitz SJ, Bassit L, Stanton RA, Ehteshami M, Schinazi RF. Approaches to hepatitis C treatment and cure using NS5A inhibitors. Infect Drug Resist. 2014;7:41-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Liu J, Shi S, Zhuang H, Luo G. Recent advance in antiviral drugs for hepatitis C. Zhongnandaxue Xuebao Yixueban. 2011;36:1025-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 10. | Chae HB, Park SM, Youn SJ. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. ScientificWorldJournal. 2013;2013:704912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Sokal EM, Bourgois A, Stéphenne X, Silveira T, Porta G, Gardovska D, Fischler B, Kelly D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol. 2010;52:827-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, Shelton M, Kerkar N, Galoppo M, Pedreira A. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010;52:501-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Schwarz KB, Gonzalez-Peralta RP, Murray KF, Molleston JP, Haber BA, Jonas MM, Rosenthal P, Mohan P, Balistreri WF, Narkewicz MR. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology. 2011;140:450-458.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2252] [Cited by in F6Publishing: 2192] [Article Influence: 146.1] [Reference Citation Analysis (1)] |
| 15. | Wirth S. Current treatment options and response rates in children with chronic hepatitis C. World J Gastroenterol. 2012;18:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Druyts E, Thorlund K, Wu P, Kanters S, Yaya S, Cooper CL, Mills EJ. Efficacy and safety of pegylated interferon alfa-2a or alfa-2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta-analysis. Clin Infect Dis. 2013;56:961-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2776] [Cited by in F6Publishing: 2666] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 18. | Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338-1345, 1345.e1-e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 850] [Cited by in F6Publishing: 854] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 19. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1505] [Cited by in F6Publishing: 1482] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 20. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1747] [Cited by in F6Publishing: 1746] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 21. | Domagalski K, Pawłowska M, Tretyn A, Halota W, Pilarczyk M, Smukalska E, Linkowska K, Grzybowski T. Impact of IL-28B polymorphisms on pegylated interferon plus ribavirin treatment response in children and adolescents infected with HCV genotypes 1 and 4. Eur J Clin Microbiol Infect Dis. 2013;32:745-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Shaker OG, Nassar YH, Nour ZA, El Raziky M. Single-nucleotide polymorphisms of IL-10 and IL-28B as predictors of the response of IFN therapy in HCV genotype 4-infected children. J Pediatr Gastroenterol Nutr. 2013;57:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Sung H, Chang M, Saab S. Management of Hepatitis C Antiviral Therapy Adverse Effects. Curr Hepat Rep. 2011;10:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Serranti D, Buonsenso D, Ceccarelli M, Gargiullo L, Ranno O, Valentini P. Pediatric hepatitis C infection: to treat or not to treat...what’s the best for the child? Eur Rev Med Pharmacol Sci. 2011;15:1057-1067. [PubMed] [Cited in This Article: ] |
| 25. | Abdel-Aziz DH, Sabry NA, El-Sayed MH, El-Gazayerly ON. Efficacy and safety of pegylated interferon in children and adolescents infected with chronic hepatitis C: a preliminary study. J Pharm Pract. 2011;24:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Yamane A, Nakamura T, Suzuki H, Ito M, Ohnishi Y, Ikeda Y, Miyakawa Y. Interferon-alpha 2b-induced thrombocytopenia is caused by inhibition of platelet production but not proliferation and endomitosis in human megakaryocytes. Blood. 2008;112:542-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Suzuki M, Inage E, Minowa K, Saito N, Naritaka N, Tsubahara M, Ohtsuka Y, Tokita A, Shimizu T. Prophylaxis for ribavirin-related anemia using eicosapentaenoic acid in chronic hepatitis C patients. Pediatr Int. 2012;54:528-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, Noventa F, Stanzial AM, Solero P, Corrocher R. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 345] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Al-Huthail YR. Neuropsychiatric side-effects of interferon alfa therapy for hepatitis C and their management: a review. Saudi J Gastroenterol. 2006;12:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmun. 2010;34:J322-J326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Doi F, Kakizaki S, Takagi H, Murakami M, Sohara N, Otsuka T, Abe T, Mori M. Long-term outcome of interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. Liver Int. 2005;25:242-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Nadeem A, Aslam M, Khan DA, Hussain T, Khan SA. Effects of combined interferon alpha and ribavirin therapy on thyroid functions in patients with chronic hepatitis C. J Coll Physicians Surg Pak. 2009;19:86-89. [PubMed] [Cited in This Article: ] |
| 33. | Narkewicz MR, Rosenthal P, Schwarz KB, Drack A, Margolis T, Repka MX. Ophthalmologic complications in children with chronic hepatitis C treated with pegylated interferon. J Pediatr Gastroenterol Nutr. 2010;51:183-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Veluru C, Atluri D, Chadalavada R, Burns E, Mullen KD. Skin rash during chronic hepatitis C therapy. Gastroenterol Hepatol (N Y). 2010;6:323-325. [PubMed] [Cited in This Article: ] |
| 35. | Mistry N, Shapero J, Crawford RI. A review of adverse cutaneous drug reactions resulting from the use of interferon and ribavirin. Can J Gastroenterol. 2009;23:677-683. [PubMed] [Cited in This Article: ] |
| 36. | Kanda T, Steele R, Ray R, Ray RB. Small interfering RNA targeted to hepatitis C virus 5’ nontranslated region exerts potent antiviral effect. J Virol. 2007;81:669-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Fauvelle C, Felmlee DJ, Baumert TF. Unraveling hepatitis C virus structure. Cell Res. 2014;24:385-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Curr Top Microbiol Immunol. 2013;369:113-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Lohmann V. Hepatitis C virus RNA replication. Curr Top Microbiol Immunol. 2013;369:167-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Morikawa K, Lange CM, Gouttenoire J, Meylan E, Brass V, Penin F, Moradpour D. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat. 2011;18:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Pan Q, Peppelenbosch MP, Janssen HL, de Knegt RJ. Telaprevir/boceprevir era: from bench to bed and back. World J Gastroenterol. 2012;18:6183-6188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Tran TT. A review of standard and newer treatment strategies in hepatitis C. Am J Manag Care. 2012;18:S340-S349. [PubMed] [Cited in This Article: ] |
| 43. | Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Müh U, Welker M, Wincheringer D, Zhou Y, Chu HM, Lin C. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767-1777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 528] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 44. | Chang MH, Gordon LA, Fung HB. Boceprevir: a protease inhibitor for the treatment of hepatitis C. Clin Ther. 2012;34:2021-2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1835] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 46. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1951] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 47. | Wyles DL. Beyond telaprevir and boceprevir: resistance and new agents for hepatitis C virus infection. Top Antivir Med. 2012;20:139-145. [PubMed] [Cited in This Article: ] |
| 48. | Vermehren J, Sarrazin C. The role of resistance in HCV treatment. Best Pract Res Clin Gastroenterol. 2012;26:487-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 49. | Halfon P, Locarnini S. Hepatitis C virus resistance to protease inhibitors. J Hepatol. 2011;55:192-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 50. | Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology. 2010;138:447-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 51. | Romano KP, Ali A, Aydin C, Soumana D, Ozen A, Deveau LM, Silver C, Cao H, Newton A, Petropoulos CJ. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog. 2012;8:e1002832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 52. | Thomas XV, de Bruijne J, Sullivan JC, Kieffer TL, Ho CK, Rebers SP, de Vries M, Reesink HW, Weegink CJ, Molenkamp R. Evaluation of persistence of resistant variants with ultra-deep pyrosequencing in chronic hepatitis C patients treated with telaprevir. PLoS One. 2012;7:e41191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Cento V, Mirabelli C, Salpini R, Dimonte S, Artese A, Costa G, Mercurio F, Svicher V, Parrotta L, Bertoli A. HCV genotypes are differently prone to the development of resistance to linear and macrocyclic protease inhibitors. PLoS One. 2012;7:e39652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Barritt AS, Fried MW. Maximizing opportunities and avoiding mistakes in triple therapy for hepatitis C virus. Gastroenterology. 2012;142:1314-1323.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Teixeira R, Nascimento Yde A, Crespo D. Safety aspects of protease inhibitors for chronic hepatitis C: adverse events and drug-to-drug interactions. Braz J Infect Dis. 2013;17:194-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45:1085-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55:1620-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 58. | Wilby KJ, Greanya ED, Ford JA, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11:179-185. [PubMed] [Cited in This Article: ] |
| 59. | Huang Y, Staschke K, De Francesco R, Tan SL. Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication? Virology. 2007;364:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Fridell RA, Qiu D, Valera L, Wang C, Rose RE, Gao M. Distinct functions of NS5A in hepatitis C virus RNA replication uncovered by studies with the NS5A inhibitor BMS-790052. J Virol. 2011;85:7312-7320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Belema M, Nguyen VN, Bachand C, Deon DH, Goodrich JT, James CA, Lavoie R, Lopez OD, Martel A, Romine JL. Hepatitis C virus NS5A replication complex inhibitors: the discovery of daclatasvir. J Med Chem. 2014;57:2013-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Gentile I, Borgia F, Coppola N, Buonomo AR, Castaldo G, Borgia G. Daclatasvir: the first of a new class of drugs targeted against hepatitis C virus NS5A. Curr Med Chem. 2014;21:1391-1404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 64. | Nettles RE, Gao M, Bifano M, Chung E, Persson A, Marbury TC, Goldwater R, DeMicco MP, Rodriguez-Torres M, Vutikullird A. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54:1956-1965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 65. | Nakamoto S, Kanda T, Wu S, Shirasawa H, Yokosuka O. Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J Gastroenterol. 2014;20:2902-2912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 103] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 66. | Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 67. | Fridell RA, Wang C, Sun JH, O’Boyle DR, Nower P, Valera L, Qiu D, Roberts S, Huang X, Kienzle B. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology. 2011;54:1924-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 68. | Paolucci S, Fiorina L, Mariani B, Gulminetti R, Novati S, Barbarini G, Bruno R, Baldanti F. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naïve patients. Virol J. 2013;10:355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Lee C. Daclatasvir: potential role in hepatitis C. Drug Des Devel Ther. 2013;7:1223-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Soriano V, Vispo E, de Mendoza C, Labarga P, Fernandez-Montero JV, Poveda E, Treviño A, Barreiro P. Hepatitis C therapy with HCV NS5B polymerase inhibitors. Expert Opin Pharmacother. 2013;14:1161-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Gentile I, Borgia F, Buonomo AR, Castaldo G, Borgia G. A novel promising therapeutic option against hepatitis C virus: an oral nucleotide NS5B polymerase inhibitor sofosbuvir. Curr Med Chem. 2013;20:3733-3742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Varshney J, Sharma PK, Sharma A. A review on an update of NS5B polymerase hepatitis C virus inhibitors. Eur Rev Med Pharmacol Sci. 2012;16:667-671. [PubMed] [Cited in This Article: ] |
| 73. | Pockros PJ. Nucleoside/nucleotide analogue polymerase inhibitors in development. Clin Liver Dis. 2013;17:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Koch U, Narjes F. Allosteric inhibition of the hepatitis C virus NS5B RNA dependent RNA polymerase. Infect Disord Drug Targets. 2006;6:31-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Herbst DA, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 603] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 77. | A special meeting review edition: Advances in the Treatment of Hepatitis C Virus Infection From EASL 2013: The 48th Annual Meeting of the European Association for the Study of the LiverApril 24-28, 2013 • Amsterdam, The NetherlandsSpecial Reporting on: • Simeprevir Plus Peginterferon/Ribavirin Is Associated with a High SVR12 Rate in Treatment-Naive Patients with Genotype 1 Hepatitis C Virus Infection• Addition of Simeprevir to Peginterferon/Ribavirin Is Associated with Faster Resolution of Fatigue in Treatment-Naive Patients• Sofosbuvir Plus Ribavirin Demonstrates Significant Efficacy in Multiple HCV Genotype 2/3 Populations• Daclatasvir Plus Sofosbuvir with or without Ribavirin Yields 100% SVR24 Rate in Genotype 1 Patients Who Fail Telaprevir or Boceprevir• Addition of TG4040 Vaccine to Peginterferon/Ribavirin Increases Sustained Virologic Response Rate at 24 Weeks in Genotype 1 Hepatitis C InfectionPLUS Meeting Abstract Summaries With Expert Commentary by: Ira M. Jacobson, MDJoan Sanford I. Weill Medical College at Cornell UniversityNew York, New York. Gastroenterol Hepatol (N Y). 2013;9:1-18. [PubMed] [Cited in This Article: ] |
| 78. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia ES, Brainard DM, Symonds WT. Sofosbuvir Ribavirin for 12 or 24 Weeks for Patients With HCV Genotype 2 or 3: the VALENCE Trial. Proceedings of the 64th Annual Meeting of the American Association for the Study of Liver Diseases. USA: Washington, DC 2013; 1-5. [Cited in This Article: ] |
| 79. | Stedman CA. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol. 2013;28:38-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Sofia MJ, Chang W, Furman PA, Mosley RT, Ross BS. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J Med Chem. 2012;55:2481-2531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 81. | Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol. 1999;6:937-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 621] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 82. | Wagner F, Thompson R, Kantaridis C, Simpson P, Troke PJ, Jagannatha S, Neelakantan S, Purohit VS, Hammond JL. Antiviral activity of the hepatitis C virus polymerase inhibitor filibuvir in genotype 1-infected patients. Hepatology. 2011;54:50-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Larrey D, Lohse AW, de Ledinghen V, Trepo C, Gerlach T, Zarski JP, Tran A, Mathurin P, Thimme R, Arastéh K. Rapid and strong antiviral activity of the non-nucleosidic NS5B polymerase inhibitor BI 207127 in combination with peginterferon alfa 2a and ribavirin. J Hepatol. 2012;57:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Pockros PJ. Drugs in development for chronic hepatitis C: a promising future. Expert Opin Biol Ther. 2011;11:1611-1622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Gallay PA, Lin K. Profile of alisporivir and its potential in the treatment of hepatitis C. Drug Des Devel Ther. 2013;7:105-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. | Frausto SD, Lee E, Tang H. Cyclophilins as modulators of viral replication. Viruses. 2013;5:1684-1701. [PubMed] [Cited in This Article: ] |
| 87. | Kaul A, Stauffer S, Berger C, Pertel T, Schmitt J, Kallis S, Zayas M, Lohmann V, Luban J, Bartenschlager R. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog. 2009;5:e1000546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 88. | Lee J. Cyclophilin A as a New Therapeutic Target for Hepatitis C Virus-induced Hepatocellular Carcinoma. Korean J Physiol Pharmacol. 2013;17:375-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Watashi K. Alisporivir, a cyclosporin derivative that selectively inhibits cyclophilin, for the treatment of HCV infection. Curr Opin Investig Drugs. 2010;11:213-224. [PubMed] [Cited in This Article: ] |
| 90. | Flisiak R, Feinman SV, Jablkowski M, Horban A, Kryczka W, Pawlowska M, Heathcote JE, Mazzella G, Vandelli C, Nicolas-Métral V. The cyclophilin inhibitor Debio 025 combined with PEG IFNalpha2a significantly reduces viral load in treatment-naïve hepatitis C patients. Hepatology. 2009;49:1460-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 91. | Schaefer EA, Chung RT. Anti-hepatitis C virus drugs in development. Gastroenterology. 2012;142:1340-1350.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Flisiak R, Horban A, Gallay P, Bobardt M, Selvarajah S, Wiercinska-Drapalo A, Siwak E, Cielniak I, Higersberger J, Kierkus J. The cyclophilin inhibitor Debio-025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology. 2008;47:817-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 93. | Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1284] [Cited by in F6Publishing: 1262] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 94. | van der Ree MH, de Bruijne J, Kootstra NA, Jansen PL, Reesink HW. MicroRNAs: role and therapeutic targets in viral hepatitis. Antivir Ther. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Baek J, Kang S, Min H. MicroRNA-targeting therapeutics for hepatitis C. Arch Pharm Res. 2014;37:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Hu J, Xu Y, Hao J, Wang S, Li C, Meng S. MiR-122 in hepatic function and liver diseases. Protein Cell. 2012;3:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 97. | Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 98. | Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1665] [Cited by in F6Publishing: 1619] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 99. | Negro F. Adverse effects of drugs in the treatment of viral hepatitis. Best Pract Res Clin Gastroenterol. 2010;24:183-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1416] [Cited by in F6Publishing: 1420] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 101. | Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63-68. [PubMed] [Cited in This Article: ] |
| 102. | Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 103. | Rauch I, Müller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2:e23820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 104. | Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Friborg J, Levine S, Chen C, Sheaffer AK, Chaniewski S, Voss S, Lemm JA, McPhee F. Combinations of lambda interferon with direct-acting antiviral agents are highly efficient in suppressing hepatitis C virus replication. Antimicrob Agents Chemother. 2013;57:1312-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 107. | Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hézode C, Lim JK, Bronowicki JP, Abrams GA. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 108. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 109. | González-Moreno J, Payeras-Cifre A. Hepatitis C virus infection: looking for interferon free regimens. ScientificWorldJournal. 2013;2013:825375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |