INTRODUCTION
Stevens-Johnson syndrome (SJS), a rare but severe dermatological condition[1], is considered a severe type of erythema exsudativum multiforme[2], characterized by erythema with bullous and eroded lesions of skin and mucous membranes. It typically occurs after ingestion of medications such as nonsteroidal drugs, antibiotics, and anticonvulsants[12]. Extracutaneous manifestations of the syndrome may involve the conjunctiva, trachea, buccal mucosa, gastrointestinal tract, and genitourinary tract[23]. Cholestatic liver disease, which may precede the cutaneous manifestations of SJS, occurs in a very limited number of patients with SJS. In the present case, SJS and acute vanishing bile duct syndrome (VBDS), the most severe cholestatic liver diseases, were identified.
CASE REPORT
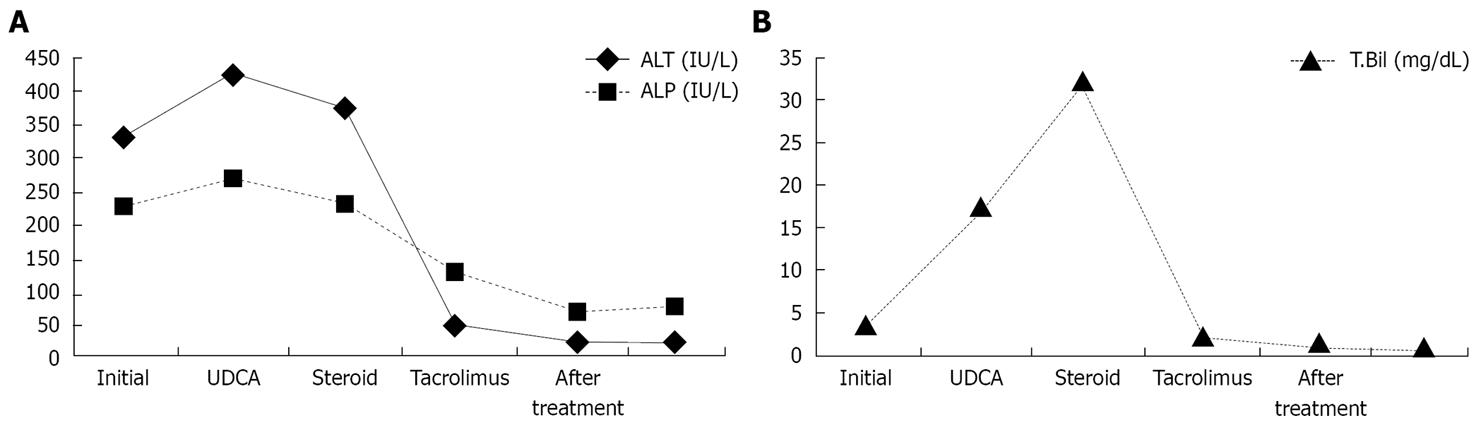
A 26-year-old woman presented to the emergency department of our hospital, complaining of dysphagia, dysuria, fever and rashes on her body. Two weeks prior to eruption, 500 mg ciprofloxacin, twice daily, was started for acute bronchitis by a pulmonologist. Her medical history was unremarkable. Physical examination revealed macular erythematous eruption on her face, back, arms and erosions on the lips, buccal and genital mucous membranes. She was febrile (39°C), her conjunctivae were icteric and injected. Laboratory investigations showed 4.07 mg/dL total biluribin (< 1.2 mg/dL), 3.58 mg/dL conjugated bilirubin (< 0.2 mg/dL), 326 IU/L alanine transaminase (ALT, < 31 IU/L), 280 IU/L gamma-glutamyl-transpeptidase (GGT, < 36 IU/L), 229 IU/L alkaline phosphatase (ALP, < 104 IU/L), and 107 mm/h erythrocyte sedimentation rate (ESR). Serologic tests were negative for viral hepatitis A-C, E, Epstein Barr virus, cytomegalovirus, Herpes simplex virus, Mycobacteria and Mycoplasma pneumonia. Abdominal ultrasound showed that liver had homogeneous texture with normal bile ducts and gallbladder. Skin biopsy from the neck showed subepidermal vesiculation and epidermal necrosis, which was consistent with SJS. Skin eruptions resolved after supportive care. However, cholestatic picture persisted. Ursodeoxycholic acid treatment (15 mg/kg per day) did not aleviate the symptoms. Antinuclear antibody, anti-neutrophil cytoplasmic antibody, anti-liver/kidney microsomal antibody and antismooth muscle antibody titers turned out to be negative. Magnetic resonance cholangiopancreatography showed no abnormality in bile ducts. Percutaneous liver biopsy 30 d after the diagnosis showed cholestasis, along with a decreased number of bile ducts and mild portal inflammation, which was consistent with VBDS (Figure 1). Prednisone (40 mg/d) was added to the treatment with ursodeoxycholic acid and discontinued after 4 wk because of lack of efficacy and iatrogenic Cushing’s syndrome. Cholestasis persisted and bilirubin level exceeded 30 mg/dL (Figure 2). After long medical discussions and repetitive literature reviews, she was put on treatment with tacrolimus (0.15 mg/kg per day in two divided doses). Significant clinical and biochemical improvements were observed 3 mo after treatment. She took tacrolimus for 3 mo, ursodeoxycholic acid (15 mg/kg per day) for 7 mo. She had a normal physical status with normal liver synthetic functions and was instructed to avoid ciprofloxacin in the future.
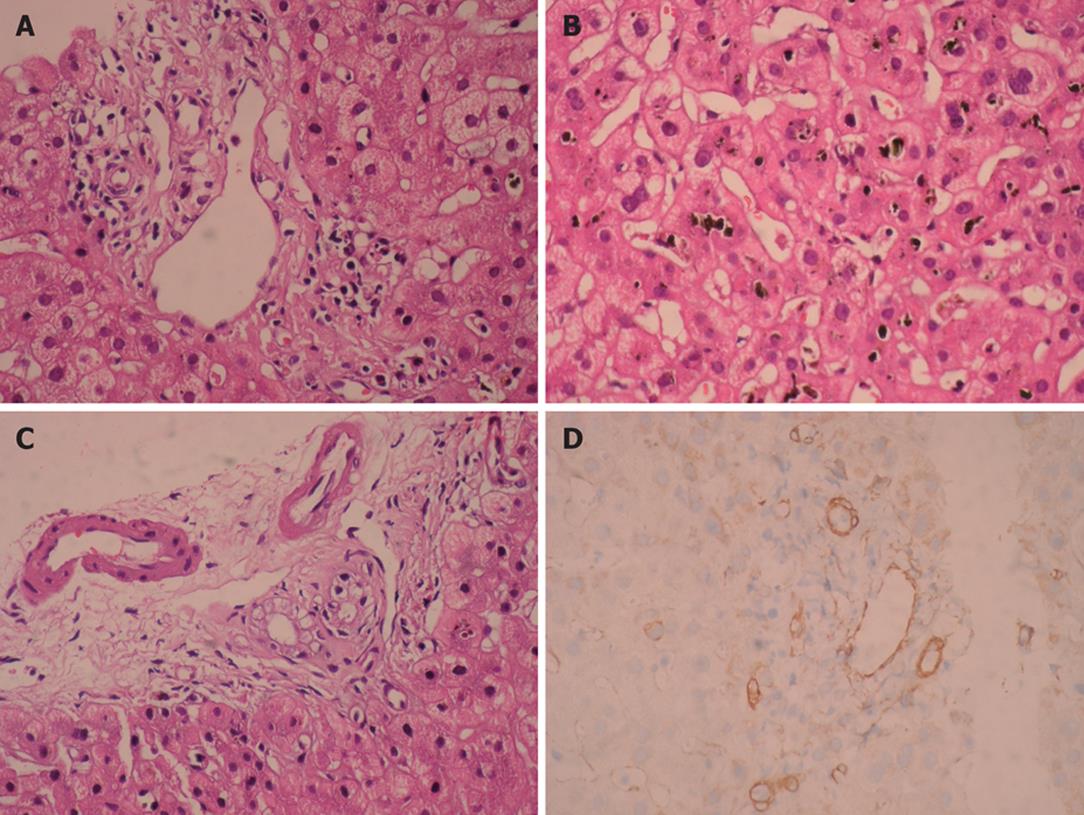
Figure 1 Moderate predominantly lymphocytic mixed portal infiltration and absence of interlobular bile duct destruction (HE, × 400) (A), intralobular severe canalicular cholestasis (HE, × 400) (B), marked cytoplasmic vacuolization in cholangiolar epithelium (HE, × 400) (C), and absence of bile ducts by cytokeratin 19 immunohistochemical staining (IHC, × 400) (D), observed in our patient.
Figure 2 ALT and ALP (A) and total biluribin (B) levels during and after treatment in our case.
DISCUSSION
SJS is a serious and potentially life-threatening disease, mainly caused by drugs. More than 100 different medications have been implicated[1]. The syndrome usually begins within 1-14 d of ingestion of the offending agent but may not manifest itself for up to three to 6 wk after ingestion[3]. In our case, the interval was 3 wk. Anticonvulsants, sulfonamides, penicillins, allopurinol, nonsteroidal anti-inflammatory drugs are the most common culprit medications while ciprofloxacin and cephalosporin are rarely reported as the causative agents of SJS[134].
SJS is a well-recognized immune complex-mediated hypersensitivity reaction that affects all age groups[5–7]. It has classic systemic, mucosal and dermatologic manifestations[5]. VBDS, a rare cause of progressive cholestasis, is mostly related with drugs. Drugs act as a hapten and produce autoantibodies against cytokeratin which is in the bile duct, skin, conjunctival epithelium and orogenital mucosa[5]. Autoantibodies destroy biliary apparatus and as a result, with resultant disappearance of intrahepatic bile duct[8].
The mechanism of biliary epithelial cell injury and interlobular duct loss in the VBDS has not been fully understood yet[5]. Toxic, idiosyncratic, metabolic, and immune etiologies have been suggested[57]. The latest evidence supports the importance of the immune system in the pathogenesis and suggests mechanisms common to both SJS and VBDS[5]. In SJS, there are immune complex formation and deposition, followed by a cytokine- and cell-mediated response[57]. Many drugs associated with VBDS are also associated with SJS like antibiotics, non-steroidal anti-inflammatory drugs and carbamazepine.
In the literature, there are less than ten reported cases of SJS and VBDS co-occurrence[157]. So it is great deal to have a standardized approach to these patients. There is no proven effective therapy for VBDS and SJS, but authors mostly agree that treatment modalities for both VBDS and SJS include withdrawal of the offending agent, supportive care, and usage of immunosuppressants[910]. Steroids, choleretic agents and immunosuppressants have been tried[1210]. Acute onset of VBDS may be unresponsive to these modalities and progress to biliary cirrhosis, thus liver transplantation may be needed[5]. Because of the paucity of cases, there are no available success and failure rates, frequency of complication and percentage of liver transplantation need. Case-based literature just gives us some clinical clues for treatment approaches.
In our case, VBDS was refractory to steroid and ursodeoxycholic acid therapies. The clinical and biochemical improvements were obtained by immune suppression with tacrolimus therapy in our patient. In the literature, it is the first case of ciprofloxacin-induced VBDS successfully treated with tacrolimus.
Tumor necrosis factor-alpha (TNF-α) has been shown to be strongly expressed in SJS lesions by an Italian group[11]. Authors suggest that IFN-gamma may play an important role in SJS. IL-2, IL-5 and IL-13 may contribute to the cutaneous immunoinflammation in this disease. Since immune complex-mediated reaction is very important in these drug-induced syndromes, anti-TNF-α antibodies may be a promising therapy in the future[1213]. However, it is not available in clinical practice, and there is no reported SJS or VBDS case treated with anti-TNF-α yet in literature.
Several recent studies have reported strong genetic associations between HLA alleles and susceptibility to drug hypersensitivity[14]. The genetic associations can be drug specific, such as HLA-B1502 being associated with carbamazepine-induced SJS, HLA-B5701 with abacavir hypersensitivity and HLA-B5801 with allopurinol-induced severe cutaneous adverse reactions[1516]. The high sensitivity and specificity of some markers provide a plausible basis for developing tests to identify individuals at risk for drug hypersensitivity. Drug specific genetic screening tests may prevent those catastrophic diseases. Prescribing medication, according to history and genetic tests, will decrease the prevalence of both diseases in the future.
Our case reminds physicians of the importance of drug reactions, their severity, techniques for diagnosis and ways of management. Prescribing any drug with ultimate care, early diagnosis and treatment of both syndromes will improve the outcome.