Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14589
Revised: May 12, 2014
Accepted: June 20, 2014
Published online: October 28, 2014
Viral hepatitis remains a worldwide public health problem. The hepatitis D virus (HDV) must either coinfect or superinfect with the hepatitis B virus (HBV). HDV contains a small RNA genome (approximately 1.7 kb) with a single open reading frame (ORF) and requires HBV supplying surface antigens (HBsAgs) to assemble a new HDV virion. During HDV replication, two isoforms of a delta antigen, a small delta antigen (SDAg) and a large delta antigen (LDAg), are produced from the same ORF of the HDV genome. The SDAg is required for HDV replication, whereas the interaction of LDAg with HBsAgs is crucial for packaging of HDV RNA. Various clinical outcomes of HBV/HDV dual infection have been reported, but the molecular interaction between HBV and HDV is poorly understood, especially regarding how HBV and HDV compete with HBsAgs for assembling virions. In this paper, we review the role of endoplasmic reticulum stress induced by HBsAgs and the molecular pathway involved in their promotion of LDAg nuclear export. Because the nuclear sublocalization and export of LDAg is regulated by posttranslational modifications (PTMs), including acetylation, phosphorylation, and isoprenylation, we also summarize the relationship among HBsAg-induced endoplasmic reticulum stress signaling, LDAg PTMs, and nuclear export mechanisms in this review.
Core tip: Hepatitis D virus (HDV) is a defective virus that depends on hepatitis B virus (HBV) to supply envelope proteins (HBsAgs) for assembling a new virion. The association of the clinical severity of hepatitis with HDV genotypes (HDV-1 to 8) has been reported, but the mechanism is unknown. Whether the combinations of HBV genotypes (A to H) with HDV genotypes cause varying clinical outcomes remains to be explored. This review focuses on HDV replication, the cross-talk between HDV and HBV, and the endoplasmic reticulum (ER) stress induced by HBsAgs in the ER which results in the promotion of large delta antigen export from the nucleus to interact with HBsAgs.
- Citation: Huang CR, Lo SJ. Hepatitis D virus infection, replication and cross-talk with the hepatitis B virus. World J Gastroenterol 2014; 20(40): 14589-14597
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14589
At least five hepatitis viruses, including the hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV), which can infect humans have been identified and their transmission routes are diverse[1]. These routes can be divided into the oral-fecal route (HAV and HEV) and the blood transmission route (HBV, HCV, and HDV). All hepatitis virus infections cause acute hepatitis; the group that is transmitted through blood can cause both acute and chronic hepatitis[2]. Four hepatitis viruses (HAV, HBV, HCV, and HEV) can infect humans independently, but HDV cannot. HDV either coinfects or superinfects with HBV because it requires the support of HBV to complete its life cycle. Therefore, HDV is known as a defective virus, or a satellite virus of HBV[3-5]. Globally, 350 million people are HBV carriers; among them, more than 15-20 million people are infected by HDV, which is associated with the most severe form of viral hepatitis[6,7]. Although HDV infection has been reported worldwide, its prevalence is not uniform. The regions with a high prevalence of HDV infection are in the northern region of South America, West and Central Africa, and in Mediterranean countries[6,8,9]. According to a survey conducted in the 1980s, the prevalence of HDV infection varied from 0% to 84.9% among HBV carriers world-wide[10]. The prevalence of HDV infection was 1.5% in Far East Asia, and 21.0% in the Middle East. A higher prevalence was discovered in the following countries: Romania (69.5%), the USSR (38.8%), Italy (24.7%), Turkey (23.4%), and France (20.2%)[10]. However, in other European countries, the rate of HDV infection among HBV carriers varied from 8.5% to 11.0%[11]. A remarkably low prevalence (< 5%) of HDV infection in HBV carriers was reported in HBV highly endemic countries, such as Vietnam, certain provinces of China, Hong Kong (China), and South Korea[12-14]. After the HBV vaccination was administered in Italy, the prevalence of HDV infection was reduced from 24.6% (1983) to 8.1% (1997)[15]. Similarly, in Taiwan, an HBV endemic region, the prevalence of HDV also decreased from 23.7% in 1983 to 4.2% in 1995[16]. Thus, the prevalence of HDV infection was efficiently reduced by the prevention of HBV infection in HBV endemic regions.
Clinically, the coinfection of HDV with HBV leads to acute hepatitis, but subsequent chronic infection is rare, whereas the superinfection of HDV in HBV carriers typically induces a severe form of hepatitis and causes chronic infection compared with the effects of HBV monoinfection[6,8]. In addition, HBV suppression is also observed as an outcome of HDV superinfection in HBV carriers, which results in the persistence of HDV infection[17-20]. Although the mechanism of HDV-induced pathogenesis has not been fully clarified, HDV infection is a critical etiological factor for serious liver diseases, such as fulminant hepatitis[6,21-23]. The role of HDV infection as a risk factor in hepatocellular carcinoma (HCC) remains debatable[23-29]. A report indicated that HDV infection could promote the risk of HCC by nearly three-fold in comparison with HBV monoinfection, which is prevalent in Western European countries[27]. Similar results were also reported in Italy[28], Sweden[24], and Sahelian Africa[23]. A 28-year follow-up study demonstrated that persistent HDV infection promotes cirrhosis and HCC at rates of 4% and 2.8% annually, respectively[26]. Conversely, several reports have indicated that HDV superinfection is not related to the development of HCC in Taiwan[30,31]. Therefore, the role of HDV in the progression of HBV-related liver diseases is a controversial topic.
Previous studies showed that the large delta antigen (LDAg) of HDV might play a role in the HDV-associated pathogenesis because LDAg can activate many eukaryotic promoters to turn on their downstream gene expression[32,33]. It is also reported that the LDAg can modulate tumor growth factor beta (TGFβ)- or c-Jun-induced signal activation[34] and trigger tumor necrosis factor alpha (TNFα)-induced nuclear factor kappa B (NF-κB) nuclear import[35]. Therefore, gene expression or signal activation is considered to contribute to HDV-induced hepatitis. Studies regarding the linkage of HDV-related pathogenesis with HBV are relatively rare. A few studies demonstrated that the LDAg could activate gene expression driven by the pre-S, S and core promoter of HBV[32], but inhibition of that gene expression was mediated by HBV enhancer 1 (Enh1)- and 2 (Enh2).[36] One report showed the X protein of HBV (HBx) induces a synergistic effect on LDAg-activated gene expression[37]. Taken together, these studies might provide some clues to understanding the connection of HDV/HBV-associated pathogenesis. Nevertheless, the contribution of HBV in HDV-related diseases and vice versa remains to be clarified.
The association between the severity of hepatitis and HDV genotypes (HDV-1 to 8) has been reported[6,38-44]; HDV-1 is distributed worldwide and causes hepatitis with a wide range of severity, whereas HDV-2 and HDV-4 infections are distributed only in Japan and Taiwan, and lead to comparatively less severe clinical manifestations[43,45,46]. HDV-3 has been reported to cause a severe form of fulminant hepatitis, which is isolated in the northern region of South America[41,44,47]. HDV-5 to -8 isolates are predominant in Africa, but the relationship between these isolates and the severity of liver diseases requires clarification[38,40,48]. An alternative observation indicated that various combinations of HBV genotypes (A to H) with HDV clades might contribute to the etiology of various clinical outcomes, suggesting that the interaction between HBV and HDV may influence pathogenesis[41,47]. Infection with HBV genotype F has been observed in patients with HDV-3-induced severe hepatitis[49], but a subsequent study demonstrated that HBV genotypes A and D were also isolated in patients with HDV-3-induced hepatitis[41]. Additionally, the influence of the HBV genotype on the replication and assembly of various HDV genotypes has been associated with various clinical outcomes[45,50,51]. The results of previous studies have indicated that the replication level of various HDV genotypes is not associated with the HBV genotype. In contrast, the assembly of various HDV genotypes is correlated with the expression level of HBsAgs, and not with HBV genotypes[50,51]. Therefore, the relationship between the combinations of HBV-HDV genotypes and the clinical outcome of HDV-induced liver diseases remains obscure.
HDV may share the same receptor as HBV for entering hepatocytes because both viruses are enveloped in a phospholipid bilayer embedded with HBsAgs. Several research groups have demonstrated that sodium taurocholate cotransporting peptide[52] and the glycoaminoglycan side chain of heparan sufate proteoglycans are functional receptors for HBV and HDV[53,54]. In addition, purinergic receptors, which are nonhepatocyte-specific receptors, have been suggested to be binding partners of HBV and HDV[55]. Therefore, the exact function of these multiple cell surface receptors for HDV-binding hepatocytes requires further verification. After the HDV attaches to the receptors and enters the hepatocytes, HDV replication requires the assistance of host RNA polymerases for genome amplification and that of HBV surface antigens for new virion assembly. Primarily, HDV replication occurs in the nucleus and new virion assembly occurs in the cytoplasm. Additional details regarding genome amplification and virion assembly are described in the following subsections.
The HDV genome is a negative-sense, which contains 1.7-kb circular RNA[56-59]. Its genome sequence is separated into the protein-coding region and the viroid-like region[60]. The protein-coding region contains the only open reading frame that can be used to produce hepatitis delta antigens (HDAgs), and the viroid-like region contains the ribozyme sequence required for the monomerization of the HDV genome from the multimer products generated during RNA replication[60,61]. During HDV replication, two types of HDAgs are produced: small delta antigen (SDAg) and LDAg[62,63]. The production of LDAg is mediated through small ADAR-1 (ADAR-1S; adenosine deaminase that acts on RNA), converting the amber stop codon (UAG) of SDAg into the tryptophan codon (UGG). Consequently, the LDAg, depending on various HDV genotypes, contains an additional 19 or 20 amino acids at its carboxyl terminus[64-67]. Both HDAgs have an identical amino acid sequence in the N-terminus, but the additional 19 or 20 amino acids of LDAg results in varying degrees of functionality between the LDAg and SDAg in the process of HDV replication. The SDAg is composed of 194 or 195 amino acids and supports HDV replication, whereas the LDAg consists of 214 amino acids, which inhibit HDV replication[63] and interact with HBsAgs to assemble new virions[68].
Both HDAgs are able to bind to HDV genomic RNA, but lack RNA polymerase or replicase activity[69-71]. Therefore, the genome replication of HDV completely depends on the cellular DNA-dependent RNA polymerases (RNAPs) and occurs in the nucleus[69,72-77]. Three species of host RNAPI, II, and III have been demonstrated to interact with the HDV genome[72,78]. However, the role of RNAPI and III in HDV replication remains undetermined, unlike that of RNAPII[69,75-77]. The role of RNAPII in HDV replication involves the de novo synthesis of HDV genomic RNA and HDAg-coding mRNA[69,75]. Whether RNAPI plays a role in HDV replication is debatable. The studies conducted by Lai and colleagues demonstrated that newly synthesized HDV antigenomic RNA is present after treatment with a specific inhibitor of RNAPII, α-amanitin. In contrast, treatment with actinomycin D, an inhibitor of RNAPI, decreases the de novo synthesis of HDV antigenomic RNA[69,76]. However, Taylor and coworkers provided evidence suggesting that RNAPII is the only host RNAP and that it is required for the amplification of both HDV genomic and antigenomic RNA[75,77]. There is no evidence supporting the role of RNAPIII in HDV replication, except for its interaction with the HDV genome[78].
In vitro transfection studies revealed that SDAg is required for HDV replication[63]. SDAg has been demonstrated to interact with RNAPII[76,79,80] and the transcription initiation factor, SL1, of RNAPI[61]. Therefore, SDAg-supporting HDV replication is mediated through the interaction with the host RNAPs to drive the replication of HDV[74,79,80]. Because of the rolling circle mechanism, the replication process produces a linear form of multimeric antigenomes or genomes, which is subsequently cleaved by the ribozyme residing in the antigenomic and genomic sequence to form a monomeric circularized unit. The total number of HDV genomes can reach 300000 copies per single cell and the ratio of genome to antigenome is approximately 5-22 in HDV-infected hepatocytes[81]. The HDV genome also serves as a template for making mRNA to produce SDAg. LDAg is subsequently produced, resulting from ADAR-1 editing, and trans-inhibits HDV replication through the interaction with SDAg to reduce the binding specificity between the HDV genome and SDAg[82]. The LDAg interacts with SDAg and genomic RNA to form RNP that can be exported from the nucleus and assembled with HBsAgs. How the amplified HDV genome, but not antigenome, is selected by HDAgs to form RNP is still unclear.
Several studies have demonstrated that the LDAg alone, or in the presence of HDV RNP, can interact with HBsAgs residing in the endoplasmic reticulum (ER) or Golgi apparatus to form empty viral-like particles or HDV virions[83-85]. The interaction between LDAgs and HBsAgs is dependent on the additional 19 or 20 amino acids at the C-terminus of LDAgs[86]. However, the predominant cellular distribution of LDAgs is in the nucleus, whereas the major location of HBsAgs is in the cytoplasm[85,87-89]; two questions are thus raised: (1) how the LDAg receives signals to export from the nucleus to the cytoplasm; and (2) how the HBsAgs participates in LDAg nuclear export. Furthermore, the exact location, ER or Golgi, where LDAgs interact with HBsAgs to form particles remains undetermined. The study conducted by Tavanez et al[90] demonstrated that the HDV RNP could be shuttled between the nucleus and cytoplasm, suggesting that the interaction between HBsAg and HDV RNP is the consequence of HDV RNP being shuttled from the nucleus to the cytoplasm. The isoprenylation of LDAg is the key event for HDV assembly when HBsAgs interact with HDV RNP[85,89,91]. However, isoprenylated LDAg has been detected inside the nucleus of cells transfected with recombinant LDAg-expressing constructs in the absence of HBsAgs[92]. Thus the role of HBsAgs in the isoprenylation of LDAg remains to be elucidated. Collectively, neither the mechanism of HDV RNP shuttling between the nucleus and cytoplasm nor the cross-talk between LDAg and HBsAg that contributes to the packaging of HDV is fully understood.
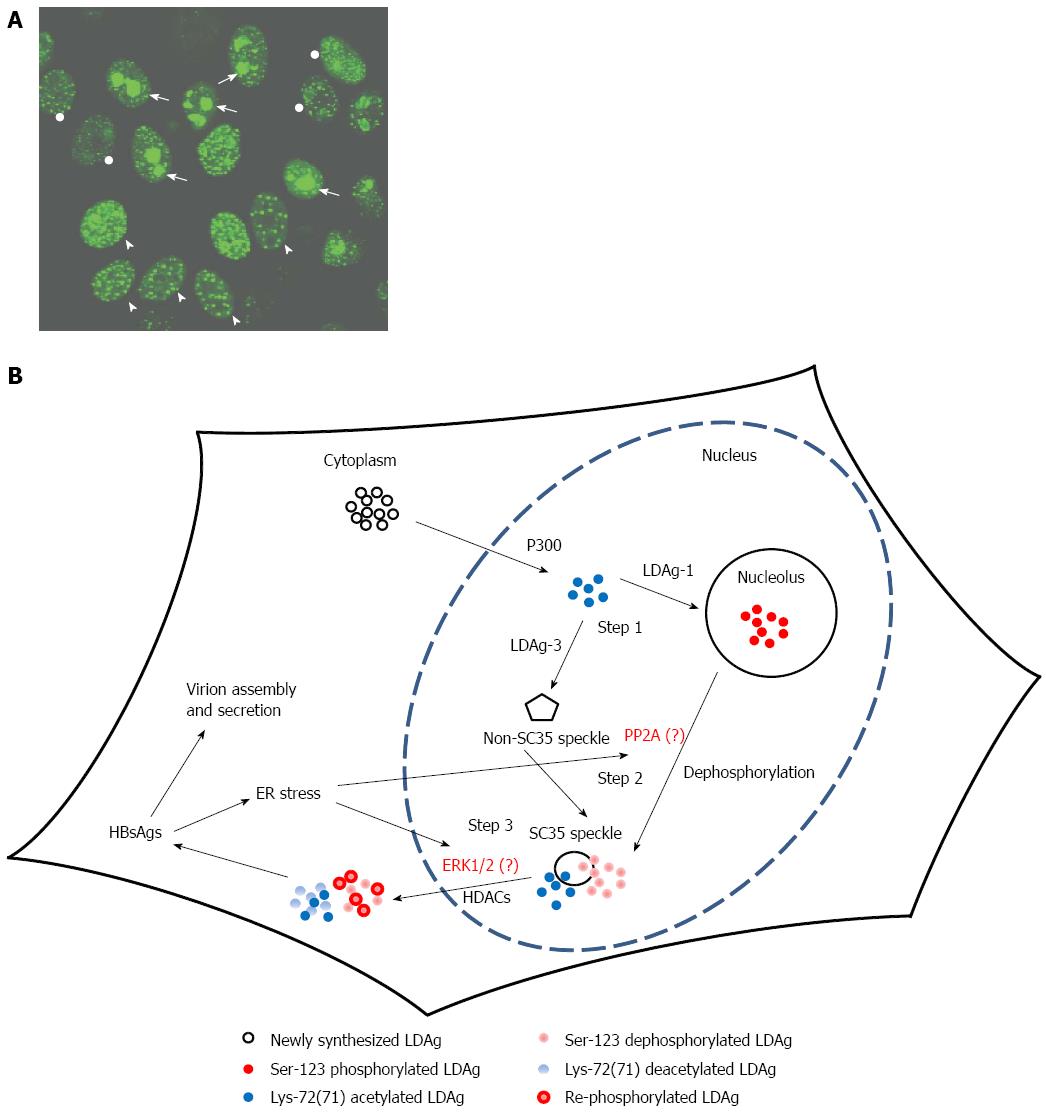
According to accumulated studies, the posttranslational modifications (PTMs) of HDAgs are required to execute various functions during HDV replication, assembly, and secretion. The isoprenylation, phosphorylation, and acetylation of LDAg have been reported to be associated with the dynamic distributions of LDAg and HDV packages[85,86,91,93,94] For example, the cysteine-211 isoprenylation of LDAg is essential for the association with HBsAgs and HDV secretion[86,91], the deficiency of isoprenylated LDAg leads to the loss of dynamic distribution from nucleoli to nuclear speckles and nuclear export, even in the presence of HBsAgs[84,89]. Although isoprenylated LDAgs have been observed inside the nucleus[89], the location where LDAg isoprenylation occurs is still debatable. The serine-123 phosphorylation of LDAgs is crucial for the nucleolus to SC35 nuclear speckle redistribution and HDV secretion[85]. The deficiency of serine-123 phosphorylation in LDAgs results in SC35 nuclear speckle-specific accumulation and leads to the reduction of LDAgs nuclear exportation and viral particle secretion[85]. The lysine-72 acetylation of LDAgs favors their nuclear retention because the lack of lysine-72 acetylation in LDAgs profoundly increases their cytoplasmic accumulation[93]. Combined, the progression of LDAg nuclear export is closely associated with PTMs. However, how the PTMs of LDAgs are regulated is unclear.
Numerous enzymes involved in the PTMs of various proteins are activated or their cellular localizations are changed under ER stress conditions. In HBV-infected cells and under the ER stress condition, the up-regulation of protein phosphatase 2A (PP2A) has been observed[95]. The dephosphorylation of LDAgs is required for the dynamic distribution of LDAgs from the nucleoli to the SC35 nuclear speckle[85]. Whether the PP2A or other protein phosphatases participate in the dephosphorylation of LDAgs for various nuclear translocations has not been clarified. In addition to PP2A, p38 MAPK or ERK1/2 is activated in the ER stress condition[96]. ERK1/2 has been reported to phosphorylate SDAg at serine-177[97], and the ERK1/2 recognition sequence is also present in the LDAg. Whether serine-177 of LDAgs is the site phosphorylated by ERK1/2 for LDAg export is still unclear. We hypothesized that various PTMs of LDAg or various amino acid sequences of LDAg encoded by different genotypes may result in different conformations of LDAg resulting in different targeting patterns to various subcellular sites (Figure 1).
Three types of HBsAg are encoded by the HBV genome, which are designated as small, middle, and large HBsAgs according to their molecular weight. The presence of a large surface antigen (LHBsAg) in virions has been shown to enhance the infectivity of both HBV and HDV[98]. An in vitro HDV package system revealed that the presence of small surface antigen (SHBsAg) is sufficient for completing HDV assembly and secretion[99]. Furthermore, the nuclear export and cytoplasmic retention of HDAgs has been observed to increase in the presence of HBsAgs[83,85,89]. Therefore, this suggests that HBsAgs play a role in the facilitation of HDAg nuclear export because HBsAgs induce ER stress[84].
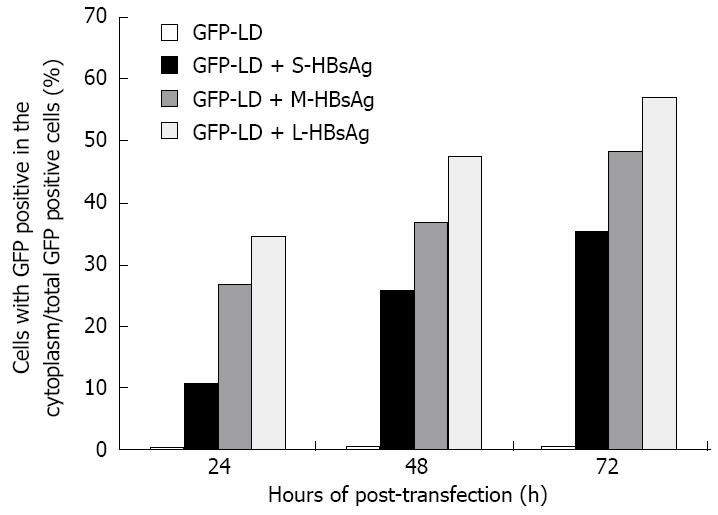
The HDV RNP shuttles back and forth between the nucleus and cytoplasm[90], but the dynamics of LDAg alone in the absence or presence of HBsAgs is poorly understood. To understand the dynamics of LDAg during HDV assembly and secretion[68,83,85,89], in vitro systems have been used in which green-fluorescent protein (GFP) fused with LDAg (GFP-LDAg) is used to trace the location of LDAg under various conditions. In the absence of HBsAg of any type, there is virtually no nuclear export of GFP-LDAg. The highest rate of GFP-LDAg export was detected in the presence of LHBsAg, whereas the lowest rate was observed in the presence of SHBsAg[84] (Figure 2). Together with a study demonstrating that a higher efficiency of HDV packaging was observed in the presence of authentic small HBsAgs than in the presence of nonglycosylated small HBsAgs[100], we hypothesize that the various degrees of glycosylation in the three HBsAgs may result in various capabilities for causing ER stress.
To mimic HBsAg-induced ER stress to facilitate LDAg nuclear export, experiments have been conducted by adding ER stress inducers (brefeldin A and tunicamycin) in the absence of HBsAgs, which resulted in a high amount of GFP-LDAg accumulation in the cytoplasm[84]. The ER stress typically results in activation of NF-κB, which is a transcription factor that facilitates NF-κB down-stream gene expression[101]. Although three types of HBsAgs individually induce varying degrees of NF-κB activation with various results in LDAg nuclear export, the study conducted by Huang et al[84] may provide a line of evidence that cross-talk occurs between HBV and HDV, via signaling through NF-κB activation.
During the course of infection, numerous cytokines, including tumor necrosis factor alpha (TNFα), interferon, and interleukins, are up-regulated in HBV patients; the up-regulated TNFα and IFNγ derived from the T cells limit HBV replication in hepatocytes[102]. The TNFα-activating signal transduction participates in the disruption of HBV capsid integrity via activation of NF-κB[103]. In the absence of HBsAgs, TNFα is reported to significantly increase LDAg nuclear export[84] However, the efficiency of LDAg nuclear export induced by TNFα treatment is unaffected by cycloheximide, a translation inhibitor[84], suggesting that the LDAg nuclear export is not dependent on de novo translation when the signals are induced by TNFα. Both TNFα and HBsAgs-induced ER stress are linked to the activation of NF-κB. However, the link between enzymes that modify LDAgs (PP2A and ERK1/2) and the downstream molecules either activated or suppressed by NF-κB remains unclear.
Coinfection and previous infection with the HBV is necessary for HDV to complete its life cycle and enter uninfected hepatocytes. Few studies have described how the two viruses cross-talk. In this review, we present two possible cross-talk pathways between HBV and HDV: one is through ER stress induced by HBsAgs and the other is through TNFα secreted from immune cells, which respond to HBV infection. Both HBV-dependent pathways can activate NF-κB, and the activation of NF-κB correlates with LDAg nuclear export and HDV particle secretion. Because various states of LDAg PTMs are also closely related to LDAg cellular distribution, identifying the target molecules of NF-κB that play a role in LDAg modifications will be necessary in order to understand the HBV-HDV cross-talk. Additional effort should be invested in studying the cross-talk between HDV and HBV to develop new regimes to control HDV replication and infection, and the resulting clinical complications caused by this virus.
We thank Wallace Academic Editing for editing the manuscript and Michael J Leibowitz (University of California-Davis and visiting Professor at Chang Gung University) for critically reading the manuscript.
P- Reviewer: Cunha C, Gong GZ, Hori T, Lesmana CRA, Pai CR, Sonzogni A, Silva LD S- Editor: Qi Y L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Purcell RH. Hepatitis viruses: changing patterns of human disease. Proc Natl Acad Sci USA. 1994;91:2401-2406. [PubMed] [Cited in This Article: ] |
| 2. | Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 715] [Cited by in F6Publishing: 723] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 3. | Rizzetto M, Canese MG, Gerin JL, London WT, Sly DL, Purcell RH. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis. 1980;141:590-602. [PubMed] [Cited in This Article: ] |
| 4. | Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci USA. 1980;77:6124-6128. [PubMed] [Cited in This Article: ] |
| 5. | Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58:945-950. [PubMed] [Cited in This Article: ] |
| 6. | Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Alves C, Branco C, Cunha C. Hepatitis delta virus: a peculiar virus. Adv Virol. 2013;2013:560105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Pascarella S, Negro F. Hepatitis D virus: an update. Liver Int. 2011;31:7-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Torres JR. Hepatitis B and hepatitis delta virus infection in South America. Gut. 1996;38 Suppl 2:S48-S55. [PubMed] [Cited in This Article: ] |
| 10. | Ponzetto A, Forzani B, Parravicini PP, Hele C, Zanetti A, Rizzetto M. Epidemiology of hepatitis delta virus (HDV) infection. Eur J Epidemiol. 1985;1:257-263. [PubMed] [Cited in This Article: ] |
| 11. | Ciancio A, Rizzetto M. Chronic hepatitis D at a standstill: where do we go from here? Nat Rev Gastroenterol Hepatol. 2014;11:68-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Abbas Z, Jafri W, Raza S. Hepatitis D: Scenario in the Asia-Pacific region. World J Gastroenterol. 2010;16:554-562. [PubMed] [Cited in This Article: ] |
| 14. | Nguyen VT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22:2093-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Rizzetto M, Ciancio A. Epidemiology of hepatitis D. Semin Liver Dis. 2012;32:211-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Huo TI, Wu JC, Lin RY, Sheng WY, Chang FY, Lee SD. Decreasing hepatitis D virus infection in Taiwan: an analysis of contributory factors. J Gastroenterol Hepatol. 1997;12:747-751. [PubMed] [Cited in This Article: ] |
| 17. | Krogsgaard K, Kryger P, Aldershvile J, Andersson P, Sørensen TI, Nielsen JO. Delta-infection and suppression of hepatitis B virus replication in chronic HBsAg carriers. Hepatology. 1987;7:42-45. [PubMed] [Cited in This Article: ] |
| 18. | Hadziyannis SJ, Sherman M, Lieberman HM, Shafritz DA. Liver disease activity and hepatitis B virus replication in chronic delta antigen-positive hepatitis B virus carriers. Hepatology. 1985;5:544-547. [PubMed] [Cited in This Article: ] |
| 19. | Chen PJ, Chen DS, Chen CR, Chen YY, Chen HM, Lai MY, Sung JL. Delta infection in asymptomatic carriers of hepatitis B surface antigen: low prevalence of delta activity and effective suppression of hepatitis B virus replication. Hepatology. 1988;8:1121-1124. [PubMed] [Cited in This Article: ] |
| 20. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis D virus genotypes in intravenous drug users in taiwan: decreasing prevalence and lack of correlation with hepatitis B virus genotypes. J Clin Microbiol. 2002;40:3047-3049. [PubMed] [Cited in This Article: ] |
| 21. | Dienstag JL. Acute Viral Hepatitis. In: Jameson L, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 17 ed. Printed in the United States of America: The McGraw-Hill Companies Inc 2008; 1932-1948. [Cited in This Article: ] |
| 22. | Govindarajan S, Valinluck B, Peters L. Relapse of acute B viral hepatitis--role of delta agent. Gut. 1986;27:19-22. [PubMed] [Cited in This Article: ] |
| 23. | Craxì A, Raimondo G, Longo G, Giannuoli G, De Pasquale R, Caltagirone M, Patti S, Squadrito G, Pagliaro L. Delta agent infection in acute hepatitis and chronic HBsAg carriers with and without liver disease. Gut. 1984;25:1288-1290. [PubMed] [Cited in This Article: ] |
| 24. | Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Abbas Z, Qureshi M, Hamid S, Jafri W. Hepatocellular carcinoma in hepatitis D: does it differ from hepatitis B monoinfection? Saudi J Gastroenterol. 2012;18:18-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, de Franchis R, Ronchi G, Colombo M. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut. 2000;46:420-426. [PubMed] [Cited in This Article: ] |
| 28. | Verme G, Brunetto MR, Oliveri F, Baldi M, Forzani B, Piantino P, Ponzetto A, Bonino F. Role of hepatitis delta virus infection in hepatocellular carcinoma. Dig Dis Sci. 1991;36:1134-1136. [PubMed] [Cited in This Article: ] |
| 29. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [Cited in This Article: ] |
| 30. | Huang YH, Wu JC, Chau GY, Tsay SH, King KL, Sheng WY, Lui WY, Lee SD. Detection of serum hepatitis B, C, and D viral nucleic acids and its implications in hepatocellular carcinoma patients. J Gastroenterol. 1998;33:512-516. [PubMed] [Cited in This Article: ] |
| 31. | Huo TI, Wu JC, Lai CR, Lu CL, Sheng WY, Lee SD. Comparison of clinico-pathological features in hepatitis B virus-associated hepatocellular carcinoma with or without hepatitis D virus superinfection. J Hepatol. 1996;25:439-444. [PubMed] [Cited in This Article: ] |
| 32. | Wei Y, Ganem D. Activation of heterologous gene expression by the large isoform of hepatitis delta antigen. J Virol. 1998;72:2089-2096. [PubMed] [Cited in This Article: ] |
| 33. | Goto T, Kato N, Ono-Nita SK, Yoshida H, Otsuka M, Shiratori Y, Omata M. Large isoform of hepatitis delta antigen activates serum response factor-associated transcription. J Biol Chem. 2000;275:37311-37316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Choi SH, Jeong SH, Hwang SB. Large hepatitis delta antigen modulates transforming growth factor-beta signaling cascades: implication of hepatitis delta virus-induced liver fibrosis. Gastroenterology. 2007;132:343-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Park CY, Oh SH, Kang SM, Lim YS, Hwang SB. Hepatitis delta virus large antigen sensitizes to TNF-alpha-induced NF-kappaB signaling. Mol Cells. 2009;28:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Williams V, Brichler S, Radjef N, Lebon P, Goffard A, Hober D, Fagard R, Kremsdorf D, Dény P, Gordien E. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol. 2009;90:2759-2767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Goto T, Kato N, Yoshida H, Otsuka M, Moriyama M, Shiratori Y, Koike K, Matsumura M, Omata M. Synergistic activation of the serum response element-dependent pathway by hepatitis B virus x protein and large-isoform hepatitis delta antigen. J Infect Dis. 2003;187:820-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Le Gal F, Gault E, Ripault MP, Serpaggi J, Trinchet JC, Gordien E, Dény P. Eighth major clade for hepatitis delta virus. Emerg Infect Dis. 2006;12:1447-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 39. | Huang CR, Lo SJ. Evolution and diversity of the human hepatitis d virus genome. Adv Bioinformatics. 2010;323654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Dény P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol. 2006;307:151-171. [PubMed] [Cited in This Article: ] |
| 41. | Gomes-Gouvêa MS, Soares MC, Bensabath G, de Carvalho-Mello IM, Brito EM, Souza OS, Queiroz AT, Carrilho FJ, Pinho JR. Hepatitis B virus and hepatitis delta virus genotypes in outbreaks of fulminant hepatitis (Labrea black fever) in the western Brazilian Amazon region. J Gen Virol. 2009;90:2638-2643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Ivaniushina V, Radjef N, Alexeeva M, Gault E, Semenov S, Salhi M, Kiselev O, Dény P. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J Gen Virol. 2001;82:2709-2718. [PubMed] [Cited in This Article: ] |
| 43. | Sakugawa H, Nakasone H, Nakayoshi T, Kawakami Y, Miyazato S, Kinjo F, Saito A, Ma SP, Hotta H, Kinoshita M. Hepatitis delta virus genotype IIb predominates in an endemic area, Okinawa, Japan. J Med Virol. 1999;58:366-372. [PubMed] [Cited in This Article: ] |
| 44. | Casey JL, Brown TL, Colan EJ, Wignall FS, Gerin JL. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci USA. 1993;90:9016-9020. [PubMed] [Cited in This Article: ] |
| 45. | Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, Chen SW, Lee PC, Lee SD, Wu JC. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Wu JC, Choo KB, Chen CM, Chen TZ, Huo TI, Lee SD. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet. 1995;346:939-941. [PubMed] [Cited in This Article: ] |
| 47. | Casey JL, Niro GA, Engle RE, Vega A, Gomez H, McCarthy M, Watts DM, Hyams KC, Gerin JL. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis. 1996;174:920-926. [PubMed] [Cited in This Article: ] |
| 48. | Radjef N, Gordien E, Ivaniushina V, Gault E, Anaïs P, Drugan T, Trinchet JC, Roulot D, Tamby M, Milinkovitch MC. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol. 2004;78:2537-2544. [PubMed] [Cited in This Article: ] |
| 49. | Quintero A, Uzcátegui N, Loureiro CL, Villegas L, Illarramendi X, Guevara ME, Ludert JE, Blitz L, Liprandi F, Pujol FH. Hepatitis delta virus genotypes I and III circulate associated with hepatitis B virus genotype F In Venezuela. J Med Virol. 2001;64:356-359. [PubMed] [Cited in This Article: ] |
| 50. | Shih HH, Shih C, Wang HW, Su CW, Sheen IJ, Wu JC. Pro-205 of large hepatitis delta antigen and Pro-62 of major hepatitis B surface antigen influence the assembly of different genotypes of hepatitis D virus. J Gen Virol. 2010;91:1004-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Shih HH, Jeng KS, Syu WJ, Huang YH, Su CW, Peng WL, Sheen IJ, Wu JC. Hepatitis B surface antigen levels and sequences of natural hepatitis B virus variants influence the assembly and secretion of hepatitis d virus. J Virol. 2008;82:2250-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1280] [Cited by in F6Publishing: 1428] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 53. | Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 2008;10:122-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Lamas Longarela O, Schmidt TT, Schöneweis K, Romeo R, Wedemeyer H, Urban S, Schulze A. Proteoglycans act as cellular hepatitis delta virus attachment receptors. PLoS One. 2013;8:e58340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Taylor JM, Han Z. Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS One. 2010;5:e15784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Bonino F, Hoyer B, Shih JW, Rizzetto M, Purcell RH, Gerin JL. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect Immun. 1984;43:1000-1005. [PubMed] [Cited in This Article: ] |
| 57. | Kos A, Dijkema R, Arnberg AC, van der Meide PH, Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 2008;323:558-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 484] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Wang KS, Choo QL, Weiner AJ, Ou JH, Najarian RC, Thayer RM, Mullenbach GT, Denniston KJ, Gerin JL, Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986;323:508-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 567] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 59. | Makino S, Chang MF, Shieh CK, Kamahora T, Vannier DM, Govindarajan S, Lai MM. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature. 1987;329:343-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 261] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Lai MM. The molecular biology of hepatitis delta virus. Annu Rev Biochem. 1995;64:259-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Taylor JM. Hepatitis delta virus. Virology. 2006;344:71-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Bergmann KF, Gerin JL. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986;154:702-706. [PubMed] [Cited in This Article: ] |
| 63. | Chao M, Hsieh SY, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990;64:5066-5069. [PubMed] [Cited in This Article: ] |
| 64. | Polson AG, Ley HL, Bass BL, Casey JL. Hepatitis delta virus RNA editing is highly specific for the amber/W site and is suppressed by hepatitis delta antigen. Mol Cell Biol. 1998;18:1919-1926. [PubMed] [Cited in This Article: ] |
| 65. | Zheng H, Fu TB, Lazinski D, Taylor J. Editing on the genomic RNA of human hepatitis delta virus. J Virol. 1992;66:4693-4697. [PubMed] [Cited in This Article: ] |
| 66. | Luo GX, Chao M, Hsieh SY, Sureau C, Nishikura K, Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990;64:1021-1027. [PubMed] [Cited in This Article: ] |
| 67. | Wong SK, Lazinski DW. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc Natl Acad Sci USA. 2002;99:15118-15123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Chang FL, Chen PJ, Tu SJ, Wang CJ, Chen DS. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci USA. 1991;88:8490-8494. [PubMed] [Cited in This Article: ] |
| 69. | Macnaughton TB, Shi ST, Modahl LE, Lai MM. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J Virol. 2002;76:3920-3927. [PubMed] [Cited in This Article: ] |
| 70. | Lai MM. RNA replication without RNA-dependent RNA polymerase: surprises from hepatitis delta virus. J Virol. 2005;79:7951-7958. [PubMed] [Cited in This Article: ] |
| 71. | Kuo MY, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945-1950. [PubMed] [Cited in This Article: ] |
| 72. | Greco-Stewart VS, Miron P, Abrahem A, Pelchat M. The human RNA polymerase II interacts with the terminal stem-loop regions of the hepatitis delta virus RNA genome. Virology. 2007;357:68-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Lehmann E, Brueckner F, Cramer P. Molecular basis of RNA-dependent RNA polymerase II activity. Nature. 2007;450:445-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Yamaguchi Y, Mura T, Chanarat S, Okamoto S, Handa H. Hepatitis delta antigen binds to the clamp of RNA polymerase II and affects transcriptional fidelity. Genes Cells. 2007;12:863-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Moraleda G, Taylor J. Host RNA polymerase requirements for transcription of the human hepatitis delta virus genome. J Virol. 2001;75:10161-10169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Li YJ, Macnaughton T, Gao L, Lai MM. RNA-templated replication of hepatitis delta virus: genomic and antigenomic RNAs associate with different nuclear bodies. J Virol. 2006;80:6478-6486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Chang J, Nie X, Chang HE, Han Z, Taylor J. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol. 2008;82:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Greco-Stewart VS, Schissel E, Pelchat M. The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III. Virology. 2009;386:12-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Yamaguchi Y, Filipovska J, Yano K, Furuya A, Inukai N, Narita T, Wada T, Sugimoto S, Konarska MM, Handa H. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science. 2001;293:124-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Hong SY, Chen PJ. Phosphorylation of serine 177 of the small hepatitis delta antigen regulates viral antigenomic RNA replication by interacting with the processive RNA polymerase II. J Virol. 2010;84:1430-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci USA. 1986;83:8774-8778. [PubMed] [Cited in This Article: ] |
| 82. | Lin BC, Defenbaugh DA, Casey JL. Multimerization of hepatitis delta antigen is a critical determinant of RNA binding specificity. J Virol. 2010;84:1406-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Huang C, Chang SC, Yu IC, Tsay YG, Chang MF. Large hepatitis delta antigen is a novel clathrin adaptor-like protein. J Virol. 2007;81:5985-5994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Huang IC, Chien CY, Huang CR, Lo SJ. Induction of hepatitis D virus large antigen translocation to the cytoplasm by hepatitis B virus surface antigens correlates with endoplasmic reticulum stress and NF-kappaB activation. J Gen Virol. 2006;87:1715-1723. [PubMed] [Cited in This Article: ] |
| 85. | Tan KP, Shih KN, Lo SJ. Ser-123 of the large antigen of hepatitis delta virus modulates its cellular localization to the nucleolus, SC-35 speckles or the cytoplasm. J Gen Virol. 2004;85:1685-1694. [PubMed] [Cited in This Article: ] |
| 86. | Hwang SB, Lai MM. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67:7659-7662. [PubMed] [Cited in This Article: ] |
| 87. | Bichko VV, Taylor JM. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J Virol. 1996;70:8064-8070. [PubMed] [Cited in This Article: ] |
| 88. | Han Z, Alves C, Gudima S, Taylor J. Intracellular localization of hepatitis delta virus proteins in the presence and absence of viral RNA accumulation. J Virol. 2009;83:6457-6463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Shih KN, Lo SJ. The HDV large-delta antigen fused with GFP remains functional and provides for studying its dynamic distribution. Virology. 2001;285:138-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Tavanez JP, Cunha C, Silva MC, David E, Monjardino J, Carmo-Fonseca M. Hepatitis delta virus ribonucleoproteins shuttle between the nucleus and the cytoplasm. RNA. 2002;8:637-646. [PubMed] [Cited in This Article: ] |
| 91. | Glenn JS, Watson JA, Havel CM, White JM. Identification of a prenylation site in delta virus large antigen. Science. 1992;256:1331-1333. [PubMed] [Cited in This Article: ] |
| 92. | Lin HP, Hsu SC, Wu JC, Sheen IJ, Yan BS, Syu WJ. Localization of isoprenylated antigen of hepatitis delta virus by anti-farnesyl antibodies. J Gen Virol. 1999;80:91-96. [PubMed] [Cited in This Article: ] |
| 93. | Huang CR, Wang RY, Hsu SC, Lo SJ. Lysine-71 in the large delta antigen of hepatitis delta virus clade 3 modulates its localization and secretion. Virus Res. 2012;170:75-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Mu JJ, Tsay YG, Juan LJ, Fu TF, Huang WH, Chen DS, Chen PJ. The small delta antigen of hepatitis delta virus is an acetylated protein and acetylation of lysine 72 may influence its cellular localization and viral RNA synthesis. Virology. 2004;319:60-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008;49:429-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 96. | Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384-46392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 97. | Mu JJ, Chen DS, Chen PJ. The conserved serine 177 in the delta antigen of hepatitis delta virus is one putative phosphorylation site and is required for efficient viral RNA replication. J Virol. 2001;75:9087-9095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Sureau C, Guerra B, Lanford RE. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993;67:366-372. [PubMed] [Cited in This Article: ] |
| 99. | Wang CJ, Chen PJ, Wu JC, Patel D, Chen DS. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J Virol. 1991;65:6630-6636. [PubMed] [Cited in This Article: ] |
| 100. | Wang CJ, Sung SY, Chen DS, Chen PJ. N-linked glycosylation of hepatitis B surface antigens is involved but not essential in the assembly of hepatitis delta virus. Virology. 1996;220:28-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63-67. [PubMed] [Cited in This Article: ] |
| 102. | Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |